Abstract
Background
This study aimed to determine the relationship between mammographic calcifications and magnetic resonance imaging (MRI) tumoral enhancement before and after neoadjuvant chemotherapy (NAC) and to assess the impact of these findings on surgical management.
Methods
This Institutional Review Board-approved, Health Insurance Portability and Accountability Act (HIPAA)-compliant retrospective study involved breast cancer patients who underwent NAC between 2009 and 2015. The study cohort comprised 90 patients with pre- and posttreatment MRI and mammograms demonstrating calcifications within the tumor bed either at presentation or after treatment. The data gathered included pre- and post-NAC imaging findings and post-NAC histopathology, particularly findings associated with calcifications. Comparisons were made using Fisher’s exact test, with p values lower than 0.05 considered significant.
Results
Complete resolution of MRI enhancement occurred for 44% of the patients, and a pathologic complete response (pCR) was achieved for 32% of the patients. No statistically significant correlation between changes in mammographic calcifications and MRI enhancement was found (p = 0.12). Resolution of enhancement was strongly correlated with pCR (p < 0.0001). The majority of the patients with pCR demonstrated complete resolution of enhancement (79%, 23/29). No statistically significant relationship was found between changes in calcifications and rates of pCR (p = 0.06). A pCR was achieved most frequently for patients with resolution of enhancement and new, increasing, or unchanged calcifications (p < 0.0001).
Conclusions
Although calcifications seen on post-NAC mammography may be associated with benign disease, loss of MRI enhancement does not predict the absence of residual tumor with sufficient accuracy to leave calcifications in place. Complete excision of tumor bed calcifications remains standard practice and a substantial limitation to NAC use for downstaging patients to be eligible for breast conservation treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neoadjuvant chemotherapy (NAC) is a valuable treatment option for patients with breast cancer.1–4 NAC can be used to downstage tumor size, allowing breast conservation treatment (BCT) for patients who would otherwise require mastectomy, and to convert unresectable to resectable disease. In addition, NAC has been shown to decrease the need for axillary dissection.5
Pre- and post-NAC imaging is used to evaluate the patient’s response to NAC and to guide the surgical approach. This generally includes mammography, ultrasound, and breast magnetic resonance imaging (MRI).
Mammography and breast ultrasound, the most commonly used imaging methods for evaluating tumor size before and after NAC, have variable accuracy.6 Several studies have demonstrated that contrast-enhanced MRI is the most accurate breast imaging technique for evaluating the extent of residual disease after NAC.6–9 De Los Santos et al.9 evaluated the ability of MRI to predict pathologic complete response (pCR) in invasive breast cancer patients receiving NAC and reported an overall accuracy of 74%. However, pCR is not necessary for BCT, and Jochelson et al. demonstrated that 88% of patients determined to be candidates for BCT based on post-NAC MRI were able to be have their breasts conserved.8
After NAC, new mammographic calcifications may develop as tumor cells die, or previously seen calcifications may decrease or increase without clear correlation with enhancement seen on MRI. The extent of residual calcifications is an unreliable indicator of response to NAC because not all calcifications represent viable tumor.10–12 Current treatment guidelines require complete excision of indeterminate or suspicious calcifications,13 sometimes necessitating a larger lumpectomy or a mastectomy for complete removal in a patient who otherwise has responded to NAC. This study aimed to determine the relationship between mammographic calcifications and MRI tumoral enhancement before and after NAC and to assess the impact of these findings on surgical treatment.
Materials and Methods
Study Population
This was an Institutional Review Board-approved, Health Insurance Portability and Accountability Act (HIPAA)-compliant study involving breast cancer patients who underwent NAC at Memorial Sloan Kettering Cancer Center between April 2009 and October 2015. Patients from 2009 to 2012 were identified retrospectively, whereas those treated from 2012 onward were included in a prospectively maintained database. Patients with both pre- and post-NAC MRI as well as mammograms demonstrating calcifications within the tumor bed either at presentation or after treatment comprised the study cohort. Patients with inflammatory breast cancer, those undergoing axillary surgery only, and those with imaging unavailable for review were excluded from the study.
Variables
Standard clinical and pathologic data were gathered, including pre-NAC imaging findings and core biopsy results as well as post-NAC imaging findings and final post-NAC histopathology results (final post-NAC histopathology results included pathologic findings specifically associated with calcifications). Pre- and postoperative pathology reports were reviewed in the electronic medical record. These reports routinely include a description of calcifications and their association with malignant or benign (and what type of benign) pathology. Hormone receptor positivity was defined as 1% or more cells staining for estrogen receptor (ER) or progesterone receptor (PR). Human epidermal growth factor 2 (HER2) positivity was defined as 3+ staining by immunohistochemistry or fluorescence in situ hybridization (FISH) amplification with a value higher than 2.
Imaging
Standard mammography included two views per breast, with additional magnification views performed at the discretion of the interpreting radiologist. A single-breast radiologist re-reviewed all pre- and post-NAC imaging. The extent of calcifications was measured in centimeters in two dimensions. To assess the change in extent of calcifications after NAC, the greatest extent of calcifications seen on a post-NAC mammogram was compared with that seen on the pre-NAC mammogram. Mammographic interpretation was reported using the American College of Radiology BI-RADS mammography lexicon.14
Breast MRI was performed with the patient prone in a dedicated surface breast coil on a 1.5- or 3.0-T commercially available system (General Electric Medical Systems, Milwaukee, WI, USA). The standard imaging protocol until 2013 included a localizing sequence followed by sagittal fat-suppressed T2-weighted, sagittal non-fat-suppressed T1-weighted, and bilateral sagittal fat-suppressed T1- weighted sequences performed before and three times after intravenous administration of a bolus of 0.1 mmol/L of gadopentetate dimeglumine (Magnevist; Bayer, Wayne, NJ, USA) per kilogram of body weight. The section thickness was 0.3 cm with no gap and a minimum matrix of 256 × 256. Unenhanced images were subtracted from the contrast-enhanced images on a pixel-by-pixel basis, producing three subtracted postcontrast subtraction sequences. Maximum-intensity projection images were created using the first post-contrast sequence and the first post-contrast-subtracted data. After 2013, imaging was performed in the axial plane with sagittal reformatting. The section thickness was reduced to 0.1 cm. On MRI, residual-enhancing tumor size after NAC was measured in the longest dimension.
Complete response seen on MRI was defined as the absence of any residual mass or non-mass enhancement. Similarly, complete response seen on mammogram was defined as the absence of residual mass, calcifications, or both.
The outcomes of interest were the extent of change on imaging after NAC, the correlation between changes seen on mammogram and MRI, and the correlation between imaging findings and the presence of residual tumor associated with calcifications. The rate of mastectomy due to residual calcifications also was ascertained.
Statistical Analysis
Comparisons were made using Fisher’s exact test. All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA), and p values lower than 0.05 were considered significant.
Results
From April 2009 to October 2015, 448 patients underwent NAC at our institution. Of these 448 women, 90 had calcifications identified on pre-and/or post-NAC mammography as well as on pre- and post-NAC breast MRI, with all imaging available for review. These 90 women comprised the study population.
The patient characteristics are summarized in Table 1. The median patient age was 49 years. The median tumor size was 4 cm, and the majority of the tumors were clinical stage 2 or higher (98%), with ductal histology (98%). Whereas HER2+ tumors comprised 49 (54%) of the 90 cases, 23 (26%) were ER−/HER2− and 28 (20%) were ER+/HER2−. Pathologic complete response in the breast, defined as the absence of any residual invasive or in situ carcinoma in the breast, was achieved for 29 (32%) of the 90 patients.
Extent of Change Seen on Imaging After NAC
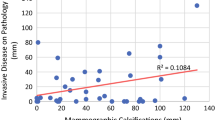
The median extent of calcifications on the pre-NAC mammograms was 3.1 cm (range 0.0–11.0 cm), whereas the median extent of calcifications on the post-NAC mammograms was 3.5 cm (range 0.0–10.0 cm). Six patients who did not have any calcifications at presentation (original extent, 0) experienced the development of calcifications after NAC. Among the 84 patients presenting with calcifications, the calcifications resolved completely for 3 patients (4%), decreased for 15 patients (18%), remained stable for 42 patients (50%), and increased for 24 patients (29%) (Table 2).
As shown on MRI, tumoral enhancement resolved completely after NAC for 40 (44%) of the 90 patients and decreased for 50 of the patients (56%). No patients experienced increased enhancement or new areas of enhancement in the index breast after NAC.
Correlation Between Changes on Mammogram and MRI
No statistically significant correlation was seen between the changes in calcifications on mammogram and the changes in enhancement on MRI (p = 0.12). However, loss of MRI enhancement was strongly correlated with pCR (p < 0.0001), with the majority of the patients with pCR demonstrating complete resolution of enhancement on MRI (23/29, 79%). In contrast, no statistically significant relationship was observed between changes in calcifications and rates of pCR (p = 0.06), with the rate of pCR ranging from 24 to 38% among the patients with decreased, increased, or stable calcifications. The relationship between the two imaging methods and the rate of pCR is shown in Table 2.
All three patients with calcifications at presentation who had complete resolution of calcifications after NAC also had complete resolution of MRI enhancement and showed a pCR on the final pathology. Among six patients with no calcifications at presentation who experienced new calcifications after NAC, three (50%) had a pCR. Only one (33%) of these three patients also showed complete resolution of enhancement by MRI. The relationship between decreasing or resolved and increasing or stable calcifications, decreasing or resolved MRI enhancement, and pCR is shown in Table 3. A statistically significant relationship is noted (p < 0.0001), with pCR the most frequent for patients with resolution of MRI enhancement and new, increasing, or unchanged calcifications.
Correlation Between Imaging Findings and the Presence of Residual Tumor Associated with Calcifications
Calcifications after NAC were associated with ductal carcinoma in situ (DCIS) or invasive ductal carcinoma (IDC) seen on the final pathology for 34 (37.8%) of the 90 patients and were benign in 56 (62.2%) of the patients. Table 4 compares characteristics between the patients with malignant calcifications and those with benign calcifications. The two groups were demographically similar, but the patients with benign calcifications after treatment were significantly more likely to have HER2+ tumors (64 vs 38%; p = 0.0002).
Among the 34 patients with malignant calcifications, MRI suggested a complete response for 6 patients (18%) and a partial response for 28 patients (82%). Meanwhile, a post-NAC mammogram showed decreased calcifications in 4 (11.8%) of the 34 patients, no change in calcifications in 19 of the patients (55.9%), and increased/new calcifications in 11 of the patients (32.4%). None of the patients with malignant calcifications demonstrated complete resolution of calcifications on the post-NAC mammogram. Of the six patients with malignant calcifications and complete resolution of MRI enhancement, three (50%) showed no change in calcifications on the post-NAC mammogram (Fig. 1), two (33%) showed increased or new calcifications, and one (17%) showed decreased calcifications.
A 52-year-old woman presented with a palpable mass in the left upper outer quadrant. Core biopsy demonstrated infiltrating ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS). a Left mediolateral oblique (MLO) view demonstrating diffuse focal asymmetry with associated calcification spanning 7 cm. b Magnetic resonance imaging (MRI) demonstrating multiple enhancing masses in two areas including a 2.6-cm mass at 12 o’clock. c Postchemotherapy magnification MLO view demonstrating a decrease in focal asymmetry with persistent calcifications spanning 7 cm. d Postchemotherapy MRI demonstrating complete resolution of enhancement. Patient underwent mastectomy with complete resolution of infiltrating carcinoma but with multiple foci of DCIS in the tumor bed
Among the 56 patients with benign calcifications, only 3 (5%) showed complete resolution of calcifications on the post-NAC mammogram, whereas 34 (61%) showed completely resolved enhancement on the post-NAC MRI.
Rate of Mastectomy Due to Residual Calcifications
Of the 90 patients in the study, 70 (78%) were candidates for BCT after NAC. Of these 70 patients, 57 (81%) had BCT and 13 (19%) had mastectomy due to the presence of a high-risk mutation or patient preference.
The remaining 20 patients (22%) were not BCT candidates after NAC. Among these 20 patients, 10 (50%) had extensive residual calcifications that precluded BCT, 3 (30%) had multifocal/multicentric disease, 1 (10%) had discordant mammographic and MRI findings, 4 (40%) had a cosmetically unfavorable tumor-to-breast size ratio, and 2 (20%) progressed with treatment. Of the 10 patients who underwent mastectomy due to extensive residual calcifications, 7 (70%) had benign calcifications, and 3 (30%) had tumor associated with calcifications.
Discussion
Downstaging of large tumors to allow BCT is a major rationale for the use of NAC in operable breast cancer. In this setting, determination of candidacy for BCT after NAC is dependent on the post-NAC evaluation of residual disease extent. The integration of discordant findings on MRI and mammography remains a significant clinical problem. Several studies have demonstrated that contrast-enhanced MRI is the most accurate technique for evaluating the extent of residual disease after NAC,6–9 yet indeterminate or malignant-appearing calcifications on mammography require surgical excision regardless of MRI findings.
In this study, we demonstrated that the combined assessment of loss of MRI enhancement and changes in calcifications does not predict the presence of pCR with sufficient accuracy to be clinically useful. Although only 38% of calcifications were associated with malignancy after NAC, neither clinical characteristics, changes in the extent of calcifications, nor the combination of changes in calcifications and loss of MRI enhancement identified a patient subset whose calcifications could be safely left in place. Loss of MRI enhancement was the most reliable predictor of pCR, but in 17 cases with resolution of enhancement, viable tumor persisted.
The utility of mammography after NAC in addition to MRI has been questioned. In a study previously published by our institution, MRI alone correctly predicted the ability to perform BCT after NAC for 88% of the patients, whereas the combination of MRI and mammography after NAC correctly predicted the ability to perform BCT for 92% of the patients8 based on using the extent of residual calcifications seen on the posttreatment mammogram to localize disease extent more accurately. The current study emphasizes the importance of identifying the calcifications in planning the appropriate extent of surgical resection.
Meanwhile, others have examined the significance of mammographically detected calcifications after NAC. Weiss et al.11 found that the extent of calcifications on seen mammography correlated poorly with tumor size on the final pathology after NAC. Adrada et al.10 reported that of 106 women, 43 (41%) had calcifications associated with benign pathology after NAC. Similar to our findings, Kim et al.12 demonstrated that the correlation between residual mammographic calcifications and residual tumor extent was lower than the correlation between MRI findings and residual tumor in all tumor subtypes after NAC. This is not particularly surprising because residual calcifications after NAC may represent necrotic material in the tumor bed area from successfully treated cancer, fat necrosis, or the sequelae of hematoma after biopsy in addition to viable tumor.15
Although clinicians widely agree that residual calcifications after NAC often are not associated with residual cancer, no consensus exists as to how these calcifications should be handled, in part because residual cancer is present in a significant number of cases and in part because following these residual calcifications may prove problematic as they may show an increase in number on subsequent imaging.
In our study of 90 patients, tumoral enhancement seen on MRI resolved after neoadjuvant chemotherapy for 40 patients (44%) and decreased for 50 patients (56%), yet only 29 patients (32%) achieved pCR (defined as no residual invasive disease or DCIS). Correlation between resolution of enhancement and pCR was 74%. However, even for the patients with a decrease in calcifications as well as resolution of enhancement, the potential for residual malignancy was high enough that surgery with complete removal of all calcifications in the region of the tumor bed still was required. The need to excise all calcifications resulted in mastectomy for 50% of the patients thought to have medical contraindications to BCT after NAC.
This study had several limitations. It was in part a retrospective, single-institution study with a relatively small sample size. However, from 2014 onward, the patients were followed in a prospectively maintained database. Hormone receptor-negative patients were overrepresented compared with prior studies, likely due to more frequent selection of these patients for neoadjuvant treatment given a better expected response.
Conclusion
Although it is clear that many of the tumor bed calcifications seen on post-NAC mammography are associated with benign disease, loss of MRI enhancement does not predict the absence of residual tumor with sufficient accuracy to allow calcifications to be left in place. Complete excision of all indeterminate or malignant-appearing calcifications remains standard practice and a substantial limitation to the use of NAC for downstaging of patients to BCT.
References
Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–9.
Teshome M, Hunt KK. Neoadjuvant therapy in the treatment of breast cancer. Surg Oncol Clin North Am. 2014;23:505–23.
Redden MH, Fuhrman GM. Neoadjuvant chemotherapy in the treatment of breast cancer. Surg Clin North Am. 2013;93:493–9.
Holmes D, Colfry A, Czerniecki B, et al. Performance and practice guideline for the use of neoadjuvant systemic therapy in the management of breast cancer. Ann Surg Oncol. 2015;22:3184–90.
Mamtani A, Barrio AV, King TA, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016;23:3467–74.
Rosen EL, Blackwell KL, Baker JA, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181:1275–82.
Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868–77.
Jochelson MS, Lampen-Sachar K, Gibbons G, et al. Do MRI and mammography reliably identify candidates for breast conservation after neoadjuvant chemotherapy? Ann Surg Oncol. 2015;22:1490–5.
De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium Trial 017. Cancer. 2013;119:1776–83.
Adrada BE, Huo L, Lane DL, Arribas EM, Resetkova E, Yang W. Histopathologic correlation of residual mammographic microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2015;22:1111–7.
Weiss A, Lee KC, Romero Y, et al. Calcifications on mammogram do not correlate with tumor size after neoadjuvant chemotherapy. Ann Surg Oncol. 2014;21:3310–16.
Kim YS, Chang JM, Moon HG, Lee J, Shin SU, Moon WK. Residual mammographic microcalcifications and enhancing lesions on MRI after neoadjuvant systemic chemotherapy for locally advanced breast cancer: correlation with histopathologic residual tumor size. Ann Surg Oncol. 2016;23:1135–42.
National Comprehensive Cancer Network. Breast Cancer Version 2.2016. Retrieved 30 August 2016 at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
American College of Radiology (ACR). ACR Breast Imaging Reporting and Data System (BI-RADS). 4th ed. Reston, VA: American College of Radiology; 2003
Moskovic EC, Mansi JL, King DM, Murch CR, Smith IE. Mammography in the assessment of response to medical treatment of large primary breast cancer. Clin Radiol. 1993;47:339–44.
Acknowledgment
This study was funded in part through NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Feliciano, Y., Mamtani, A., Morrow, M. et al. Do Calcifications Seen on Mammography After Neoadjuvant Chemotherapy for Breast Cancer Always Need to Be Excised?. Ann Surg Oncol 24, 1492–1498 (2017). https://doi.org/10.1245/s10434-016-5741-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5741-y