Abstract
Background
Approximately 8–17 % of patients with von Hippel–Lindau (VHL) syndrome develop pancreatic neuroendocrine tumors (PNETs), with 11–20 % developing metastases. Tumor grade is predictive of prognosis.
Objective
The aim of this study was to determine if preoperative metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were associated with metastatic disease and tumor grade.
Methods
Sixty-two patients with VHL-associated PNETs prospectively underwent 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT). MTV, TLG, and maximum standardized uptake value (SUVmax) were measured using a semi-automatic method. Surgically resected PNETs were classified according to 2010 World Health Organization tumor grade classification. MTV, TLG, and SUVmax were analyzed by metastatic disease and tumor grade using the Mann–Whitney test.
Results
A total of 88 PNETs were identified by CT and 18F-FDG PET/CT, 10 of which were non-FDG-avid. Histologic grading was available for 20 surgical patients. Patients with metastatic PNETs had a higher TLG (median 25.9 vs. 7.7 mean SUV [SUVmean]*mL; p = 0.0092) compared with patients without metastasis, while patients with grade 2 PNETs had a higher MTV (median 6.9 vs. 2.6 mL; p = 0.034) and TLG (median 41.2 vs. 13.1 SUVmean*mL; p = 0.0035) compared with patients with grade 1 PNETs. No difference in tumor size or SUVmax was observed between the groups.
Conclusions
Patients with metastatic PNETs have a higher TLG compared with patients without metastasis. Grade 2 PNETs have a higher MTV and TLG compared with grade 1 PNETs. Tumor size and SUVmax were not associated with grade. Volumetric parameters on 18F-FDG PET/CT may be useful in detecting higher grade PNETs with a higher malignant potential that may need surgical intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Von Hippel–Lindau (VHL) disease, an autosomal dominant disorder, results from an inactivating germline mutation in the VHL tumor suppressor gene.1,2 Patients with VHL are predisposed to develop a variety of tumors and cysts, including hemangioblastomas of the central nervous system and retina, renal cell carcinoma, pheochromocytomas, pancreatic cysts and tumors, and endolymphatic sac neoplasms. The most common pancreatic manifestations of VHL are cystic lesions, which include asymptomatic simple and complex cysts (30–91 %) and serous cystadenomas (10–12 %).3–6 These lesions are rarely symptomatic and do not have malignant potential. In contrast, pancreatic neuroendocrine tumors (PNETs), which occur in 8–17 % of patients with VHL over their lifetime,1,6 are indolent and slow growing in nature, but approximately 11–20 % of patients have, or will develop, metastatic disease.6,7
The goal in the management of PNETs in patients with VHL is to resect malignant disease (metastatic) or potentially malignant disease.8,9 Current criteria for surgical resection of PNETs in VHL are based on tumor size, with resection recommended for lesions >3 cm, based on larger tumors having a higher rate of metastases.9 In addition to tumor size, Blansfield and colleagues found exon 3 mutation status and doubling time less than 500 days were also associated with a higher rate of metastatic disease.10
The histologic grade of PNETs is an important prognostic factor.11–15 The grading system developed by the World Health Organization (WHO) in 2010 classifies PNETs into low grade (G1), intermediate grade (G2), and high grade (G3) based on the Ki67 index and mitotic count.16 Morin and colleagues examined a cohort of 119 patients with sporadic and non-sporadic PNETs, and found that higher grade was significantly associated with progression-free survival (G1 vs. G2 vs. G3, median 79.3 vs. 144 vs. 21 months, respectively).11 Similarly, Strosberg and colleagues found tumor grade, as defined by mitotic count, was prognostic for overall survival, showing that the 5-year survival rates for intermediate- (62 %) and high-grade tumors (7 %) were significantly worse compared with low-grade tumors (75 %).13 Given the valuable prognostic information, investigators have suggested an endoscopic ultrasound (EUS)-guided biopsy of the lesion could help in selecting individuals for surgery. Hasegawa and colleagues evaluated Ki-67 index in EUS-guided fine-needle aspiration (EUS–FNA) specimens and found the concordance of EUS–FNA specimens and resected tumor was only 74.0 % due to tumor heterogeneity. Furthermore, of the 50 patients who underwent EUS–FNA, 18 % had insufficient sample, subjecting nearly one in five patients to a non-diagnostic invasive procedure.17 An invasive biopsy has risks, including pancreatitis, abscess formation, fistulas, and hemorrhage, and may not provide an accurate assessment of the grade of the tumor due to heterogeneity of tumor tissue.17–19
Positron emission tomography/computed tomography (PET/CT) using 18F-fluorodeoxyglucose (18F-FDG) has been shown to be a prognostic imaging modality in pancreatic,20 lung,21 head and neck,22 and ovarian cancer,23,24 In cases of sporadic PNETs,18F-FDG PET/CT parameters, such as maximum standardized uptake values (SUVmax) and tumor avidity have been associated with tumor aggressiveness and grade.25–27 While SUVmax provides single voxel information, volumetric parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG), defined as the product of MTV and mean SUV (SUVmean), provide a comprehensive assessment of the whole tumor.28 These parameters have been utilized to predict the prognosis of esophageal,29 multiple myeloma,29 and small cell lung cancer.30 Furthermore, these parameters may predict treatment response in pancreatic20,31 and non-small cell lung cancer.32 Automatic and semi-automatic methods have been developed to decrease inter- and intraobserver variation in tumor volume delineation, which allows data to be more accurate.33
The objective of this study was to determine whether volumetric parameters measured on 18F-FDG PET/CT predict the metastatic potential and WHO histologic grade in VHL-associated PNETs, and can thus serve as a tool to better inform surgical intervention for patients with VHL.
Methods
Patients
A retrospective analysis was performed on 62 of 314 patients with VHL-associated PNETs who were prospectively evaluated by 18F-FDG PET/CT at the National Institutes of Health (NIH) Clinical Center (NCT00062166). Twenty-seven patients underwent an operation. Seven of the surgical patients were excluded due to the lack of histologic information or a > 1-year interval between 18F-FDG PET/CT and surgical intervention. The decision to undergo surgical resection was based on our institutional operative criteria (≥2 cm in the head of the pancreas or ≥3 cm in the body or tail of the pancreas by anatomical imaging). Patients with tumors <2 cm in the head and <3 cm in the body or tail by imaging (median follow-up 33.3 months, range 7.9–42.4 months) and patients with surgically resected tumors without evidence of regional lymph node metastases or distant metastases to the liver or lung (median follow-up 25.6 months, range 2.9–42.8 months) were classified as patients without evidence of metastatic disease. Patients with imaging suggestive of metastatic disease, as well as patients with surgically resected tumors with evidence of regional lymph node metastases or distant metastases to the liver, were classified as patients with metastatic disease. All patients gave written informed consent to participate in the imaging protocol, and the study was approved by the Institutional Review Board.
Pathology and Histology
Tumors were reviewed by board-certified pathologists and were classified according to the WHO 2010 tumor grade classification system: G1: Ki67 <2 % and/or <2 mitoses per 10 high-power fields (hpf); G2: Ki67 3–20 % and/or 2–20 mitoses/10 hpf; G3: Ki67 > 20 % and/or > 20 mitoses/10 hpf. The greatest diameter of each pancreatic lesion was recorded for each patient.
Positron Emission Tomography/Computed Tomography Imaging
18F-FDG PET/CT scans were performed approximately 1 h following intravenous administration of 10 mCi of 18F-FDG for patients with a body weight <90 kg, and 15 mCi for patients with a body weight >90 kg. Patients were required to fast 4–6 h before the scan was performed. Blood glucose levels, measured before administration of the radiotracer, were confirmed to be <200 mg/dL. Patients were scanned from the base of the skull to the mid-thigh. A low-dose non-contrast CT scan was used for anatomical localization and attenuation correction, and an estimate of the patient’s lean body mass, body on weight, height and sex, was used to calculate SUVs.
Image Analysis
18F-FDG PET/CT images were analyzed using custom software written in IDL (Excelis Visual Information Solutions, Boulder, CO, USA). A semi-automated method was used to delineate tumors with post-construction smoothing with a Gaussian filter (FWHM, 5 mm) in an operator-defined region of interest (ROI). Tumor boundaries were determined using a threshold of 45 % of the voxel of maximum filtered value within the ROI. Areas of physiologic uptake were excluded in order to limit measurements to the tissue of interest. MTV, SUVmax and mean SUVmean (SUV lean body mass) were measured for each tumor lesion, and TLG was determined as a product of MTV and mean SUVmean. Tumor doubling time was calculated based on measurements of the largest pancreatic lesions from two sequential CT scans 1 year apart. The following equation was used: \(T_{\text{i}} \times {\text{log2}}/ 3 \times { \log }\left( {D_{\text{i}} /D_{0} } \right)\), where T i is the time interval, D i is the initial diameter, and D 0 is the final diameter.34
Statistical Analyses
Differences in MTV and TLG between groups were assessed using the Mann–Whitney test, cut-off values to differentiate grades were calculated using receiver operator characteristic (ROC) curves, and associations were assessed using Spearman rank correlation coefficients. Statistical significance was defined as a p value < 0.05. Outliers were identified and removed using the ROUT method.35 All calculations were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Results
The cohort consisted of 62 patients with VHL-associated PNETs who underwent 18F-FDG PET/CT and CT scan with intravenous contrast (Table 1). The majority of patients in the cohort were female (female:male ratio 44:18) and the mean age was 44.7 years. A total of 88 solid pancreatic lesions were identified on CT, of which 78 were found to be 18F-FDG-avid. The MTV and SUVmax were measured for each pancreatic lesion identified on 18FDG PET/CT (Fig. 1). The median MTV and TLG of all pancreatic lesions was 2.8 mL (interquartile range [IQR] 2.0–5.2) and 9.6 SUVmean*mL (IQR 5.3–17.1), respectively. In patients who did not undergo surgical intervention, the median MTV and TLG of pancreatic lesions was 2.4 mL (IQR 1.6–3.5) and 5.9 SUVmean*mL (IQR 4.3–10.9).
Thirty-five patients in the non-surgical cohort and 14 patients in the surgical cohort without metastatic disease at the time of operation had a total of 63 PNETs identified by 18F-FDG PET/CT. Four patients with metastatic disease had four PNETs associated with metastatic disease and two patients with metastatic disease had two PNETs per patient associated with metastatic disease. A total of eight PNETs were associated with metastatic disease and were identified by 18F-FDG PET/CT. Sixty-three PNETs not associated with metastatic disease by imaging were compared with the eight PNETs associated with metastatic disease. PNETs associated with metastatic disease had a significantly higher TLG (median 25.9 SUVmean*mL vs. 7.7 SUVmean*mL; p = 0.0092) [Fig. 2a]. An outlier analysis was completed showing a continued difference between the two groups (p = 0.0080) [Fig. 2b]. No statistical difference was observed between PNETs not associated with metastatic disease and PNETs associated with metastatic disease when comparing the groups by MTV and SUVmax.
Twenty-seven patients underwent surgical resection of their PNETs, 20 of whom had histological tumor grade and imaging data (Table 2). A total of 33 pancreatic lesions were identified on CT, of which 29 were found to be 18F-FDG-avid (four lesions were non-avid, detected by intraoperative ultrasound, and not resected). Of these 29 pancreatic lesions, 26 were resected and graded according to 2010 WHO classifications, with 13 lesions (50 %) classified as G1 and 13 lesions (50 %) classified as G2.
G2 lesions resected from the pancreas had a significantly higher MTV (median 6.9 vs. 2.6 mL; p = 0.034) [Fig. 3a], and TLG (median 41.2 SUVmean*mL vs. 13.1 SUVmean*mL; p = 0.0035) [Fig. 3b]. There was no difference in tumor size (median greatest diameter 2.5 vs. 2.0 cm; p = 0.09) or SUVmax (median SUVmax 8.4 vs. 9.0; p = 0.54) between G1 and G2 lesions. After an outlier analysis, TLG remained significantly higher in G2 lesions compared with G1 lesions (median 39.4 SUVmean*mL vs. 13.3 SUVmean*mL; p = 0.0073) and there was a trend for MTV (median 2.6 vs. 6.3 mL; p = 0.0510).
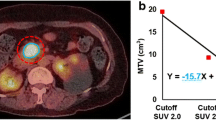
MTV, TLG, and tumor size had an area under the curve (AUC) of 0.75, 0.83, and 0.73, respectively, when determining grade. The positive predictive values (PPV) and negative predictive values (NPV) for G2 lesions were 75 and 71.4 % for MTV (cut-off 3.59 mL), 76.9 and 76.9 % for TLG (cut-off 17.2 SUVmean*mL), and 57.1 and 83.3 % for tumor size (cut-off >3 cm), respectively. Metastatic disease, exon 3 mutational status, and tumor doubling time <500 days were not associated with higher MTV or TLG. Finally, no correlation was observed between whole-body MTV or TLG with preoperative serum chromogranin A levels.
Discussion
TLG measured on 18F-FDG PET/CT was found to be higher in PNETs with metastatic disease compared with PNETs without metastatic disease. MTV and TLG measured on 18F-FDG PET/CT were found to be higher in G2 PNETs compared with G1 PNETs in patients with VHL, while SUVmax showed no significant difference. The findings of this study demonstrate that volumetric parameters on 18F-FDG PET/CT may be able to predict the metastatic potential and grade of PNETs. These findings may help determine whether patients have high-risk PNETs that would benefit from surgical intervention in VHL-associated PNETs.
Tumor grade is an important prognostic factor in patients with PNETs.11–15 The WHO classification system developed in 2010 reflects these findings, classifying PNETs based on mitotic count and/or Ki67 index, which has been validated by multiple studies.14,36,37 The findings of our study suggest that 18F-FDG PET/CT volumetric parameters may be useful in determining the grade of PNETs, and may provide a non-invasive method of detecting higher-grade lesions. The current 2010 WHO grading classification requires the examination of a large number of cells for the determination of grade due to high intratumoral heterogeneity within PNETs; however, it is often difficult to obtain sufficient cells on biopsy for a comprehensive analysis for tumor grade.38,39 Studies assessing the use of EUS–FNA for the diagnosis of PNETs have further found that there is often discordance in cytology and histology findings for G2 tumors in samples obtained by EUS–FNA.17,40 Thus, the ability to accurately detect tumor grade using 18F-FDG PET/CT without the need for biopsy may be valuable for the management of PNETs.
Studies on sporadic PNETs have shown 18F-FDG PET/CT to be clinically useful in the determination of tumor grade and overall survival; however, the utility of parameters such as SUVmax has produced conflicting results.41,42 In comparison, our study of VHL-associated PNETs found that there was no association between SUVmax and grade, yet MTV and TLG were significantly different between G1 and G2 tumors. This reveals the advantage of using volumetric parameters that may better reflect the biology of the tumor. To our knowledge, this is the first time the utility of volumetric parameters on 18F-FDG PET/CT have been shown to be associated with VHL-associated PNET WHO tumor grade.
Metabolic volumetric parameters were analyzed to identify if these could differentiate between patients with and without metastatic disease, and potentially identify patients who would benefit from earlier intervention. Several factors have been proposed for PNETs, including stage, exon 3 mutation status, serum chromogranin A levels, and doubling time associated with metastatic disease.11,13 There was no association between tumor doubling time and exon 3 mutation status and metastatic disease in this study. Blansfield and colleagues used these two criteria, in addition to tumor size, to develop their treatment algorithm for VHL-associated PNETs. A lesion is defined as having high metastatic potential only if it meets two or more criteria.10 An association of TLG was seen with metastatic disease and this may be explained by tumors with higher metabolic activity being more aggressive. A combination of tumor size and TLG may better select patients who would benefit from surgical intervention for potential metastatic disease, but this needs to be confirmed. Analyzing a change in either MTV or TLG with two discrete 18F-FDG PET/CT scans may also identify patients with metastatic potential.
There are several limitations to this study. The study cohort consisted of 20 patients with histologically classified PNETs imaged on 18F-FDG PET/CT, which is a relatively small sample size. 18F-FDG PET/CT, while increasingly found to be useful in the evaluation of PNETs, is not the standard imaging modality for detection.43,44 Newer modalities such as the -68Gallium-DOTATATE scan may be more accurate than our study and will require further study. Another limitation is that pancreatic solid lesions identified on CT are not –always avid on 18F-FDG PET/CT. On the other hand, pancreatic lesions such as microcystic serous cystadenomas can also take up 18F-FDG and can be mistaken for PNETs.45
Conclusions
The results from our study indicate that preoperative 18F-FDG PET/CT volumetric parameters such as MTV and TLG may be useful in the differentiation of G1 and G2 PNETs, and TLG may be useful in determining the metastatic potential of VHL-associated PNETs. Therefore, 18F-FDG PET/CT may play an important role in determining grade and metastatic potential in selecting patients with VHL-associated PNETs who would benefit from surgical intervention.
References
Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067.
Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320.
Hammel PR, Vilgrain V, Terris B, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119(4):1087–1095.
Hough DM, Stephens DH, Johnson CD, Binkovitz LA. Pancreatic lesions in von Hippel-Lindau disease: prevalence, clinical significance, and CT findings. AJR Am J Roentgenol. 1994;162(5):1091–1094.
Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194(3):629–642.
Charlesworth M, Verbeke CS, Falk GA, Walsh M, Smith AM, Morris-Stiff G. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg. 2012;16(7):1422–1428.
Yamasaki I, Nishimori I, Ashida S, Kohsaki T, Onishi S, Shuin T. Clinical characteristics of pancreatic neuroendocrine tumors in Japanese patients with von Hippel-Lindau disease. Pancreas. 2006;33(4):382–385.
Tamura K, Nishimori I, Ito T, Yamasaki I, Igarashi H, Shuin T. Diagnosis and management of pancreatic neuroendocrine tumor in von Hippel-Lindau disease. World J Gastroenterol. 2010;16(36):4515–4518.
Libutti SK, Choyke PL, Bartlett DL, et al. Pancreatic neuroendocrine tumors associated with von Hippel Lindau disease: diagnostic and management recommendations. Surgery. 1998;124(6):1153–1159.
Blansfield JA, Choyke L, Morita SY, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery. 2007;142(6):814-818; discussion 818 e811–812.
Morin E, Cheng S, Mete O, et al. Hormone profiling, WHO 2010 grading, and AJCC/UICC staging in pancreatic neuroendocrine tumor behavior. Cancer Med. 2013;2(5):701–711.
Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40(9):1262–1268.
Strosberg JR, Cheema A, Weber J, Han G, Coppola D, Kvols LK. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29(22):3044–3049.
Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23(6):824–833.
Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20(11):2633–2642.
Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39(6):735–752.
Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46(1):32–38.
Yang M, Zeng L, Zhang Y, et al. TNM staging of pancreatic neuroendocrine tumors: an observational analysis and comparison by both AJCC and ENETS systems from 1 single institution. Medicine. 2015;94(12):e660.
Goldin SB, Bradner MW, Zervos EE, Rosemurgy AS 2nd. Assessment of pancreatic neoplasms: review of biopsy techniques. J Gastrointest Surg. 2007;11(6):783–790.
Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55(6):898–904.
Park SY, Cho A, Yu WS, et al. Prognostic value of total lesion glycolysis by 18F-FDG PET/CT in surgically resected stage IA non-small cell lung cancer. J Nucl Med. 2015;56(1):45–49.
Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55(6):884–890.
Lee JW, Cho A, Lee JH, et al. The role of metabolic tumor volume and total lesion glycolysis on (1)(8)F-FDG PET/CT in the prognosis of epithelial ovarian cancer. Eur J Nucl Med Mol Imaging. 2014;41(10):1898–1906.
Delbeke D, Martin WH. Positron emission tomography imaging in oncology. Radiol Clin North Am. 2001;39(5):883–917.
Bombardieri E, Aktolun C, Baum RP, et al. FDG-PET: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30(12):B115–B124.
Masui T, Doi R, Ito T, et al. Diagnostic value of (18)F-fluorodeoxyglucose positron emission tomography for pancreatic neuroendocrine tumors with reference to the World Health Organization classification. Oncol Lett. 2010;1(1):155–159.
Pasquali C, Rubello D, Sperti C, et al. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg. 1998;22(6):588–592.
Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159–171.
Fonti R, Larobina M, Del Vecchio S, et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012;53(12):1829–1835.
Gimm O, DeMicco C, Perren A, Giammarile F, Walz MK, Brunaud L. Malignant pheochromocytomas and paragangliomas: a diagnostic challenge. Langenbecks Arch Surg. 2012;397(2):155–177.
Dholakia AS, Chaudhry M, Leal JP, et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89(3):539–546.
Satoh Y, Onishi H, Nambu A, Araki T. Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology. 2014;270(1):275–281.
Moon SH, Choi JY, Lee HJ, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck. 2013;35(1):15–22.
Schwartz M. A biomathematical approach to clinical tumor growth. Cancer. 1961;14:1272–1294.
Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123.
Pape UF, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113(2):256–265.
Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104(10):764–777.
Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35(6):853–860.
Alexiev BA, Darwin PE, Goloubeva O, Ioffe OB. Proliferative rate in endoscopic ultrasound fine-needle aspiration of pancreatic endocrine tumors: correlation with clinical behavior. Cancer. 2009;117(1):40–45.
Weynand B, Borbath I, Bernard V, et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology. 2014;25(6):389–395.
Tomimaru Y, Eguchi H, Tatsumi M, et al. Clinical utility of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography in predicting World Health Organization grade in pancreatic neuroendocrine tumors. Surgery. 2015;157(2):269–276.
Kim HS, Choi JY, Choi DW, et al. Prognostic value of volume-based metabolic parameters measured by (18)F-FDG PET/CT of pancreatic neuroendocrine tumors. Nucl Med Mol Imaging. 2014;48(3):180–186.
Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000;216(1):163–171.
Viola KV, Sosa JA. Current advances in the diagnosis and treatment of pancreatic endocrine tumors. Curr Opin Oncol. 2005;17(1):24–27.
Sadowski SM, Weisbrod AB, Ellis R, et al. Prospective evaluation of the clinical utility of 18-fluorodeoxyglucose PET CT scanning in patients with von hippel-lindau-associated pancreatic lesions. J Am Coll Surg. 2014;218(5):997–1003.
Funding
This research was made possible through the NIH Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Newport Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program.
Disclosures
Kei Satoh, Samira M. Sadowski, William Dieckmann, Martha Quezado, Naris Nilubol, Electron Kebebew, and Dhaval Patel have no conflicts of interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satoh, K., Sadowski, S.M., Dieckmann, W. et al. 18F-FDG PET/CT Volumetric Parameters are Associated with Tumor Grade and Metastasis in Pancreatic Neuroendocrine Tumors in von Hippel–Lindau Disease. Ann Surg Oncol 23 (Suppl 5), 714–721 (2016). https://doi.org/10.1245/s10434-016-5541-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5541-4