Abstract
Background
Recent trials have suggested the feasibility of performing a sentinel lymph node biopsy (SNB) following neoadjuvant chemotherapy (NAC). The selection of suitable patients for this approach remains controversial. We developed a predictive model to identify patients most likely to benefit from SNB following NAC.
Methods
The National Cancer Data Base was used to identify patients with clinically node positive (cN+) breast cancer undergoing NAC followed by breast surgery and axillary lymph node dissection (ALND). Patients were randomly assigned to a 70% testing or 30% validation cohort for model development. A predictive model was built based on significant factors associated with pathologic nodal response (pN0) and breast response.
Results
Using the testing cohort (n = 13,396), multivariate regression was used to identify predictors of pN0 based on preoperative factors. Younger age, hormone receptor (HR)-negative/Her2-negative, HR-positive/Her2-positive, HR-negative/Her2-positive, high-grade, ductal histology, cN1 versus cN2, and extent of breast response were all significant independent predictors of pN0 on adjusted analysis. The odds ratios translated into a 10-point scale correlating to a stepwise increase in pN0 response. The area under the curve for the ROC curves for the testing and validation cohorts was 0.781 and 0.788, respectively (p < 0.01).
Conclusions
Our model incorporates known preoperative factors to predict the likelihood of pN0 response in patients with cN+ disease who undergo NAC. For patients with high scores, SNB should be considered over ALND, because these patients have a greater likelihood of having negative nodes at final pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For patients with clinically node-negative breast cancer, a sentinel node biopsy (SLNB) provides comparable regional node staging information with less morbidity than axillary lymph node dissection.1,2 However, for patients initially presenting with clinically node-positive breast cancer, the appropriate use of SLNB in the neoadjuvant chemotherapy (NCT) setting is debated.1,3,4 The use of NCT in breast cancer has expanded from its initial role of facilitating resectability to providing important prognostic information in breast cancer management. No data have yet demonstrated a survival advantage of receiving NCT compared with chemotherapy in the adjuvant setting. However, patients who achieve a pathologic complete response (pCR) to NCT have better prognosis compared with those with residual invasive disease in the breast or lymph nodes at the completion of NCT.5,6,7 This is especially true for triple negative and HER2 amplified breast cancer, where pCR is associated with improved survival.8,9
The potential advantage of SLNB in this setting is the avoidance of the morbidity of an axillary dissection in cases where there is an axillary pCR. The SENTINA and ACOSOG 1071 trials both affirmed that SLNB can be performed for clinically node positive women who become clinically node negative following neoadjuvant therapy, with the stipulation that a minimum of three sentinel nodes are obtained to lower the false-negative rate sufficiently. However the overall false-negative rate is still a concern, and axillary node dissection remains a standard option in the management.1,4
Given these concerns, changes in the approach and patient selection that result in greater sensitivity have been recommended as necessary to support further the use of SLNB as an alternative to ALND following neoadjuvant therapy.1 Ideally, surgeons would be able to predict which patients would demonstrate response in the axilla and select patients for SLNB in whom an axillary pCR following NCT was most probable. With this goal in mind, we sought to develop a clinical model to predict which patients were most likely to downstage in the axilla and therefore become optimal candidates for SLNB following NCT for breast cancer, based on variables associated with axillary pCR.
Methods
The axillary response predictive model was generated from clinical and pathologic data available in the National Cancer Data Base (NCDB). The NCDB is a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons.10 Variables include specific information on patient demographics, tumor characteristics, stage, treatment course, and timing of treatment. Data are compliant with the privacy requirements of the Health Insurance Portability and Accountability Act.
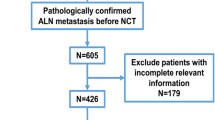
The 2013 Participant User File of the NCDB was used to identify retrospectively women with nonmetastatic, invasive breast cancer between the years 2010 and 2013 (n = 589,378) who underwent neoadjuvant chemotherapy (NAC) followed by breast surgery (n = 59,158), were found to be clinically node positive (cN+), and who underwent an axillary lymph node dissection (ALND) (n = 19,115). Table 1 demonstrates the cohort characteristics. Clinical node status was defined based on the clinical stage designation as recorded in the database. Hormone receptor (HR) positive is defined as either estrogen or progesterone positivity in the data set. Her2 status is defined as reported. Axillary lymph node dissection was defined as a minimum of ten nodes examined on pathology. NAC was defined as a start date of chemotherapy prior to the date of surgery. Specific chemotherapy regimens and number of cycles of therapy are not available in the NCDB. The patient cohort was randomly assigned to two groups using random number generation. The two groups were statistically equivalent in all demographics and tumor characteristics. The randomization process resulted in 70% being assigned to a “testing” group (used in creating our initial model) and 30% being assigned to a “validation” group for confirmation of model strength. Univariate analysis was used to determine factors associated with ypN0 response and clinical breast response (data not shown). The significant factors were then entered into a multivariate analysis (Table 2 – testing cohort MVR), and a predictive model was built based on significant preoperative factors associated with pathologic nodal response (pN0). A numerical score (1–10) was then assigned to corresponding odds ratio from multivariate analysis of increasing ypN0 response, as well as the addition of points for clinical breast response due to the high correlation of pathologic breast and nodal response in our population. The validation cohort was used for validation of the model. ROC analysis was used to evaluate model fit.
All analysis was performed using SPSS software version 19.0 (IBM Corp, Armonk, NY). Statistical test were two-sided, and a p value ≤ 0.05 was considered statistically significant. Random number generation and sorting was used to create randomization to the testing and validation cohorts. Chi square (χ2) tests and multivariate logistic regression models were used to examine factors associated with likelihood of pN0 response. Odds ratio (OR) > 1 signified a higher odds of pN0. Model fit was tested using ROC analysis. All confidence intervals (CI) are reported at a 95% level of significance.
Results
This study identified 19,115 patients between the years 2010 and 2013 with nonmetastatic, clinically node-positive breast cancer who underwent neoadjuvant chemotherapy followed by breast surgery and an axillary lymph node dissection. Of these patients, 27.3% had a complete pathologic nodal response after NAC, and 17.6% had a complete pathologic response in the breast. The distribution of cohort characteristics can be seen in Table 1. First, the randomly selected “testing” and “validation” cohorts were compared and found to be statistically equivalent with respect to patient, facility, tumor, and treatment factors (data not shown).
Characteristics of patients with pathologic node response (pN0) and those with residual positive nodes (pN+) were compared (Table 1). Using the testing cohort, Chi square and univariate analyses were used to identify significant preoperative factors associated with pN0 response (Table 1; data not shown for univariate analysis). These factors were then entered into an adjusted multivariable regression to identify predictors of pN0 response (Table 2). Younger age (OR 1.41 for < 50 y/o), tumor subtype (OR 2.80 for HR-negative/Her2-negative; OR 3.67 for HR-positive/Her2-positive; OR 5.51 for HR-negative/Her2-positive), grade 3 tumors (OR 1.68), ductal histology (OR 1.66), and cN1 tumors (OR 1.28 compared with cN2) were all significant independent predictors of pathologic nodal response on adjusted analysis. The odds ratios of significant independent predictors were used to translate into points for the model (Table 3).
The National Cancer Database collects data on clinical tumor size before neoadjuvant therapy but does not collect data on clinical tumor size following neoadjuvant therapy. Therefore, pathologic tumor size following neoadjuvant therapy was used as a surrogate. Additional analysis, including the variable pathologic breast response was highly significant when added into the model (OR 20.37 for complete response, OR 2.36 for partial response). However, because this could not be determined preoperatively, we did not use the full odds ratio in our model construction but rather used the point value that was found to correlate best with pathologic nodal response when fitted to the model as a surrogate of clinical breast response: 4 points for a complete response in the breast and 2 for a partial response.
Because the cumulative model score was from 5 to 15, the model was adjusted to a 1–10 numeric scale by subtracting 5 from the total score which simplified it into categories of 1–10. Increasing point scores were found to correlate with a stepwise increase in the rate of pathologic complete response. A score of 1 correlated with a 5.7% rate of pathologic complete response in the testing cohort (4.5% in the validation cohort), whereas a score of 10 correlated with an 81.5% rate of pathologic complete response in the testing cohort (76.4% in the validation cohort; Table 4). This is represented graphically in Fig. 1. The area under the curve for the ROC curves for the testing and validation cohorts was 0.781 and 0.788, respectively (both p < 0.001).
Discussion
This model was designed to predict which patients with clinically node-positive breast cancer treated with neoadjuvant chemotherapy would be ideal candidates for SLNB in an attempt to avoid ALND, based on a greater predictive likelihood of achieving ypN0 status. The model incorporated a large, nationwide patient population from a wide variety of facility types. The generated data demonstrated that younger age (OR 1.41 for < 50 y/o), HR-negative/Her2-negative (OR 2.80), HR positive/Her2 positive (OR 3.67), HR negative/Her2 positive (OR 5.51), high-grade (OR 1.68), ductal histology (OR 1.66), cN1 versus cN2 tumors (OR 1.28), and extent of pathologic breast response (OR 20.37) were all significant independent predictors of ypN0 on adjusted analysis.
Of note, pathologic response in the breast was so highly predictive that it heavily outweighed other factors. However, pathologic response in the breast was not incorporated into our final predictive model due to the fact that this pathologic information is not available preoperatively during the time of surgical decision-making. Instead, an estimate of clinical breast response was used for the model. Although not ideal, it may be reasonable to posit that a number of patients who demonstrated a significant pathological response also had a detectable clinical response on breast exam and/or imaging. In fact, studies have demonstrated that despite some limitations, radiographic assessment correlated with pCR after neoadjuvant chemotherapy.11,12 Still, if available, it is possible that using actual clinical response data could produce a distinct predictive model. Ideally, future studies might focus on better assessments of clinical response that would yield new models determining risk of residual node positivity.
Interestingly, no association was found between pretreatment (i.e., clinical) T stage and nodal outcome. This may reflect that it is the degree of response to treatment, rather than initial tumor size, which predicts biological response in the axilla. This further supports the understanding that molecular/biological factors of breast cancer supersede anatomical features in driving behavior of breast cancer.
Our findings are consistent with other models generated that found axillary pCR to be associated with initial clinical nodal status, negative ER status, HER2 amplification, and clinical response observed in the nodes and primary tumor.13,14 Our model, however, benefits from a large sample size, and a national cohort comprised of relatively diverse treatment facilities. The predictive model may serve as a means of identifying which patients are most eligible to undergo SLNB following NCT. It provides a useful clinical tool to predict, a priori, which patients should be considered for NCT due to a higher likelihood of achieving nodal pCR and avoiding unnecessary ALND. The model could arguably also be used to predict those least likely to have pCR and who therefore should go directly to ALND. Used in this manner, the model may help to prepare patients and surgeons for instances where there is greater likelihood of encountering residual axillary disease requiring an ALND. This could help to minimize the risk of false negatives from SLNB in patients for whom pCR in the nodes is improbable.
Further clinical studies using this model are required to determine specific recommendations and guidelines regarding when exactly a SLNB versus ALND may be best indicated following NCT. The question remains about what the appropriate “cutoffs” should be for these clinical decisions. For example, it may be reasonable to omit SLN biopsy in patients with a model score of 1–2 who have less than a 10% chance of a pCR in the nodes. Conversely, a 50% likelihood of pCR may be high enough to dictate an attempted SLNB over ALND following NCT in a patient who was previously clinically node-positive. A prospective clinical trial further investigating the predictive capability of the model and its role in clinical decision making may help to answer these questions. With further study, the predictive model may be incorporated along with the minimum threshold of obtaining three sentinel nodes, using dual tracer, and marking the biopsy-positive node(s) with clips to enable targeted SLNB to improve the accuracy of nodal assessment in this patient population. Whether or not predictive models can or should eliminate these other recommendations is not known at this time.
Our developed model has limitations inherent to the National Cancer Database (NCDB). Patients were excluded from this study, because there were missing and incomplete data, and the data were only collected from commission on cancer accredited centers. These data also were retrospective in nature. Additionally, although the NCDB includes all biopsy-proven positive nodes as clinically node-positive, we were not able to determine whether clinical N stage was designated on clinical findings alone. Our model therefore may be limited by variables not accounted for. As discussed previously, not having clinical response as a variable in the database is another recognized limitation. Pathologic response was used as a surrogate. Finally, external validation of this model is required.
Conclusions
We have generated a model that incorporates measurable preoperative factors to stratify the likelihood of ypN0 response in patients with cN+ disease who undergo NAC. For patients with high scores, there is a greater expectation that they will have an axillary clinical response and be suitable candidates for performing SLNB over ALND, because they have a greater likelihood of having achieved axillary pCR at the time of surgery.
References
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. Alliance for Clinical Trials in Oncology. JAMA. 2013;310(14):1455–61. https://doi.org/10.1001/jama.2013.278932.
Mautner SK, Cody HS 3rd. Sentinel node biopsy after neoadjuvant chemotherapy for node-positive breast cancer: does axillary ultrasound improve performance? J Clin Oncol. 2015;33(30):3375–8. https://doi.org/10.1200/jco.2014.60.3316.
Morrow M, Dang CT. Sentinel node biopsy after neoadjuvant chemotherapy: a new standard for patients with axillary metastases? JAMA. 2013;310(14):1455–61.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18. https://doi.org/10.1016/s1470-2045(13)70166-9.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85.
van Nijnatten TJ, et al. Prognosis of residual axillary disease after neoadjuvant chemotherapy in clinically node-positive breast cancer patients: isolated tumor cells and micrometastases carry a better prognosis than macrometastases. Breast Cancer Res Treat. 2017 Feb 17. https://doi.org/10.1007/s10549-017-4157-0.
Mougalian SS, et al. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016;2(4):508–16. https://doi.org/10.1001/jamaoncol.2015.4935.
Kim MM, et al. Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer. Ann Oncol. 2013;24(8):1999–2004. https://doi.org/10.1093/annonc/mdt131.
Broglio KR, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2(6):751–60. https://doi.org/10.1001/jamaoncol.2015.6113.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–900.
Peintinger F, Kuerer HM, Anderson K, et al. Accuracy of the combination of mammography and sonography in predicting tumor response in breast cancer patients after neoadjuvant chemotherapy. Ann Surg Oncol. 2006;13(11):1443–9.
Marinovich ML, Houssami N, Macaskill P, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105(5):321–33.
Kim JY, Park HS, Kim S, et al. Prognostic nomogram for prediction of axillary pathologic complete response after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Medicine. 2015;94(43):e1720.
Schipper RJ, Moossdorff M, Nelemans PJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno)therapy in patients with clinically node-positive breast cancer, clinical breast cancer, 2014;14:315–22, ISSN 1526-8209, http://dx.doi.org/10.1016/j.clbc.2013.12.015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kantor, O., Sipsy, L.M., Yao, K. et al. A Predictive Model for Axillary Node Pathologic Complete Response after Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg Oncol 25, 1304–1311 (2018). https://doi.org/10.1245/s10434-018-6345-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6345-5