Abstract
Purposes
Clinical predictive markers for the malignant potential of pancreatic neuroendocrine tumors (PNETs) are limited without histological investigation. We reported previously that a loss of the regular enhancement pattern in preoperative computed tomography (CT) was correlated with the malignant tumor phenotype. This study aimed to establish whether the metabolic aspect of the tumor evaluated by fludeoxyglucose (18F) positron emission tomography/computed tomography 18F-FDG PET/CT is associated with the tumor imaging characteristics and postoperative oncological outcome.
Methods
Among 77 patients who underwent surgery with curative intent for a PNET at our institution between 2001 and 2017, 24 who received 18F-FDG PET/CT before surgery were enrolled in this study. The clinical importance of the standardized uptake value (SUVmax) was investigated with regard to tumor progression and prognosis after curative surgery.
Results
There were four (16%) patients with insulinoma. The mean tumor size was 17 mm and when the median value of the SUVmax (= 2.0) was measured as the cut-off value, the SUVmax ≥ 2.0 group (n = 12) was associated with large tumor size (p = 0.021), high tumor grade (p = 0.015), and irregular pattern on CT (p = 0.0055). The SUVmax was not correlated with age, gender, whether the tumor was functioning or non-functioning, or lymph node metastasis. The SUVmax ≥ 2.0 group had significantly poorer disease-free survival (median, 3.5 vs 16.2 months; p = 0.023) and poorer overall survival (median, 8.8 vs 16.2 months; p = 0.042).
Conclusion
An SUVmax ≥ 2.0 on 18F-FDG PET/CT might be associated with higher malignant potential of PNETs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumors (PNETs) are the second most common primary pancreatic malignancy, accounting for 1–2% of newly diagnosed pancreatic tumors each year [1, 2]. The incidence of PNETs is 2.5–5 in 100,000 individuals per year worldwide and has increased rapidly, in line with developments in imaging modalities [3, 4].
Although there have been remarkable advances in the diagnosis and treatment of PNET, there has not been a comparable progression in the ability to predict disease progression and prognosis. Some reports have suggested prognostic factors for predicting survival and disease progression of PNET, including tumor size, whether the tumor is functioning or non-functioning, Ki67 index, distant metastases, and treatment modality, but these studies have been inconclusive about the non-invasive indicators of malignant potential [5, 6].
Positron emission tomography/computed tomography (PET/CT) using 18F-fluorodeoxyglucose (18F-FDG) has been shown to be valuable for initial staging and for detecting the recurrence of many tumor types including PNETs. 18F-FDG PET/CT is a non-invasive imaging technique based on the principle of specific tissue metabolism [7, 8]. The prognostic value of 18F-FDG PET/CT has been investigated for several cancers, such as non-small cell lung cancer [9], metastatic colorectal cancer [10, 11], and gastric cancer [12, 13].
We reported previously that the radiologic characteristics of PNET are associated with tumor progression [14]. In that report, the attenuation of enhancement and irregular-shaped expansion on enhanced CT were correlated with recurrence and poor prognosis after curative surgery. Based on this finding, we assumed that tumor progression under hypoxic conditions might be relevant to malignant characteristics. Previous reports have demonstrated an association between standardized uptake value (SUVmax) and the stage and metastatic status of PNETs [15, 16].
We conducted this study to clarify whether “irregular shape and enhancement pattern”, which was defined in a previous report [14], is dependent on glucose metabolism; and the clinical significance of 18F-FDG PET/CT for predicting the malignant potential of PNETs.
Patients and methods
Patients
Between April 2001 and October 2017, 77 patients with a preoperative diagnosis of PNET underwent curative pancreatic resection at the Department of Gastroenterological Surgery, Kumamoto University. Twenty-four of these patients received 18F-FDG PET/CT preoperatively and were the subjects of this study. Tumors were evaluated preoperatively by different diagnostic imaging modalities, including endoscopic ultrasonography, abdominal CT, and magnetic resonance imaging (MRI). In patients with multiple tumors, the diameter of the largest tumor was measured. This study was approved by the Human Ethics Review Committee of the Graduate School of Medicine, Kumamoto University.
FDG/PET-CT
18F-FDG PET/CT was performed by injecting 10–12 mCi of 18F-FDG after an overnight fast. The blood glucose level was measured just before tracer administration and was confirmed within 140 mg/ml. For quantitative analysis, the SUVmax was calculated at the sites of suspected tumor foci on CT scans. The positive uptake of FDG was defined as the focal uptake with a SUVmax of ≥ 2.0, which was the median value of this cohort.
Histological examination
Histological grading and staging were based on the World Health Organization (WHO) classification for PNET (2010) and the TNM classification of the Union for International Cancer Control (UICC) and European Neuroendocrine Tumor Society (ENETS). Histological examination routinely included hematoxylin and eosin staining, and additional staining with neuroendocrine markers (synaptophysin, chromogranin A). Tumor grade was determined by the mitotic count in ten high-power fields and by the percentage of tumor cells that were immunohistochemically Ki-67 positive.
Tumor shape and enhancement pattern on enhanced CT
The definition of tumor shape and enhancement characteristics were described previously [14]. Briefly, the tumor shape and enhancement characteristics were evaluated in the arterial or portal phase. Tumors with characteristics that met all of the following criteria were defined as having “regular tumor shape and enhancement”
-
1.
A round or oval shape with a distinct or indistinct margin. For small tumors, obtaining robust contrast between the tumor and background pancreas is sometimes difficult.
-
2.
Homogeneous tumor enhancement.
-
3.
Enhancement is greater than that of the background pancreatic parenchyma. Tumors not meeting the criteria were defined as having “irregular shape and/or enhancement”.
Statistical analysis
All p values were two sided. Qualitative variables were compared using χ2 tests, and quantitative variables were compared using Wilcoxon tests. For overall survival (OS) and relapse-free survival (RFS), we used the log-rank test and the Kaplan–Meier method. Commercial software (JMP Version 10®, SAS Institute, Cary, NC, USA) was used for all statistical analyses, with p < 0·05 defined as significant.
Results
Patients’ characteristics
The median age was 60 (17–81) years. Nineteen patients had non-functioning tumors (79.2%), four had insulinomas (16.7%), and one had a glucagonoma (4.2%). One patient (4.2%) had multiple endocrine neoplasia 1 (MEN1) disease. All patients underwent pancreatic surgery with curative intent. Two patients (8.4%) were found to have liver metastases at surgery. Table 1 gives details of the surgical procedure, tumor size, lymph node status and pathological findings. The median follow-up for the 24 patients who were still alive at the time of last follow-up was 12 (1–101) months. Four patients (16.7%) died during follow-up: one (4.2%) of advanced esophageal cancer and three of liver metastases. Recurrence was found in six patients after surgery. All six had liver metastases, one also had lymph node metastasis, and one also had lung metastasis.
Relationship between clinical variables and the SUVmax
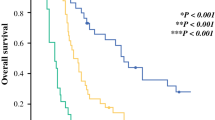
With the median SUVmax (n = 2.0) defined as the cut-off value, an SUVmax ≥ 2.0 was significantly correlated with clinical variables relevant to disease progression, such as large tumor size ≥ 3.0 cm (p = 0.0255), histological grade 2/3 (p = 0.0066), and pathological capsular invasion (p = 0.0066) (Table 2). There was a high correlation between tumor size and SUVmax (r = 0.553, p = 0.0204; Fig. 1a). Grade 1 tumors had a significantly lower correlation with an SUVmax ≥ 2.0 than grade 2 and grade 3 tumors (p = 0.0204 and 0.0258, respectively; Fig. 1b). An SUVmax ≥ 2.0 was significantly correlated with poor RFS and poor OS (p = 0.0255 and 0.0424, respectively; Fig. 2a, b).
Relationship between radiological features and the SUVmax
Based on our previous investigation, patients were divided into a regular shape and enhancement pattern group (regular type, n = 12) and an irregular shape and/or enhancement pattern group (irregular type, n = 12). Figure 3a presents typical cases of PNET. The regular type showed a low SUVmax, whereas the irregular type showed high SUVmax. The SUVmax was significantly higher in the irregular type group than in the regular type group (mean 4.34 vs 1.23; p = 0.0033; Fig. 3b).
Consistency of imaging characteristics determined by enhanced computed tomography (CT) and fludeoxyglucose (18F) positron emission tomography/CT (18F-FDG PET/CT). a Representative image of enhanced CT and 18F-FDG PET/CT in regular and irregular tumors. b Preoperative SUVmax of the regular tumor and irregular tumor groups by the Wilcoxon test
Discussion
The results of this study suggest that the SUVmax is associated with tumor progression in patients with PNET. The 2010 WHO guidelines recommend assessment of the malignant potential of PNET by tumor size and histological grade, which require invasive investigation. A previous study suggested that the histological grade of PNET obtained by fine-needle aspiration was inconsistent with that obtained from resected specimens in 3 of 22 patients [17]. A non-invasive biomarker that would enable us to assess malignant potential and monitor the treatment effect is still being sought. Of note, the SUVmax is significantly lower in histological grade 1 PNET than in grade 2 or grade 3 PNET and is highly correlated with tumor size. This suggests that a high SUVmax in 18F-FDG PET/CT could be a non-invasive predictor of malignant potential.
Although few investigations have focused on the role of 18F-FDG PET/CT in patients with PNET, a previous study showed that a hypoxic condition, assessed by enhanced CT, was correlated with recurrence and poor prognosis. This type of hypoxic condition is consistent with the increase in the SUVmax value in the current study. Cancers exhibit altered glucose metabolism, defined as the Warburg effect, and in some cancers, the regulators are reported to be a hypoxia-inducible factor (HIF) and c-Myc [18]. Whole genome analysis revealed that there was a subgroup associated with hypoxia and HIF signaling in patients with PNET [19]. Transcriptomic analysis of both a mouse model of PNET and human PNET revealed that a metastasis-like primary subtype, one of three major subtypes, had a remarkably high number of Ki67 + cells and a low number of CD31 + cells [20]. Patients with small tumors do not seem to have this altered glucose metabolism, which accompanies an increase in the size of the tumor. It is assumed that the oncogenic switch that induces the Warburg effect does not occur at the beginning of tumorigenesis, but later in tumor development. Because the number of patients in the current study was limited, studies with a larger patient sample are needed to confirm the clinical outcomes.
We analyzed the current cohort by the SUVmax and established that the cut-off of 2.0 was useful for discriminating aggressive tumors from less aggressive ones. Previous research suggests that the metabolic tumor volume measured by volumetric analysis with 18F-FDG PET/CT can predict the prognosis of patients with PNET [15]. In the previous research, the mean tumor size was 31 mm and the median SUVmax value was 4.0, whereas in our cohort, the mean tumor size was 17 mm and the median SUVmax was 2.0. We assume that our cohort included more patients with small and non-metastatic tumors, resulting in good correlation of the SUVmax and disease progression. Thus, metabolic tumor volume as suggested by the previous investigation might be useful to predict their prognosis of patients with advanced PNET.
Four of our patients with small tumors of < 2.0 cm had an SUVmax of ≥ 2.0. Two patients with hereditary diseases, including Hippel–Lindau disease and MEN1, had a 10-mm tumor with an SUVmax of 5.2 and recurrence in the remnant pancreas. An irregular pattern on enhanced CT was not seen in any of the patients, which suggests that 18F-FDG PET/CT might play a clinical role, even in patients with small tumors < 2.0 cm in diameter and a regular shape/enhancement pattern on CT.
In conclusion, SUVmax is correlated with radiologic features as well as tumor progression in patients with PNET. An SUVmax ≥ 2.0 in 18F-FDG PET/CT might be useful for assessing the malignant potential of PNET.
References
Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115:741–51.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–72.
Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008;37:134–8.
Keutgen XM, Nilubol N, Kebebew E. Malignant-functioning neuroendocrine tumors of the pancreas: A survival analysis. Surgery. 2016;159:1382–9.
Madeira I, Terris B, Voss M, Denys A, Sauvanet A, Flejou JF, et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 1998;43:422–7.
Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798–803.
Delbeke D, Martin WH. Positron emission tomography imaging in oncology. Radiol Clin North Am. 2001;39:883–917.
Hustinx R, Benard F, Alavi A. Whole-body FDG-PET imaging in the management of patients with cancer. Semin Nucl Med. 2002;32:35–46.
Kwon W, Howard BA, Herndon JE, Patz EF. FDG uptake on positron emission tomography correlates with survival and time to recurrence in patients with stage i non-small-cell lung cancer. J Thorac Oncol. 2015;10:897–902.
Engelmann BE, Loft A, Kjaer A, Nielsen HJ, Gerds TA, Benzon EV, et al. Positron emission tomography/computed tomography and biomarkers for early treatment response evaluation in metastatic colon cancer. Oncologist. 2014;19:164–72.
Shim JR, Lee SD, Han SS, Lee SJ, Lee DE, Kim SK, et al. Prognostic significance of (18)F-FDG PET/CT in patients with colorectal cancer liver metastases after hepatectomy. Europ J Surg Oncol. 2018;44:670–6.
Chung HW, Lee EJ, Cho YH, Yoon SY, So Y, Kim SY, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol. 2010;136:1929–35.
Song BI, Kim HW, Won KS, Ryu SW, Sohn SS, Kang YN. Preoperative standardized uptake value of metastatic lymph nodes measured by 18F-FDG PET/CT improves the prediction of prognosis in gastric cancer. Medicine. 2015;94:e1037.
Okabe H, Hashimoto D, Chikamoto A, Yoshida M, Taki K, Arima K, et al. Shape and enhancement characteristics of pancreatic neuroendocrine tumor on preoperative contrast-enhanced computed tomography may be prognostic indicators. Ann Surg Oncol. 2017;24:1399–405.
Kim HS, Choi JY, Choi DW, Lim HY, Lee JH, Hong SP, et al. Prognostic value of volume-based metabolic parameters measured by (18)F-FDG PET/CT of pancreatic neuroendocrine tumors. Nucl Med Mol Imaging. 2014;48:180–6.
Masui T, Doi R, Ito T, Kami K, Ogawa K, Harada D, et al. Diagnostic value of (18)F-fluorodeoxyglucose positron emission tomography for pancreatic neuroendocrine tumors with reference to the World Health Organization classification. Oncol Letters. 2010;1:155–9.
Farrell JM, Pang JC, Kim GE, Tabatabai ZL. Pancreatic neuroendocrine tumors: accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol. 2014;122:770–8.
Sawayama H, Ishimoto T, Sugihara H, Miyanari N, Miyamoto Y, Baba Y, et al. Clinical impact of the Warburg effect in gastrointestinal cancer (review). Int J Oncol. 2014;45:1345–54.
Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65–71.
Sadanandam A, Wullschleger S, Lyssiotis CA, Grotzinger C, Barbi S, Bersani S, et al. A cross-species analysis in pancreatic neuroendocrine tumors reveals molecular subtypes with distinctive clinical, metastatic, developmental, and metabolic characteristics. Cancer Disc. 2015;5:1296–313.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Takashi Matsumoto and his co-authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Matsumoto, T., Okabe, H., Yamashita, Yi. et al. Clinical role of fludeoxyglucose (18F) positron emission tomography/computed tomography (18F-FDG PET/CT) in patients with pancreatic neuroendocrine tumors. Surg Today 49, 21–26 (2019). https://doi.org/10.1007/s00595-018-1703-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-018-1703-2