Abstract
Background
Occult breast cancer (OBC) is rare and optimal local–regional (LR) management has not been defined. Using a patient registry database, we examine factors associated with treatment and outcomes in OBC.
Methods
Female patients with cT0 N1/2 M0 BC were selected from the National Cancer Database (2004–2013) and categorized into four treatment groups: MAST = mastectomy with axillary lymph node dissection (ALND) ± radiation (RT); RT + ALND = RT with ALND, no breast surgery; ALND = ALND alone; OBS = no breast surgery, RT, or ALND. Patient characteristics and overall survival (OS) were compared between groups, and multivariable analysis was used to identify factors associated with treatment and OS.
Results
Among 2.03 million BC cases, 1853 females (0.09%) with cT0 N1/2 M0 disease were identified and 1231 patients were categorized into a treatment group: MAST = 592, RT + ALND = 342, ALND = 106, OBS = 191. On logistic regression, care at an academic center was associated with a higher likelihood of RT + ALND compared with MAST (odds ratio 2.03, 95% confidence interval [CI] 1.50–2.74, p < 0.001). Patients treated with RT + ALND had significantly better OS on univariate survival analysis compared with patients treated with MAST (hazard ratio [HR] 0.475, 95% CI 0.306–0.736, p = 0.001). RT + ALND was independently associated with OS on multivariable survival analysis (HR 0.509, 95% CI 0.321–0.808, p = 0.004), after adjusting for covariates.
Conclusions
Patients with OBC were more likely to undergo RT + ALND if they received care at an academic center. Patients treated with RT + ALND had significantly better OS compared with patients treated with MAST, after adjusting for covariates. This supports the use of RT + ALND as LR treatment for patients with OBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Occult breast cancer (OBC), which generally presents as axillary metastases without clinical or radiologic evidence of a primary breast tumor, accounts for only 0.1% of all breast cancer cases. Despite ongoing advances in diagnostic breast imaging, the management of OBC remains a clinical challenge. The majority of the literature on OBC consists of small, single-institution series and case reports. Most studies show the importance of local–regional (LR) treatment in OBC and, to date, have demonstrated similar outcomes for mastectomy or breast conservation with radiation therapy (RT).1,2
Few published guidelines address the treatment of OBC, and there is no clear consensus on the optimal management of these patients. The current National Comprehensive Cancer Network (NCCN) guidelines emphasize the diagnostic importance of magnetic resonance imaging (MRI) and suggest either mastectomy with axillary lymph node dissection (ALND) with or without RT, or ALND with RT.3 The American Society of Breast Surgeons lists OBC as an indication for ALND.4 In this study, we utilized a large patient registry database (National Cancer Database [NCDB]) to examine factors associated with LR treatments and their respective outcomes in patients with OBC, with a primary focus on comparing breast-directed treatment with RT versus mastectomy in patients who underwent ALND.
Methods
Study Design
The NCDB was utilized for this study. The NCDB is a clinical oncology database sponsored by the American College of Surgeons and the American Cancer Society. Information in this database includes patient demographics, tumor characteristics, treatment facilities, types of treatment, and outcomes, and is collected from the cancer registries of over 1500 Commission on Cancer-accredited facilities. The NCDB contains information on approximately 70% of all newly diagnosed malignancies in the US.5 Institutional Review Board approval was not required for this study since no protected health information was utilized.
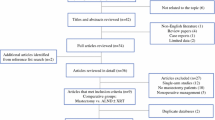
Female patients with clinical T0 N1/2 M0 breast cancer were selected from the NCDB (2004–2013). Patients were categorized into four treatment groups: MAST = mastectomy + ALND ± RT; RT + ALND = no breast surgery + ALND + adjuvant RT; ALND = no breast surgery + no RT + ALND; OBS = no breast surgery or RT or ALND. Following Walker et al.,1 ALND was considered regional lymph node (LN) surgery with the removal of four or more nodes. Patients who received neoadjuvant chemotherapy were included, whereas patients were excluded from analysis if any partial breast or unspecified/unknown breast surgery was performed, the clinical N stage was 2B (internal mammary lymph node involvement without axillary node involvement), no breast surgery was performed and RT was not documented, regional LN surgery was not documented for ALND groups, an unknown number of LNs were removed, and mastectomy or RT was performed with removal of fewer than four nodes.
Variables examined included age, comorbid conditions (Charlson/Deyo comorbidity score [CDCC]), income, insurance status, race, patient location, clinical and pathologic N stage, number of LNs examined, histology, laterality of tumor, estrogen receptor (ER) and progesterone receptor (PR) status, use of chemotherapy and endocrine therapy, timing of chemotherapy, use of RT, facility type, facility location, distance from the treatment facility, and year of diagnosis. Multivariable analysis (MVA) included the following variables: age (continuous variable), CDCC score, income (<US$48 K vs. ≥US$48 K), insurance status (private vs. other), race (White vs. non-White), patient location (urban vs. other), clinical N stage (1 vs. 2), pathologic N stage (0, 1, 2, or 3), number of LNs examined (<10, 10–19, ≥20), ER status (positive vs. negative), use of chemotherapy (yes vs. no), facility type (academic vs. other), and distance from the treatment facility (<50 miles vs. ≥50 miles). Facility location was divided into four regions and analyzed as a categorical variable: Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TX, VA, WV), Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Statistical Analysis
All statistical analyses were performed using SPSS statistical software version 22 (SPSS for Windows, IBM Corporation, Armonk, NY, USA). Patient, tumor, and treatment characteristics were compared between treatment groups using a χ 2 test. Binary logistic regression was used for MVA to identify factors associated with treatment with MAST or RT + ALND among age, CDCC score, income, insurance, race, patient location, clinical N stage, ER status, facility type, facility location, and distance from the treatment facility. Cox regression MVA considering age, CDCC score, race, clinical and pathologic N stage, number of LNs examined, use of chemotherapy, ER status, facility type, facility location, and treatment with either MAST or RT + ALND or MAST ± RT as covariates was performed to identify factors associated with overall survival (OS). Kaplan–Meier estimates and a log-rank test were used to examine the impact of treatment type on OS. A two-tailed p value of <0.05 was considered statistically significant.
Results
Patient, Tumor and Treatment Characteristics
All Treatment Groups
From 2004 to 2013, 2.03 million BC cases were reported to the NCDB, and 1853 female patients with clinical T0 N1/2 M0 disease (0.09%) were identified. There were 1231 patients who met our inclusion criteria. Overall, the majority of patients were White (76%), had private insurance (55%), and had no comorbid conditions (85%). ER-positive tumors (58%) and clinical N1 disease (75%) were more common. Chemotherapy was administered to 77% of patients and endocrine therapy was administered to 46% of patients. The total number of patients and median age by treatment group were as follows: MAST: n = 592, 58 years; RT + ALND: n = 342, 60 years; ALND: n = 106, 63.5 years; and OBS, n = 191, 65 years. Patient, tumor and treatment characteristics for all treatment groups are listed in electronic supplementary Table 1.
When comparing all four treatment groups, significant differences were observed in age, insurance, clinical and pathologic N stage, use of chemotherapy and endocrine therapy, and facility type (electronic supplementary Table 1). Younger patients were more likely to be treated with MAST, whereas older patients were more often treated with OBS. A higher percentage of patients with clinical N1 disease were treated with OBS. The use of chemotherapy and endocrine therapy were more common in patients treated with MAST or RT + ALND compared with ALND or OBS. Patients were less likely to be treated with MAST if they received care at an academic center.
Treatment with MAST Versus Radiation Therapy (RT) + Axillary Lymph Node Dissection (ALND)
Significant differences were also observed between treatment with MAST and RT + ALND (Tables 1, 2). Patients were more likely to be treated with RT + ALND than MAST if they received treatment at an academic center (39.5 vs. 25.2%, p < 0.001), lived in an urban location (76.0 vs. 68.9%, p = 0.014), or were treated in the Northeast (20.5 vs. 13.5%, p = 0.041). MAST was more common in patients treated in the South (34.6 vs. 29.5%, p = 0.041), in those who had PR-positive tumors (46.3 vs. 37.7%, p = 0.017), and in younger patients (age <40 years; 6.8 vs. 2.9%, p = 0.043). The vast majority of MAST (N = 451) and RT + ALND (N = 280) patients who received chemotherapy also received multiagent therapy (95.3 vs. 97.2%, p = 0.198). On MVA, the only factor associated with type of treatment was care at an academic center, with a higher likelihood of treatment with RT + ALND (odds ratio 2.03, 95% confidence interval [CI] 1.50–2.74, p < 0.001).
Use of RT in MAST Patients
RT was documented in 571 patients treated with MAST, with 323 (56.6%) receiving RT. Patients were more likely to receive RT if they had a higher clinical N stage (cN2, 33.1 vs. 14.1%, p < 0.0001) and pathologic N stage (pN2/3, 56.3 vs. 26.3%, p < 0.0001). Patients who received RT were also more likely to receive chemotherapy (62.9 vs. 37.1%, p < 0.0001) and endocrine therapy (60.7 vs. 39.3%, p = 0.029). The groups were similar in terms of CDCC score, income, insurance, race, patient location, ER and PR status, facility type, facility location, and distance from the treatment facility.
Survival Analysis
All Treatment Groups
Univariate survival analysis comparing all four treatment groups showed that patients treated with MAST or RT + ALND had better OS compared with patients treated with ALND or OBS. The 5- and 8-year estimates of OS for each of the treatment groups were MAST 80.0 ± 2.2 and 72.8 ± 3.2%; RT + ALND 90.8 ± 1.9 and 84.7 ± 3.6%; ALND 76.2 ± 5.0 and 65.1 ± 6.7%; OBS 56.5 ± 4.8 and 49.0 ± 5.9%, respectively.
Treatment with MAST versus RT + ALND
Univariate survival analysis showed that patients treated with RT + ALND had a significantly better OS compared with patients treated with MAST (Fig. 1; hazard ratio [HR] 0.475, 95% CI 0.306–0.736, p = 0.001). Multivariable survival analysis showed that RT + ALND was independently associated with OS (HR 0.509, 95% CI 0.321–0.808, p = 0.004), in addition to age, CDCC score, pathologic N stage, number of LNs examined, and ER status (Table 3).
When we limited our ALND inclusion criteria to those patients who had ≥10 axillary LNs removed (total patients 738; MAST = 463, RT + ALND = 275), significantly better OS was still shown on univariate survival analysis in patients treated with RT + ALND compared with MAST (HR 0.475, 95% CI 0.288–0.786, p = 0.004), and treatment with RT + ALND was independently associated with OS on multivariable survival analysis (HR 0.591, 95% CI 0.349–0.998, p = 0.049).
Similarly, when patients who received neoadjuvant chemotherapy were excluded (MAST = 185, RT + ALND = 11), a significantly better OS was observed in patients treated with RT + ALND compared with MAST on univariate survival analysis (HR 0.466, 95% CI 0.293–0.743, p = 0.001), and, on multivariable survival analysis, treatment with RT + ALND was independently associated with OS (HR 0.512, 95% CI 0.310–0.847, p = 0.009).
Treatment with MAST (±RT)
No significant difference in OS was shown on univariate survival analysis for patients treated with MAST with and without RT (HR 0.995, 95% CI 0.648–1.528, p = 0.982). Multivariable survival analysis showed that the use of RT was not independently associated with OS in patients treated with MAST; however, age, CDCC score, pathologic N stage, number of LNs examined, and ER status were all independently associated with OS.
Pathologic Findings on Mastectomy
Of the 592 MAST patients, 397 (67%) had primary tumors that were classified as T0 or TX on final pathology. However, tumors were identified in the breast after mastectomy in 195 patients (33%). The majority of these were in situ or T1 tumors (n = 124, 63.6%), while 71 patients (36.4%) had tumors that were T2 or greater.
Discussion
In this NCDB analysis examining treatment and outcomes in patients with OBC, we show that patients treated with RT + ALND had a significant improvement in OS compared with patients treated with MAST after adjusting for other covariates. Other factors that typically influence outcomes in patients with breast cancer, including hormone receptor status and pathologic N stage, were also independently associated with OS in patients treated with MAST or RT + ALND. This analysis lends support to the use of RT + ALND as a treatment choice for OBC. This study also reinforces the importance of LR therapy in OBC, as definitive LR treatment with MAST or RT + ALND conferred a survival advantage over treatment with either ALND or OBS.
Our analysis is consistent with existing data in terms of the importance of LR therapy.1,2 Multiple single-institutional studies have shown a clear advantage of RT and/or mastectomy with ALND in the management of OBC compared with less aggressive LR treatment approaches.6–10 In a recent meta-analysis that evaluated therapeutic options for OBC, LR recurrence rates were decreased and there was a trend toward improvement in mortality with the addition of RT to ALND compared with ALND alone.2 A population-based analysis of OBC using the Surveillance, Epidemiology, and End Results (SEER) database showed that LR treatment with either mastectomy + ALND ± RT or breast-conserving therapy (BCT) + RT + ALND resulted in an improvement in survival compared with ALND alone or observation.1 Both of these studies showed similar survival when comparing patients treated with mastectomy or RT, which is in contrast to the results of our study.
The use of adjuvant RT in patients with breast cancer as part of multimodal treatment has increased as studies have shown improvements in local control and breast cancer survival in patients treated with breast conservation or mastectomy with positive lymph nodes.11,12 These studies highlight the importance of RT in node-positive patients, regardless of local therapy with mastectomy or BCT. In the present study, the addition of RT to ALND resulted in a better OS compared with ALND alone. In patients who underwent mastectomy, the use of adjuvant RT did not improve OS and was not independently associated with OS on MVA. These results are likely due to patient selection since patients treated with postmastectomy RT had higher clinical and pathologic N stage.
One major obstacle when optimizing therapeutic management of OBC is the low incidence of the disease, which makes the conduct of randomized controlled trials challenging. As a result, no consensus exists on the optimal LR therapy. A survey conducted by the American Society of Breast Surgeons on the management of patients with OBC highlights this lack of consensus, with 43% of surgeons preferring mastectomy and 37% recommending whole-breast irradiation.13
Historically, modified radical mastectomy was the recommended treatment for patients with OBC. This was likely driven by the high percentage of patients with positive pathology in the breast after mastectomy and high local recurrence rates when no treatment was administered to the breast.7,8,14 However, advanced imaging techniques, especially breast MRI, have significantly improved our ability to identify OBCs that are not detected on mammography.15–18 A study by Olson et al. from Memorial Sloan Kettering Cancer Center demonstrated that breast MRI improved breast cancer detection over mammography and physical examination and that a negative MRI predicted a low tumor yield at mastectomy.15 More recent studies have demonstrated positive pathology in the breast at mastectomy in approximately 30% of OBC patients.6,10,16 This is consistent with our findings of 33% and may reflect the increased use of MRI in these patients.
As with other large database analyses, our study was limited to the reported data, leaving several questions unanswered. For example, use of breast MRI imaging is not included in the NCDB, therefore we were unable to evaluate what percentage of patients had baseline assessment with breast MRI and whether this correlated inversely with positive pathology on mastectomy. Since human epidermal growth factor receptor 2 (HER2) has only been recently reported in the NCDB, we did not evaluate this as a prognostic or treatment factor. Likewise, the use of neoadjuvant chemotherapy was not well documented in patients treated with RT + ALND. Finally, since information on LR recurrences is also not available to researchers, we were unable to evaluate the impact of LR treatment on this outcome.
Conclusion
The results of our study support the use of RT + ALND as a treatment option for patients with OBC, and emphasize the importance of LR treatment and multimodality therapy in these patients.
References
Walker GV, Smith GL, Perkins GH, et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer. 2010;116(17):4000–4006.
Macedo FI, Eid JJ, Flynn J, Jacobs MJ, Mittal VK. Optimal surgical management for occult breast carcinoma: a meta-analysis. Ann Surgical Oncol. 2016;23(6):1838–1844.
National Comprehensive Cancer Network. NCCN Guidelines Version 1.2017 Invasive Breast Cancer. 2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 26 Mar 2017.
American Society of Breast Surgeons. Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients. 2014. Available at: https://www.breastsurgeons.org/statements/guidelines/PerformancePracticeGuidelines_ALND.pdf. Accessed Mar 26 2017.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690.
He M, Tang LC, Yu KD, et al. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol. 2012;38(11):1022–1028.
Shannon C, Walsh G, Sapunar F, A’Hern R, Smith I. Occult primary breast carcinoma presenting as axillary lymphadenopathy. Breast. 2002;11(5):414–418.
Foroudi F, Tiver KW. Occult breast carcinoma presenting as axillary metastases. Int J Radiat Oncol Biol Phys. 2000;47(1):143–147.
Wang X, Zhao Y, Cao X. Clinical benefits of mastectomy on treatment of occult breast carcinoma presenting axillary metastases. Breast J. 2010;16(1):32–37.
Blanchard DK, Farley DR. Retrospective study of women presenting with axillary metastases from occult breast carcinoma. World J Surg. 2004;28(6):535–539.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716.
McGale P. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135.
Khandelwal AK, Garguilo GA. Therapeutic options for occult breast cancer: a survey of the American Society of Breast Surgeons and review of the literature. Am J Surg. 2005;190(4):609–613.
Rosen PP, Kimmel M. Occult breast carcinoma presenting with axillary lymph node metastases: a follow-up study of 48 patients. Hum Pathol. 1990;21(5):518–523.
Olson JA Jr, Morris EA, Van Zee KJ, Linehan DC, Borgen PI. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 2000;7(6):411–415.
Buchanan CL, Morris EA, Dorn PL, Borgen PI, Van Zee KJ. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol. 2005;12(12):1045–1053.
Morris EA, Schwartz LH, Dershaw DD, van Zee KJ, Abramson AF, Liberman L. MR imaging of the breast in patients with occult primary breast carcinoma. Radiology. 1997;205(2):437–440.
de Bresser J, de Vos B, van der Ent F, Hulsewe K. Breast MRI in clinically and mammographically occult breast cancer presenting with an axillary metastasis: a systematic review. Eur J Surg Oncol. 2010;36(2):114–119.
Diclosure
Lindsay K. Hessler, Jason K. Molitoris, Paula Y. Rosenblatt, Emily C. Bellavance, Elizabeth M. Nichols, Katherine H.R. Tkaczuk, Steven J. Feigenberg, Soren M. Bentzen, and Susan B. Kesmodel declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Lindsay Hessler and Jason Molitoris have contributed equally to this article.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hessler, L.K., Molitoris, J.K., Rosenblatt, P.Y. et al. Factors Influencing Management and Outcome in Patients with Occult Breast Cancer with Axillary Lymph Node Involvement: Analysis of the National Cancer Database. Ann Surg Oncol 24, 2907–2914 (2017). https://doi.org/10.1245/s10434-017-5928-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5928-x