Abstract
Background
Many patients with clinically node-positive breast cancer receive neoadjuvant chemotherapy (NAC). Recent trials suggest the potential for limiting axillary surgery in patients who convert to pathologically node-negative disease. The authors developed a nomogram to predict axillary response to NAC in patients with cN1 disease that can assist clinicians in treatment planning.
Methods
Patients with cT1–4N1M0 breast cancer who received NAC and underwent axillary lymph node dissection from 2001 through 2013 were identified (n = 584). Uni- and multivariate logistic regression analyses were performed to determine factors predictive of nodal conversion. A nomogram to predict the likelihood of nodal pathologic complete response (pCR) was constructed based on clinicopathologic variables and validated using an external dataset.
Results
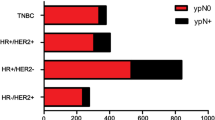
Axillary pCR was achieved for 217 patients (37 %). Patients presenting with high nuclear grade [grade 3 vs. 1, odds ratio (OR) 13.4], human epidermal growth factor receptor 2-positive (OR 4.7), estrogen receptor (ER)-negative (OR 3.5), or progesterone receptor-negative (OR 4.3) tumors were more likely to achieve nodal pCR. These factors, together with clinically relevant factors including presence of multifocal/centric disease, clinical T stage, and extent of nodal disease seen on regional nodal ultrasound at diagnosis were used to create nomograms predicting nodal conversion. The discrimination of the nomogram using ER+ status (>1 % staining) versus ER− status [area under the curve (AUC) 78 %] was improved slightly using the percentage of ER staining (AUC 78.7 %). Both nomograms were validated using an external cohort.
Conclusion
Nomograms incorporating routine clinicopathologic parameters can predict axillary pCR in node-positive patients receiving NAC and may help to inform treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Axillary lymph node status provides prognostic information and guides treatment decisions for patients with breast cancer. Patients presenting with axillary nodal metastases often receive neoadjuvant chemotherapy (NAC), which eradicates nodal disease in 40–75 % of patients.1 – 5 Patients who achieve nodal pathologic complete response (pCR) after NAC have improved locoregional and survival outcomes.1 , 3 , 6 In fact, findings have shown nodal pCR to be a more important prognostic indicator than primary tumor response.3 , 6
Historically, clinically node-positive patients have undergone axillary lymph node dissection (ALND) after NAC regardless of nodal response. Recently, interest has focused on minimally invasive approaches to identify patients who convert to pathologic node-negative status after NAC who might avoid the morbidities associated with ALND.5 , 7 – 10 With the possibility of less extensive axillary surgery for patients who achieve nodal pCR, predicting nodal response to NAC has clinical implications.
This study aimed to evaluate potential predictors of nodal conversion after NAC for patients presenting with cN1 breast cancer and to generate nomograms predicting the probability of achieving a nodal pCR that might aid clinicians in treatment decisions.
Methods
Study Population
This study was approved by the Institutional Review Board. Patients with clinical T1–4, N1, M0 breast cancer who received NAC followed by ALND between 2001 and 2013 at the University of Texas MD Anderson Cancer Center were identified from a prospectively maintained database. All the patients had nodal metastases confirmed by needle biopsy.11 Patients presenting with recurrence, a history of axillary surgery including sentinel lymph node dissection (SLND), or no NAC treatment were excluded from the study. All the patients received anthracycline- and/or taxane-based chemotherapy. To reflect current practice patterns, only patients with human epidermal growth factor receptor 2+ (HER2+) tumors who received HER2-targeted therapy were included in the study.
Clinicopathologic and treatment data from 584 patients were used to determine factors predictive of nodal conversion after NAC. Complete data related to the significant factors were available for 578 patients. These data were used to create nomograms predicting conversion to pathologic node-negative status. Data from 315 cN1 patients who completed NAC followed by ALND at The European Institute of Oncology (EIO) were used as an external validation cohort.
Data Collection and Analysis
Clinic and pathology reports were reviewed to determine pretreatment data. Estrogen receptor (ER) status and progesterone receptor (PR) status were recorded as the percentage of cells staining positive by immunohistochemistry (IHC). The HER2 status was recorded as positive if immunostaining was scored as 3+ or the amplification ratio was higher than 2 as shown by fluorescence in situ hybridization. Radiology reports were reviewed to determine clinical tumor size, radiographic evidence of multifocal or multicentric disease, and number and size of abnormal axillary nodes on ultrasonography. The number of suspicious axillary lymph nodes was recorded as fewer than four or as four or more. Clinical T and N status were defined according to the seventh edition of the American Joint Committee on Cancer cancer staging system.12 Nodal status after NAC was determined from surgical pathology reports after ALND.
End Point and Statistical Analysis
Nodal pCR after NAC was defined as no evidence of residual tumor in the axillary lymph nodes. Any nodal metastases, including isolated tumor cells (ITCs), were considered to be positive. Data were summarized using standard descriptive statistics and frequency tabulation. Univariate logistic regression analysis was performed to determine the association of axillary pCR with clinicopathologic and treatment variables.
Both statistically significant and clinically relevant features were used to create multivariable logistic regression models and nomograms. One nomogram was developed using ER status as a binary variable, with ER+ defined as 1 % or more staining and ER− as less than 1 % staining. A second nomogram then was created using the percentage of cells with ER+ staining as a continuous variable to explore the possibility that the degree of ER positivity might influence the response to chemotherapy. We also created a nomogram that did not include the number of abnormal nodes for use at institutions in which this is not available [area under the curve (AUC) 77.6 %] (Supplemental Fig. 1).
The discrimination of the nomograms was evaluated using the AUC of the receiver operating characteristic (ROC) curve. The AUC and corresponding 95 % confidence interval (CI) are provided for each nomogram. In addition, to adjust for bias, the nomograms were validated with 10,000 bootstrap samples, and the bootstrap-adjusted AUC was estimated for each. A calibration curve was generated to show the association between the observed outcome frequencies and the predicted probabilities by categorizing patients on the basis of their predictive probability of converting to pathologic node-negative status. The nomogram was subsequently validated using data from the external cohort. Statistical analyses were performed using R version 3.2.0 software (http://www.r-project.org, R Foundation for Statistical Computing, Vienna, Austria). All statistical tests used a significance level of 5 %.
Results
Patient, Tumor, and Treatment Characteristics
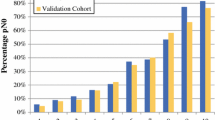
The clinicopathologic characteristics of the development and validation cohorts are depicted in Table 1. The nodal pCR rates were 37.2 % (217/584) in the MD Anderson cohort and 37.3 % (118/315) in the EIO cohort.
Uni- and Multivariate Analyses
Table 2 summarizes the univariate analyses for the MD Anderson cohort used to identify factors predictive of achieving nodal pCR. The odds of nodal conversion were significantly improved for tumors with a high nuclear grade [grade 3 vs. 1: odds ratio (OR) 13.4, 95 % CI 3.1–57.6], higher Ki-67 scores (OR 1.03 per unit, 95 % CI 1.02–1.04), ER− status (OR 3.5, 95 % CI 4.4–5.1), PR− status (OR 4.3, 95 % CI 3.0–6.2), or HER2+ status (OR 4.7, 95 % CI 3.1–7.3). The odds of conversion to pathologic node-negative status decreased as the amount of ER percentage staining increased (OR 0.98 per unit, 95 % CI 0.975–0.984). In addition, patients with multifocal/centric disease (OR 0.7, 95 % CI 0.4–1.0) had decreased odds of achieving nodal pCR (p = 0.05).
The aforementioned factors and others thought to be clinically relevant (clinical T stage, number of suspicious nodes on ultrasound categorized as <4 vs. ≥4 nodes) were included in the multivariate analysis (Table 3) and nomogram models. The analysis did not include Ki-67 because it was not known in a large number of patients (n = 181).
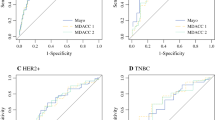
These variables then were used to create two nomogram models to predict the likelihood of achieving nodal pCR after NAC. The first model categorized ER status as positive or negative. The predicted accuracy as estimated by the AUC was 78 % (95 % CI 74.2–81.7 %). The bootstrap-adjusted AUC, which corrects for optimism, was 76.5 % (Fig. 1). A nomogram using the percentage of ER staining as a continuous variable then was created to investigate whether the extent of ER positivity might influence the nodal response to chemotherapy. Using this approach, the predictive ability of the model slightly improved, with an AUC of 78.7 % (95 % CI 75.0–82.5 %) (Fig. 2a) and a bootstrap optimism-corrected AUC of 77.3 %. An ROC curve plotting the tradeoff between sensitivity and specificity for different probability cutoffs is shown in Fig. 2b.
a Nomogram for predicting axillary pathologic complete response after neoadjuvant chemotherapy using estrogen receptor categorized as positive (≥1 % of cells staining) or negative (0 % of cells staining). To calculate the individual probability of nodal conversion, the number of points for each factor is first determined by drawing a vertical line from the specific characteristic to the points scale and figuring the sum of total points. A line drawn from the total points line to the lower line shows the individual probability of achieving an axillary pathologic complete response. b Receiver operative characteristic curve for the nomogram. The predicted accuracy-unadjusted area under the curve (AUC) was 78 % [95 % confidence interval (CI) 74.2–81.7 %]. The bootstrap-adjusted AUC for this model was 76.5 %
a Nomogram for predicting axillary pathologic complete response after neoadjuvant chemotherapy using estrogen receptor as the percentage of cells stained (continuous variable from 0 to 100 %). b Receiver operative characteristic curve for the nomogram. The unadjusted area under the curve (AUC) for this model was 78.7 %, 95 % confidence interval (CI) 75.0–82.5 %]. The bootstrap-adjusted AUC was 77.3 %
External Validation of the Nomogram
Data from 315 patients treated at the EIO were used to determine the external validity of the nomogram. The validation cohort differed from the MD Anderson cohort, showing a greater percentage of clinical T4 tumors (34 vs. 7 %, p = 0.0005), a higher proportion with multifocal/centric disease (32 vs. 24 %, p = 0.01), and a higher nuclear grade (grade 3: 65 vs. 52 %, p = 0.0005). It was more likely to be ER− (43 vs. 25 %, p < 0.0001), PR− (58 vs. 36 %, p < 0.0001), and HER2+ (31 vs. 20 %, p = 0.0004).
The validation cohort was used in the first model, which categorized ER status as positive or negative with an unadjusted AUC of 79.4 % (95 % CI 74.6–84.3 %). When the nomogram using percentage of ER staining as a continuous variable was evaluated using the validation cohort, it continued to show robust accuracy, with an unadjusted AUC of 82.2 % (95 % CI 77.6–86.8 %).
Discussion
In this study, we identified high nuclear grade, high Ki-67 score, ER and PR negativity, and HER2 positivity as factors associated with achieving a nodal pCR in patients with cN1 disease receiving NAC. In addition, we developed nomograms that estimate the likelihood of axillary pCR in these patients. Because many institutions worldwide assess ER status categorized as positive or negative, a nomogram was constructed using ER status as a dichotomous variable, with an AUC of 78 %, suggesting the utility of this predictive tool when details regarding the absolute percentage of ER+ staining are not available. Using ER staining percentage as a continuous variable slightly improved the nomogram (AUC 78.7 %). Both models were validated using an independent cohort of patients demonstrating that the nomogram is reliable for use at other institutions.
Nodal response to chemotherapy has been established as an important prognostic feature. In a study of 403 patients with biopsy-confirmed axillary metastases who received NAC, Hennessy et al.3 showed that patients who achieved a nodal pCR had better 5-year overall survival (OS 93 %) and relapse-free survival (RFS 87 %) than those who had residual nodal disease (OS 72 %, RFS 60 %). For patients not achieving a nodal pCR, outcomes are related to the number of nodes harboring residual disease as well as the tumor subtype. One study showed that patients with hormone receptor-positive (HR+)/HER2− tumors with zero to three positive nodes after NAC had a 5-year locoregional recurrence rate (LRR) of 2 % compared with 7 % if four or more positive nodes were identified. In contrast, those with HR−/HER2− tumors had 5-year LRR of 9 % if there were zero to three positive nodes compared with 44 % if there were four or more positive nodes.13
Traditionally, patients with axillary nodal metastases have undergone ALND, a procedure with considerable morbidity such as chronic pain, decreased range of motion, and lymphedema.14 – 16 Just as NAC can eradicate disease in the breast,2 , 17 , 18 it also can convert clinically node-positive patients to pathologic node-negative status in 40–75 % of cases.2 – 5 Unfortunately, accurate identification of these patients likely to have a nodal pCR who may not benefit from extensive axillary surgery has been difficult. Single-institution and retrospective reports using SLND to restage the axilla after NAC have shown false-negative rates of 5–20 %, which has limited its use in this setting.19 – 22
Several prospective trials have recently addressed this issue, showing that the false-negative rate of SLND in cN1 patients after NAC is 12.6–14.1 %.5 , 7 , 8 However, subset analyses of these trials show that the accuracy of SLND can be improved using a dual-tracer technique and retrieval of two or more SLNs, with ensured removal of the node initially confirmed to contain metastases (marked with a clip), and using IHC for pathologic evaluation.5 , 7 , 8 , 23 , 24 Several groups currently are working on ways to ensure removal of the clipped nodes using techniques such as targeted axillary dissection.9 , 10 , 25 – 27 As techniques to ensure reliable axillary staging are developed, predicting axillary nodal response to therapy will become increasingly important in treatment planning.
Similar to the primary tumor, nodal response to therapy varies according to tumor biology. The American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, which reflects current chemotherapy approaches including the use of trastuzumab for patients with HER2+ tumors, showed differential nodal responses based on tumor biology. The overall nodal conversion rate was 41.1 %, but this varied from 21.1 % in patients with HR+/HER2− tumors to 49.4 % in patients with triple-negative breast cancer to 64.7 % in patients with HER2+ disease (p < 0.0001).28 Our study corroborated these findings, with ER, PR, and HER2 status strongly predictive of nodal response.
This nomogram may be used as an adjunct to clinical decision making about appropriateness of NAC but not as a replacement for current decision paradigms. For instance, the decision to use NAC often is driven by the goal to downsize the primary tumor, making breast-conserving therapy possible. Considering omission of ALND with eradication of nodal disease, clinicians may currently consider NAC as a route to downstaging of the axilla as well. Although the overall probability of nodal conversion in recent trials has been about 40 %,28 certain subgroups, such as patients with ER+ tumors, have a much lower chance of conversion, as described earlier. This nomogram may be especially helpful in predicting which patients with ER+ tumors might gain a benefit from NAC with regard to nodal response, allowing a more individualized assessment of nodal conversion probability than that defined by tumor receptor status alone. It also may be used to counsel patients on the expected outcomes after chemotherapy. However, many other factors go into the decision to administer chemotherapy in the neoadjuvant setting that are not related to nodal status. For some patients, tumor biology or primary tumor characteristics such as skin or chest wall involvement may drive the decision to administer NAC rather than the probability of nodal conversion.
A Dutch group has developed a similar model predicting the likelihood of nodal conversion based on 291 clinically node-positive breast cancer patients treated with NAC.29 Their model includes age, clinical T stage, tumor histology, ER, PR, and HER2 status, as well as whether the patients received trastuzumab or a taxane, with a reported AUC of 0.77 (95 % CI 0.71–0.82). Several differences need to be noted between their model and those in the current study.
First, in the Dutch cohort, only 54.2 % (52/96) of the patients with HER2+ tumors received trastuzumab, whereas 78.4 % (228/291) of the entire cohort received neoadjuvant taxane-based therapy. Because the use of HER2-targeted therapy currently is a standard component of neoadjuvant therapy for patients with HER2+ disease, to reflect contemporary practice patterns, we excluded patients with HER2+ tumors who did not receive trastuzumab. Additionally, we included only patients who received the entire planned course of chemotherapy. Our model is designed to help the clinician seeing a patient with cN1 disease to decide on the optimal order of therapy. Thus, we wanted to model the probability of nodal conversion with the expectation that patients would receive all therapy.
Second, the Dutch group considered less than 10 % ER staining to be ER−. Our study corroborated the notion that low ER expression influences nodal conversion rates both when included as a continuous variable and when categorized using a cutoff of 1 %, consistent with the current American Society of Clinical Oncology guidelines.30
Finally, our model considered any residual nodal disease as positive, even in cases of ITCs, in contrast to the model presented by Schipper et al.,29 which considered ITCs as negative. Both the ACOSOG Z1071 trial and the sentinel node biopsy following NAC trial showed that defining ITCs found in SLNs as positive decreased the false-negative rate of SLND. Although ITCs identified in a patient undergoing upfront surgery are treated as node-negative,31 , 32 the impact of ITCs after NAC is unclear, and consideration should be given to performing completion ALND.8 , 33 Our model has the added advantage of an external validation, which performed similarly to the MD Anderson cohort although it represented a subgroup with larger tumors and higher proportions of ER−, PR−, and HER2+ tumors.
In conclusion, as the paradigm shifts to omission of extensive axillary surgery after response to NAC, using NAC to eradicate nodal disease is a clinically relevant strategy. These nomograms can be used to predict the probability of achieving nodal pCR after NAC with readily available clinicopathologic features. Therefore, they may have utility for informing treatment decisions.
References
Kuerer H, Sahin A, Hunt K, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230:72–8.
Buzdar A, Ibrahim N, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–84.
Hennessy B, Hortobagyi G, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–11.
Dominici L, Negron Gonzalez V, Buzdar A, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116:2884–9.
Boughey J, Suman V, Mittendorf E, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61.
Rouzier R, Extra J, Klijanienko J, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20:1304–10.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33:258–64.
Caudle A, Yang W, Mittendorf E, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg. 2014;34:1072–8.
Mittendorf E, Caudle A, Yang W, et al. Implementation of the American College of Surgeons Oncology Group Z1071 trial data in clinical practice: Is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014;21:2468–73.
Krishnamurthy S, Sneige N, Bedi D, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–8.
Edge S, Byrd D, Compton C. AJCC cancer staging manual. 7th ed. Springer, New York; 2009.
Caudle A, Yu T, Tucker S, et al. Local–regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14:R83.
Kakuda J, Stuntz M, Trivedi V, Klein S, Vargas H. Objective assessment of axillary morbidity in breast cancer treatment. Am Surg. 1999;65:995–8.
Lucci A, McCall A, Beitsch P, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–63.
Fleissig A, Fallowfield L, Langridge C, et al. Postoperative arm morbidity and quality of life: results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–93.
Bear H, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–74.
Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local–regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93.
Alvarado R, Yi M, Le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012;19:3177–83.
Classe J, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009;27:726–32.
Newman E, Sabel M, Nees A, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol. 2007;14:2946–52.
Mamounas E, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–702.
Caudle A, Yang W, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–8.
Boughey J, Ballman K, Le-Petross H, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263:802–7.
Choy N, Lipson J, Porter C, et al. Initial results with preoperative tattooing of biopsied axillary lymph nodes and correlation to sentinel lymph nodes in breast cancer patients. Ann Surg Oncol. 2015;22:377–82.
Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261:378–82.
Caudle A, Yang W, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;10:1072–8.
Boughey J, McCall L, Ballman K, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260:608–14.
Schipper R, Moossforff M, Nelemens P, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno)therapy in patients with clinically node-positive breast cancer. Clin Breast Cancer. 2014;14:315–22.
Hammond M, Hayes D, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–22.
Giuliano A, Hawes D, Ballman K, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011;306:385–93.
Weaver D, Ashikaga T, Krag D, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412–21.
Boughey J, Ballman K, Symmans W, et al. Methods impacting the false-negative rate of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0–T4, N1–2) who receive neoadjuvant chemotherapy: results from a prospective trial—ACOSOG Z1071 (Alliance). In: San Antonio breast cancer symposium 2014, Poster Presentation, 2014. http://eposter.abstractsonline.com/sabcs. Accessed 31 Jan 2015.
Acknowledgment
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672). Dr. Jose Vila is supported by a Grant from Umberto Veronesi Foundation and the Carlos III Health Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material. Supplemental Fig. 1. Nomogram for predicting axillary pathologic complete response after neoadjuvant chemotherapy using estrogen receptor categorized as positive (>1 % of the cells staining) or negative (0 % of cells staining) without using the number of abnormal nodes on US. The unadjusted AUC is 77.6 %
Rights and permissions
About this article
Cite this article
Vila, J., Mittendorf, E.A., Farante, G. et al. Nomograms for Predicting Axillary Response to Neoadjuvant Chemotherapy in Clinically Node-Positive Patients with Breast Cancer. Ann Surg Oncol 23, 3501–3509 (2016). https://doi.org/10.1245/s10434-016-5277-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5277-1