Abstract
Purpose
Management of the axilla in patients with early-stage breast cancer (ESBC) has evolved. Recent trials support less extensive axillary surgery in patients undergoing mastectomy. We examine factors affecting regional lymph node (RLN) surgery and outcomes in patients with ESBC undergoing mastectomy.
Methods
Women with clinical T1/2 N0 M0 invasive BC who underwent mastectomy with 1–2 positive nodes were selected from the National Cancer Database (2004–2015). Axillary surgery was defined by number of RLNs examined: 1–5 sentinel LN dissection (SLND), and ≥ 10 axillary LND (ALND). Binary logistic regression and survival analyses were performed to assess the association between axillary surgery and clinical characteristics, and overall survival (OS), respectively.
Results
34,243 patients were included: 13,821 SLND (40%) and 20,422 ALND (60%). SLND significantly increased from 21% (2004) to 45% (2015) (p < .001). Independent factors associated with SLND were treatment year, non-Academic centers, geographic region, tumor histology, and postmastectomy radiotherapy (PMRT). Multivariable survival analysis showed that ALND was associated with better OS (HR 0.78, 95% CI 0.72–0.83, p < .001) relative to SLND; however, there was no difference in patients with LN micrometastases treated without RT (HR 0.87, 95% CI 0.73–1.05, p = .153) or patients receiving PMRT (HR 0.92, 95% CI 0.76–1.13, p = .433).
Conclusions
SLND has significantly increased in patients undergoing mastectomy with limited axillary disease and is influenced by patient, tumor, and treatment factors. Survival outcomes did not differ by axillary treatment for patients with LN micrometastases treated without RT or patients who received PMRT. SLND may be considered in select patients with ESBC and limited axillary disease undergoing mastectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Management of the axilla in patients with early-stage breast cancer (ESBC) has evolved significantly over the last several decades. This has been influenced by improvements in systemic and radiation therapy, a better understanding of BC subtypes and tumor biology, the introduction of sentinel lymph node dissection (SLND), and continued efforts to reduce the morbidity of axillary lymph node dissection (ALND). Overall, these advancements have resulted in a significant decline in the use of ALND in patients with ESBC [1].
The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial was practice changing in patients with ESBC undergoing breast-conserving therapy (BCT), and multiple studies confirm a significant decrease in the use of ALND in eligible patients after publication of this trial [2,3,4]. However, management of the axilla in patients with ESBC with limited ALN involvement undergoing mastectomy has been more challenging.
Recent trials support less extensive axillary surgery and alternatives to ALND in patients with ESBC with limited axillary disease undergoing mastectomy [5, 6]. In this study, we utilize a large patient cancer registry to examine changes in practice patterns of axillary surgery, factors influencing the number of regional lymph nodes (RLN) removed, and the impact of RLN surgery on outcomes in patients with ESBC with limited ALN metastasis undergoing mastectomy.
Materials and methods
Data source
The National Cancer Database (NCDB) Participant User File (PUF) for breast tumors 2004–2015 was reviewed. The NCDB is a nationwide hospital-based cancer registry sponsored by the American College of Surgeons Commission on Cancer and the American Cancer Society. The NCDB collects de-identified data on over 70% of newly diagnosed cancer cases, including patient demographics, clinical factors, treatment, and outcomes [7]. This study was reviewed as exempt by the local Institutional Review Board.
Patient selection
Female patients with clinical T1/2 N0 M0 invasive BC who underwent mastectomy with 1–2 positive LNs on final pathology were selected. Cohorts were stratified according to extent of axillary surgery defined by number of RLNs removed: SLND (1–5 LNs), and ALND (≥ 10 LNs) [8]. Patients were excluded from analysis if they received neoadjuvant radiation or chemotherapy, had positive surgical margins, or underwent surgery at a facility other than the reporting facility. Patients with 6–9 lymph nodes examined were included as part of the entire cohort for analysis of extent of axillary surgery; however, they were excluded from subsequent analyses comparing SLND to ALND since we were unable to classify the type of axillary surgery in this group of patients. (Fig. 1) The NCDB PUF 2015 data dictionary describes other variables used [9].
Statistical analysis
Descriptive statistics were calculated for clinical characteristics using frequencies (percentage) for categorical data and mean (standard deviation) or median (interquartile rage Q1–Q3) for continuous data. The association of clinicopathologic factors among treatments was analyzed. For categorical variables, differences in percentages were compared across groups using chi-squared (X2) or Fisher’s exact tests. For continuous variables, the distributions across axillary surgery were compared using student’s t test or Wilcoxon test.
The primary endpoint was trend analysis of the extent of axillary surgery and factors associated with use of SLND. Cochran–Armitage trend test was used for comparison of treatment proportions. Odds ratios (OR) with 95% confidence intervals (CI) were calculated using a multivariable binary logistic regression model to identify factors associated with SLND versus ALND. Covariates in the model included age (< 50 vs. ≥ 50), year of diagnosis (2004–2010 vs. 2011–2015), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), insurance status (insured vs. uninsured), facility type (Academic vs. others), facility location, Charlson–Deyo comorbidity score (0 vs. ≥ 1), histology (ductal, lobular, mixed ductal/lobular), estrogen receptor (ER) status (positive vs. negative), T stage (1 vs. 2), number of positive LNs (1 vs. 2), LN disease burden (micrometastatic vs. macrometastatic), and use of postmastectomy radiotherapy (PMRT) to the chest wall ± LNs (yes vs. no). Facility location was grouped into 4 regions as defined by the U.S. Census (Northeast, South, Midwest, and West) [10].
The secondary endpoint was overall survival (OS), measured from date of diagnosis until death. The Kaplan–Meier method was used to derive survival curve estimates across treatments, and the log-rank test was used to make comparisons of the survival rates [11]. Hazard ratios (HR) with 95% CI were calculated using the Cox proportional hazards model [12, 13]. Analyses were adjusted to aforementioned covariates in addition to adjuvant systemic treatments and type of axillary surgery (SLND vs. ALND). Statistical significance was determined at an alpha level of ≤ 0.05. Statistical analyses were carried out using SAS Software version 9.4 (SAS Institute Inc., Cary, NC) and R Software (RStudio. Inc. Boston, MA).
Results
Patient, tumor, and treatment characteristics
A total of 34,243 patients met our inclusion criteria: 13,821 (40%) underwent SLND, and 20,422 (60%) ALND. Mean age was 58 years. The majority of patients were non-Hispanic white (81%), were treated at Comprehensive Community Cancer Programs (46%), and had private insurance (58%). Most tumors were ductal adenocarcinoma (81%), ER+ (86%), clinical T1 (58%), with LN macrometastases (67%), and moderately differentiated (48%). Sixty-two percent of patients received adjuvant chemotherapy, 25% PMRT, and most ER+ patients (86%) received hormonal therapy.
Trends in regional lymph node surgery
A significant increase in SLND was observed from 2004 to 2015, 21% in 2004 to 45% in 2015 (p < .001). This increase was particularly evident after 2010. Similar trends were observed in both patients with macrometastatic (SLND increase 18–37%, p < .001) and micrometastatic (SLND increase 40–66%, p < .001) disease. In addition, in patients who received PMRT, SLND increased from 16 to 48% during the same time period (p <.001) and the use of PMRT overall in patients undergoing SLND increased from 9 to 35% (p <.001) (Fig. 2).
Analysis of SLND versus ALND
Patients treated with SLND were slightly older (60 vs. 57 years, p < .001) and as expected, were more likely to have Medicare insurance (36 vs. 29%, p < .001) compared to those treated with ALND. They also received care more often at Comprehensive Community Cancer Programs (50 vs. 43%, p < .001). Among patients treated with ALND, there were higher proportions of ductal carcinoma (82 vs. 78%, p < .001), T2 tumors (43 vs. 39%, p < .001), poorly differentiated tumors (34 vs. 27%, p < .001), and LN macrometastases (75 vs. 54%, p < .001) compared to SLND. Adjuvant chemotherapy was given to 69% of patients who underwent ALND compared to 52% of patients who underwent SLND (p < .001). (Table 1).
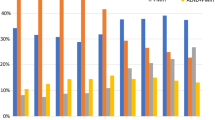
Independent factors associated with SLND were year of diagnosis (2011–2015; OR 2.21, 95% CI 2.09–2.33, p < .001), treatment at non-Academic centers (OR 1.40, 95% CI 1.32–1.48, p < .001), Western region (OR 1.22, 95% CI 1.12–1.33, p < .001), lobular (OR 1.40, 95% CI 1.26–1.56, p < .001) or mixed ductal/lobular (OR 1.16, 95% CI 1.07–1.27, p = .001) histology, and PMRT (OR 1.54, 95% CI 1.45–1.63, p < .001). Factors associated with lower use of SLND were younger age (< 50 years, OR 0.76, 95% CI 0.72–0.81, p = .001), non-Hispanic black race (OR 0.80, 95% CI 0.73–0.87, p < .001), Hispanic ethnicity (OR 0.83, 95% CI 0.74–0.93, p = .002), Midwest region (OR 0.88, 95% CI 0.82–0.95, p = .001), ER- tumors (OR 0.83, 95% CI 0.77–0.89, p < .001), T2 tumors (OR 0.88, 95% CI 0.84–0.92, p < .001), two positive LNs (OR 0.38, 95% CI 0.35–0.40, p < .001), and LN macrometastases (OR 0.25, 95% CI 0.23–0.26, p < .001) (Fig. 3).
Survival analysis
The median follow-up was 53 months (IQR 33–77 months). Five-year OS was 88% in the ALND group and 85% in the SLND group (log rank p < .001). Factors associated with better OS on multivariable survival analysis were younger age (< 50 years; HR 0.47, 95% CI 0.42–0.53, p < .001), year of diagnosis (2011–2015; HR 0.76, 95% CI 0.70–0.82, p < .001), Hispanic ethnicity (HR 0.67, 95% CI 0.55–0.80, p < .001), lobular (HR 0.83, 95% CI 0.74–0.93, p < .001) or mixed ductal/lobular histology (HR 0.85, 95% CI 0.74–0.96, p = .010), ALND (HR 0.78, 95% CI 0.72–0.83, p < .001), adjuvant chemotherapy (HR 0.42, 95% CI 0.38–0.45, p < .001), PMRT (HR 0.83, 95% CI 0.76–0.92, p < .001), and hormonal therapy (HR 0.52, 95% CI 0.48–0.57, p < .001). Negative prognostic factors were lack of insurance (HR 1.24, 95% CI 1.02–1.51, p < .001), treatment at non-Academic centers (HR 1.26, 95% CI 1.16–1.36, p < .001), Midwest region (HR 1.14, 95% CI 1.03–1.26, p = .012), comorbid conditions (HR 1.79, 95% CI 1.67–1.92, p < .001), ER- tumors (HR 1.52, 95% CI 1.37–1.69, p < .001), T2 tumors (HR 1.77, 95% CI 1.65–1.90, p < .001), two positive LNs (HR 1.22, 95% CI 1.13–1.31, p < .001), and LN macrometastases (HR 1.12, 95% CI 1.01–1.23, p = .024) (Table 2).
Subgroup survival analysis of patients with LN macrometastases treated without RT (n = 16 070) and LN micrometastases treated without RT (n = 5 883) showed better 5-year OS in patients undergoing ALND (LN macrometastases: 85 vs. 79%, log rank p < .001; LN micrometastases: 88 vs. 86%, log rank p = .050). However, in patients who received PMRT (n = 8429), no difference was observed (PMRT: 91 vs. 90%, log rank p = .193).
Adjusted models showed similar OS regardless of type of axillary surgery for patients with LN micrometastases treated without RT (HR 0.87, 95% CI 0.73–1.05, p = .153) or patients treated with PMRT (HR 0.92, 95% CI 0.76–1.13, p = .433), though, for patients with LN macrometastases treated without RT, better OS was observed with ALND (HR 0.73, 95% CI 0.66–0.80, p < .001).
Discussion
The results of this NCDB analysis show that the use of SLND alone in patients with ESBC with limited ALN metastasis undergoing mastectomy has significantly increased. This is particularly evident since publication of the Z0011 trial, even though patients undergoing mastectomy were not included in this trial. Patients were less likely to get SLND if they were younger (< 50 years), were non-Hispanic black or Hispanic, had greater tumor burden, or ER- tumors. On multivariable survival analysis, ALND was independently associated with better OS. Other factors that affect survival in patients with breast cancer including hormone receptor status, tumor burden, and receipt of adjuvant systemic therapy were also significant. In our subgroup survival analysis, type of axillary surgery was not associated with OS for patients with LN micrometastases treated without RT or patients treated with PMRT. These findings contribute to the surgical management of the axilla in patients with ESBC undergoing mastectomy and provide support for less extensive axillary surgery in select patients.
A significant evolution in the management of the axilla in patients with ESBC has occurred [14,15,16,17,18]. Multiple studies demonstrate SLND to be an accurate predictor of disease burden in the axilla in patients with clinically negative LNs [14,15,16,17]. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial demonstrated similar outcomes in patients with negative SLNs treated with SLND compared to ALND [18]. This trial also demonstrated low regional recurrence (RR) rates in patients with occult SLN metastases who underwent SLND and helped established the basis for less extensive axillary surgery in patients with ESBC and limited axillary disease [18].
The Z0011 trial was practice changing in patients with ESBC with 1–2 positive SLNs undergoing BCT, showing similar RR rates, disease-free survival (DFS), and OS in patients treated with SLND compared to SLND and completion ALND (CLND) [2, 15, 19]. Since publication of this trial, a significant decline in the rate of CLND has occurred in patients meeting Z0011 criteria [3, 4, 20]. A prior analysis of the NCDB that examined practice patterns 1 year before and after publication of Z0011 demonstrated a significant increase in the use of SLND in Z0011 eligible patients 1 year after publication [3]. Interestingly, a significant increase in SLND was also observed in multiple groups who did not meet Z0011 criteria, including those undergoing mastectomy [3]. The findings from our study confirm this early analysis and demonstrate an even further increase in the use of SLND alone in patients with ESBC undergoing mastectomy, although the safety of this approach in patients who fall outside of Z0011 criteria is not known.
Several single institution series have examined outcomes of patients undergoing mastectomy with positive SLNs who did not receive additional axillary surgery and show low RR rates [21,22,23]. An analysis of 210 patients with ESBC who underwent mastectomy with positive SLNs and received no further axillary surgery showed a 4-year RR rate of 1.2%, DFS of 94.8%, and OS of 97.8% [22]. A large proportion of patients in this study had isolated tumor cells only (54%) or micrometastatic disease (37%). A similar study that examined 10-year RR rates showed no difference between treatment groups, 3.8% with no further axillary treatment, 1.6% with CLND, 1.8% for CLND and RT, and 0% for RT alone [21]. However, there were inherent differences between the treatment groups with patients who did not undergo CLND being older and having more favorable tumor characteristics.
In the present analysis, we demonstrated that for all patients, ALND was independently associated with better OS. This is true even though patients who underwent ALND had greater disease burden, although they were also significantly more likely to receive adjuvant chemotherapy. However, the impact of ALND on recurrence-free survival and OS is controversial, particularly in patients with ESBC given the long-term results from the NSABP B-04 trial [24] and with the use of contemporary systemic therapies [25,26,27]. Several meta-analyses of randomized clinical trials that evaluated the effect of ALND on OS show differing results [25, 27], and there are several ongoing clinical trials that are examining this question [28, 29]. Unfortunately, due to the limitations of information available in the NCDB, we were unable to evaluate breast cancer-specific survival (BCSS) which may be a more informative outcome in this patient population.
Since publication of Z0011, four randomized trials have been reported which examined less extensive axillary surgery or use of axillary RT (AxRT) as an alternative to CLND in patients with ESBC and limited axillary disease [5, 6, 30, 31]. Although these trials were not specifically designed to answer the question of axillary management in patients undergoing mastectomy, they all included some patients treated with mastectomy. The International Breast Cancer Study Group (IBCSG) 23-01 trial compared ALND to no ALND in patients with ESBC with SLN micrometastases [5]. While the majority of patients had breast conserving surgery, 9% in each group underwent mastectomy [5]. With a median follow-up of 9.8 years, there was no significant difference in DFS or OS between the treatment groups [5, 32]. Of the 86 patients who underwent mastectomy, 44 ALND and 42 no ALND, there was one axillary recurrence (1.2%) in each group and only 6% (5/86) received adjuvant RT [5, 32]. The After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) trial compared ALND to AxRT in patients with ESBC with positive SLNs, and included 248 (17%) patients who underwent mastectomy, 127 (17%) ALND and 121 (18%) AxRT [6]. At a median follow-up of 6.1 years, there was no significant difference in DFS or OS between the treatment groups [6]. The RR rates were extremely low at 5 years, 4/744 (0.43%) ALND group and 7/681 (1.19%) AxRT group [6]. These trials provide support for SLND alone or AxRT as alternatives to ALND in patients with ESBC with positive SLNs undergoing mastectomy [5, 6]. The results from these trials are consistent with our subgroup analysis, which showed that for patients with LN micrometastases treated without RT or for patients who received PMRT, type of axillary surgery was not independently associated with OS.
Another interesting finding from our study is the significant increase in PMRT use from 2004 to 2015. This occurred in both patients treated with SLND and ALND (data not shown) and PMRT was also associated with better OS on multivariable analysis. This likely reflects the increasing use of PMRT in patients with 1–3 positive LNs based on the improvements in LR control and survival observed in randomized clinical trials in patients with ESBC [33, 34]. It is also possible that the use of PMRT increased due to the concomitant decrease in the extent of axillary surgery that occurred over this time period, although the exact criteria for patient selection for PMRT in the present analysis are unknown.
One of the benefits of using the NCDB is that we were able to examine RLN management in a large number of patients with ESBC undergoing mastectomy. Until recently, however, the NCDB has not categorized patients by type of axillary surgery, and therefore, we used the number of RLNs examined as a proxy. This made it difficult to classify patients who had 6–9 lymph nodes removed and may actually underestimate the number of patients who underwent SLND since surgeon intent for the axillary surgery was not known. Also, we were unable to analyze outcomes according to specific breast cancer molecular subtypes because human epidermal growth factor receptor-2 (HER-2) status has only been included in the NCDB since 2010, and therefore we used ER status alone. In addition, data for other significant oncologic outcomes such as RR and DFS rates were not available to examine from the NCDB, which is important when considering management of the axilla.
In conclusion, the use of SLND alone in patients with ESBC with 1–2 positive SLNs undergoing mastectomy has significantly increased and is influenced by multiple patient, tumor, and treatment factors. While ALND was associated with better OS on multivariable analysis, type of axillary surgery was not significant in patients with LN micrometastases treated without RT or patients who received PMRT. The findings from this analysis are consistent with the results from recent clinical trials and provide support for the use of SLND alone in select patients with ESBC with limited ALN metastasis undergoing mastectomy.
References
Jatoi I, Benson JR, Toi M (2016) De-escalation of axillary surgery in early breast cancer. Lancet Oncol 17(10):e430–e41
Giuliano AE, Hunt KK, Ballman K, Beitsch P, Whitworth PW, Blumencranz P et al (2011) Sentinel lymph node dissection with and without axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305(6):569–575
Yao K, Liederbach E, Pesce C, Wang C, Winchester DJ (2015) Impact of the American College of Surgeons Oncology Group Z0011 randomized trial on the number of axillary nodes removed for patients with early-stage breast cancer. J Am Coll Surg 221(1):71–81
Caudle AS, Hunt KK, Tucker SL, Hoffman K, Gainer SM, Lucci A et al (2012) American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol 19(10):3144–3151
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P et al (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 14(4):297–305
Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE et al (2014) Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 15(12):1303–1310
Bilimoria KY, Stewart AK, Winchester DP, Ko CY (2008) The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15(3):683–690
Ong CT, Thomas SM, Blitzblau RC, Fayanju OM, Park TS, Plichta JK et al (2017) Patient age and tumor subtype predict the extent of Axillary Surgery among breast cancer patients eligible for the American College of Surgeons Oncology Group Trial Z0011. Ann Surg Oncol 24(12):3559–3566
NCDB PUF (2015) Data Dictionary. http://ncdbpuf.facs.org/node/259
United States Census Bureau GD. Census Regions and Divisions of the United States.https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Breslow NE (1975) Analysis of survival data under the proportional hazards model. Int Stat Rev 43(1):45–57
Cox D (1972) Regression models and life tables. J R Stat Soc 34:187–220
Bergkvist L, de Boniface J, Jonsson PE, Ingvar C, Liljegren G, Frisell J et al (2008) Axillary recurrence rate after negative sentinel node biopsy in breast cancer: three-year follow-up of the Swedish Multicenter Cohort Study. Ann Surg 247(1):150–156
Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P et al (2016) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg 264(3):413–420
Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W et al (2000) Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol 18(13):2553–2559
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V et al (2003) A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349(6):546–553
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP et al (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11(10):927–933
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR et al (2017) Effect of axillary dissection versus no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 318(10):918–926
Robinson K, Pockaj BA, Wasif N, Kaufman K, Gray (2014) Richard. Have the American College of Surgeons Oncology Group Z0011 trial results inluenced the number of lymph nodes removed during sentinel lymph node dissection? Am J Surg 208(6):1060–1064
FitzSullivan E, Bassett RL, Kuerer HM, Mittendorf EA, Yi M, Hunt KK et al (2017) Outcomes of sentinel lymph node-positive breast cancer patients treated with mastectomy without axillary therapy. Ann Surg Oncol 24(3):652–659
Milgrom S, Cody H, Tan L, Morrow M, Pesce C, Setton J et al (2012) Characteristics and outcomes of sentinel node-positive breast cancer patients after total mastectomy without axillary-specific treatment. Ann Surg Oncol 19(12):3762–3770
Snow R, Reyna C, Johns C, Lee MC, Sun W, Fulp WJ et al (2015) Outcomes with and without axillary node dissection for node-positive lumpectomy and mastectomy patients. Am J Surg 210(4):685–693
Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N (2002) Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 347(8):567–575
Joyce DP, Manning A, Carter M, Hill AD, Kell MR, Barry M (2015) Meta-analysis to determine the clinical impact of axillary lymph node dissection in the treatment of invasive breast cancer. Breast Cancer Res Treat 153(2):235–240
Houvenaeghel G, Boher JM, Reyal F, Cohen M, Garbay JR, Classe JM et al (2016) Impact of completion axillary lymph node dissection in patients with breast cancer and isolated tumour cells or micrometastases in sentinel nodes. Eur J Cancer 67:106–118
Sanghani M, Balk EM, Cady B (2009) Impact of axillary lymph node dissection on breast cancer outcome in clinically node negative patients: a systematic review and meta-analysis. Cancer 115(8):1613–1620
de Boniface J, Frisell J, Andersson Y, Bergkvist L, Ahlgren J, Ryden L et al (2017) Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer 17(1):379
van Roozendaal LM, de Wilt JH, van Dalen T, van der Hage JA, Strobbe LJ, Boersma LJ et al (2015) The value of completion axillary treatment in sentinel node positive breast cancer patients undergoing a mastectomy: a Dutch randomized controlled multicentre trial (BOOG 2013-07). BMC Cancer 15:610
Savolt A, Peley G, Polgar C, Udvarhelyi N, Rubovszky G, Kovacs E et al (2017) Eight-year follow up result of the OTOASOR trial: the Optimal treatment of the axilla—surgery or radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: a randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol 43(4):672–679
Sola M, Alberro JA, Fraile M, Santesteban P, Ramos M, Fabregas R et al (2013) Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol 20(1):120–127
Galimberti V, Cole BF, viale G, veronesi P, Vicini E, Intra M et al (2017) Axillary dissection vs. no axillary dissection in patients with cT1-T2cN0M0 breast cancer and only micrometastases in the sentinel node(s): ten-year results of the IBCSG 23-01 Trial. San Antonio Breast Cancer Symposium
Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS et al (2005) Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 97(2):116–126
Overgaard M, Nielsen HM, Overgaard J (2007) Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 82(3):247–253
Funding
There was no external funding for this work.
Author information
Authors and Affiliations
Contributions
OP and SK contributed for study conception and design; OP and SK analyzed the data. OP, KK, CA, and SK prepared the manuscript. DY, EA, and SK provided critical revisions to the manuscript. OP, KK, CA, DY, EA, and SK reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Informed consent
This study utilizes retrospective, de-identified information. Informed consent was not necessary.
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Picado, O., Khazeni, K., Allen, C. et al. Extent of regional lymph node surgery and impact on outcomes in patients with early-stage breast cancer and limited axillary disease undergoing mastectomy. Breast Cancer Res Treat 171, 461–469 (2018). https://doi.org/10.1007/s10549-018-4840-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4840-9