Abstract
Background
The role of glucose transporter 14 (GLUT-14/SLC2A14) in tumor biology is entirely unknown, and the significance of hypoxia inducible factor 1-alpha (HIF1-α) for gastric adenocarcinoma is controversial. The impact of GLUT-1/SLC2A1 has never been confirmed in a Caucasian cohort.
Methods
Between 1996 and 2007, 124 patients underwent gastrectomy for gastric adenocarcinoma. Tumor sections were incubated with GLUT-1, GLUT-14, and HIF1-α antibodies. Expression was analyzed for correlations with histopathology, marker coexpression, and patient survival by uni- and multivariate analyses.
Results
Expressions of GLUT-1, GLUT-14, and HIF1-α were detectable in 50, 77.4, and 27.1 %, respectively. Expression of GLUT-1 was associated with pT-category (p = 0.019), pN-category (p = 0.019), tubular (WHO, p = 0.008), and intestinal (Lauren classification; p = 0.002) histologic subtypes. Expression of GLUT-14 was correlated with pT category (p = 0.043), whereas HIF1-α did not show any correlation with histopathology or survival. The median survival period was 14 months (95 % confidence interval [CI] 9.2–18.8 months) for GLUT-1-positive patients and 55 months (95 % CI 25.8–84.2; p = 0.01) for GLUT-1-negative patients. An inferior prognosis also was seen for GLUT-14-positive cases compared with GLUT-14-negative cases (p = 0.004). Thus, worst survival was seen with both GLUT-1- and GLUT-14-positive expression followed by single-positive and then double-negative cases (p = 0.004). In multivariate analysis including International Union Against Cancer (UICC) stages, R category, Lauren classification, surgery alone versus neoadjuvant/perioperative chemotherapy, and marker expression as covariates, GLUT-1 (p = 0.011) and GLUT-14 (p = 0.025) kept their prognostic independence.
Conclusions
The study findings suggest that detection of GLUT-1 and GLUT-14 is of high prognostic value. It gives additional information to UICC stages and identifies patients with inferior prognosis. If confirmed in prospective studies, these markers need to be considered for future classification systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is the fourth most common cancer, with approximately 800,000 new cases per year, and the second leading cause of cancer-related death worldwide.1 Many patients have advanced disease at the time of diagnosis, resulting in poor prognosis and high mortality.2–4
Of particular interest are prognostic factors, which can identify high-risk patients with poor prognosis. Identification of patients with a poorer outcome can help in setting up novel treatment strategies at the beginning of treatment, which might lead to better and more individualized therapy strategies with superior survival.2 Therefore, current efforts are focused on detection and validation of markers that provide additional information about prognosis to well-established prognostic factors such as the tumor-node-metastasis (TNM) classification. Ideally, such markers should be feasible and useful for clinical routine.5–8
To date, 14 members of the mammalian glucose transporter (GLUT) family have been identified. On the basis of sequence similarities and structural elements, this family is divided into three subfamilies: class 1 (GLUT-1 to -4, GLUT-14), class 2 (GLUT-5, -7, -9, and -11), and class 3 (GLUT-6, -10, -12, and -13).9,10
The human erythrocyte glucose transporter, GLUT-1, is the first identified protein of the GLUT family.11 Responsible for basal glucose uptake, GLUT-1 has been identified as an important functional transporter of glucose in most transformed cells.12,13 For gastric cancer, Kim et al.14 have shown that GLUT-1 is correlated with the intestinal type, and Kawamura et al.15 found higher tumor stages and decreased survival in GLUT-1-positive cases. However, these findings never led to usage of this marker in clinical practice or to its inclusion in classification systems.
In addition, GLUT-14 is a class 1 protein and a splice variant of GLUT-3. High GLUT-14 expression is seen in tissue of the human testis.10,16 Preliminary data on 12 samples indicate expression of GLUT-14 also in gastric adenocarcinoma, but the eventual impact of GLUT-14 in tumor biology, especially in gastric adenocarcinoma, is entirely unknown to date.17
Hypoxia inducible factor 1-alpha (HIF1-α) is a protein induced by hypoxia. In models, the HIF complex has been shown to induce a translational cascade itself, including GLUT-1 and other proteins for which a major role in tumor biology is known such as hepatocyte growth factor receptor (c-MET), vascular endothelial growth factor (VEGF), and matrix metalloproteinase-2 (MMP-2).18 For gastric adenocarcinoma, studies have delivered controversial results concerning the prognostic impact of HIF1-α and its impact of other marker expressions.19–21
The current study is the first to analyze GLUT-14 expression in gastric adenocarcinoma in a large single-center cohort. The impact of GLUT-1 expression also has never been confirmed in a Caucasian patient cohort, and more importantly, previous studies did not include patients with neoadjuvant treatment.22
Materials and Methods
Patients
The subjects of this study were 124 patients [93 men (75 %) and 31 women (25.0 %)] treated surgically with curative intention between March 1997 and September 2008 for primary gastric adenocarcinoma in the Department of General, Visceral, and Cancer Surgery at the University of Cologne. The mean age of the patients was 66.6 years (range 19–85 years), and the median follow-up period was 24 months (range 3–154 months). The study was performed according to the local Research Ethics Guidelines and Helsinki-Ethical Principles, and informed consent for treatment and research was obtained from the patients at the time of admission.
The patients underwent gastrectomy with D2-lymphadenectomy (compartments 1 and 2) in 112 cases (90.3 %) and a subtotal gastrectomy with D2-lymphadenectomy in 12 cases (9.7 %). The median number of dissected lymph nodes was 33. Of the 124 patients, 115 (92.7 %) underwent R0 resection and 9 (7.3 %) underwent R1 resection. Neoadjuvant therapy was administered to 28 patients (22.6 %), who were included for subgroup analyses to determine the impact of marker expression on pretreated patients. Patients with diffuse metastases were excluded. A total of 19 patients (15.3 %) with localized peritoneal carcinosis, distant lymph node metastasis (M1 lymph), or single liver metastasis (M1 Hep) were treated with curative intention and therefore were not excluded.
Neoadjuvant chemotherapy was administered to 28 patients with cT3/4 tumors shown by preoperative endosonography, whereas 15 patients received only a neoadjuvant regimen, and 13 patients in a perioperative setting had the same number of cycles during adjuvant therapy. Chemotherapy was performed with cisplatin + 5-FU + leucovorin (PLF) or epirubicin + cisplatin + 5-FU (ECF) or epirubicin + oxaliplatin + capecitabine (EOX) based on the actual guidelines and recommendations at that time. The neoadjuvant treatment (n = 15) was performed for eight patients with ECF, four patients with PLF, and three patients with EOX. Perioperative treatment (n = 13) was performed for four patients with ECF, four patients with PLF, and five patients with EOX.
Histopathology
Specimens were removed en bloc, and lymph nodes were marked according to a standardized protocol. Resected specimens were fixed in 5 % phosphate-buffered formalin and embedded in paraffin.
Histopathologic examination consisted of a thorough and standardized evaluation of tumor stage, residual tumor (R) category, grading, and number of resected and infiltrated lymph nodes. Gastric lymph nodes were documented according to the classification of the Japanese Research Society of Gastric Cancer with lymph node groups 1–13.23 Tumor localization was defined according to the International Classification of Diseases for Oncology. Lesions were further classified and graded in accordance with World Health Organization (WHO) recommendations, Lauren classification, differentiation (G1–G3), and the 7th edition of the TNM classification.
Immunohistochemistry
Resected tumor samples were cut (5 µm thick) and deparaffinized. Peroxidase activity was blocked by 3 % H2O2/methanol for 20 min. Sections were incubated with primary polyclonal antihuman GLUT-1 antibody (GLUT-1, RB-9052-P0; Thermo Fisher Scientific, Cheshire, UK), monoclonal mouse antihuman HIF1-α antibody (HIF1-α, H1α-67), or polyclonal rabbit anti-human GLUT-14 antibody (GLUT-14, P94354Hu01; Live Science Inc.) overnight at 4 °C. Antibodies were guaranteed to be specific without any cross-reactivity with proteins of the same family. We diluted GLUT-1, GLUT-14, and HIF1-α respectively at 1:200, 1:50, and 1:20 in Tris-buffered saline (TBS, containing 2.5 % bovine serum albumin).
The next day, after a double rinse with TBS, the polymer secondary antibody-horseradish-peroxidase complex (Envision system; Dako) was applied, incubated for 30 min, and rinsed twice. Then enzyme substrate AEC+ (Dako) was applied. After incubation for 30 min, slides were counterstained with hematoxylin for 2 min and mounted in glycerol jelly.
Semiquantitative Analysis
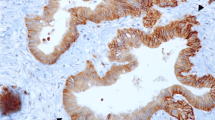
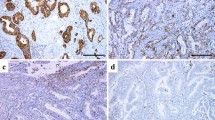
Expression of GLUT-1, GLUT-14, and HIF1-α of the primary tumor was determined by semiquantitative evaluation and divided into two groups as follows: 0 (no expression or expression in <10 % of tumor cells) and 1 (GLUT-1, GLUT-14 or HIF1-α expression in ≥10 % primary tumor cells; Fig. 1). Analyses were performed by an experienced pathologist (U.D.), who was blinded to all clinical data.
Statistical Analysis
Associations between clinicopathologic parameters and GLUT-1, GLUT-14, and HIF1-α were evaluated using Pearson’s Chi square-test. Survival analysis was performed by the Kaplan–Meier approach using the log-rank test. Independent prognostic factors were determined by multiple stepwise regression analysis using the Cox-model. The level of significance was set at a p value lower than 0.05, and p values are for two-sided testing. The tests were performed with SPSS 22.0 (SPSS, Chicago, IL, USA).
Results
Demographic Characteristics and Marker Expression
Gender and both WHO and Lauren classifications as well as tumor grade and stage overall and in correlation with protein expression of GLUT-1, GLUT-14, and HIF1-α are presented in Table 1. The most frequent histologic type was the tubular type (WHO, 44.4 %), followed by the signet-ring carcinoma type (WHO, 31.5 %), the diffuse type (Lauren, 46 %), and the intestinal type (Lauren, 44.4 %). Most tumors showed a poor differentiation (G3, 55.6 %) and were diagnosed as pT3 or pT4 carcinoma (36.3 and 35.5 %, respectively). No lymph node infiltration was found in 38 patients (pN0, 30.6 %), whereas pN1, pN2, and pN3 were diagnosed respectively in 25 (20.2 %), 22 (17.7 %), and 39 (31.5 %) patients. According to the 2009 UICC staging, 22 patients had stage 1, 16 patients had stage 2, 31 patients had stage 3, and 55 patients had stage 4 disease.
GLUT-1
The findings showed GLUT-1 to be partly homogeneous and partly focal cytoplasmatic with a membranous staining pattern (Fig. 1a, b). Significant correlation of GLUT-1 expression with nodal status was noted (p = 0.049). In only 13 cases (34.2 %), GLUT-1 expression was detected in N0 patients, whereas 49 (57 %) of 86 lymph node-positive patients showed GLUT-1 expression in the primary tumor (p = 0.019).
A significant correlation of GLUT-1 expression with higher stages of disease also was seen for the pT category (p = 0.019). As such, 12 (85.7 %) of 14 pT1 patients did not show any GLUT-1 expression. In addition, the intestinal and mixed subtypes of gastric adenocarcinoma also showed higher GLUT-1 expression than the diffuse type (p = 0.002).
A significant difference (p < 0.008) also was detected for the subtypes according to the WHO classification. Whereas 30 (76.9 %) of 39 signet-ring cell carcinomas did not show any GLUT-1 expression, 33 (60.0 %) of 55 tubular tumors showed a positive expression. No correlation of GLUT-1 with HIF1-α was found (p = 1.0).
GLUT-14
The staining pattern of GLUT-14 was mostly homogeneous cytoplasmatic (Fig. 1c, d). Of all three markers, GLUT-14 showed the highest expression, with 96 (77.4 %) positive cases. A significant correlation of GLUT-14 expression was seen for the pT category in terms of a higher pT category for GLUT-14-positive tumors (p = 0.043). Remarkably, GLUT-14 expression was associated with GLUT-1 expression (p = 0.001).
HIF1-α
In this study, HIF1-α showed a perinuclear cytoplasmatic staining pattern (Fig. 1e, f). The only significant correlation of HIF1-α was seen for gender (p = 0.018). No association with histology or TNM was found for HIF1-α.
Survival Analysis
Kaplan–Meier survival analyses based on GLUT-1 and GLUT-14 expression are shown in Fig. 2a, b. The patients with positive GLUT-1 expression had a significantly worse prognosis than those without GLUT-1 expression (p = 0.01). The median survival time for the GLUT-1-positive patients was 14 months (95 % CI 9.2–18.8 months) compared with 55 months (95 % CI 25.8–84.2 months) for the GLUT-1-negative patients (Fig. 2a).
The patients with positive GLUT-14 expression also showed a significant worse outcome than the patients without GLUT-14 expression (p = 0.004). The median survival time for the GLUT-14-positive patients was 18 months (95 % CI 11.7–24.3 months) compared with 105 months (95 % CI 23.4–186.7 months) for the patients without GLUT-14 expression in tumor tissue (Fig. 2b). Expression of HIF1-α, however, did not correlate with patient survival (Fig. 2c; p = 0.830).
For further survival analyses, we divided the patients into the following three groups based on their GLUT-1 and GLUT-14 status: (1) double-GLUT-positive patients, (2) single-GLUT-positive patients (GLUT-1 or GLUT-14 positive), and (3) double-GLUT-negative patients. The significantly worst survival rates were seen for the double-GLUT-positive patients, followed by the single-GLUT-positive patients, and the highest survival rates were seen for the double-GLUT-negative patients (p = 0.004; Fig. 2d).
Multivariate Analysis
In the multivariate analyses, known prognostic factors for gastric cancer (UICC, R category, Lauren classification, and surgery alone vs neoadjuvant/perioperative chemotherapy), GLUT-1 and GLUT-14 status were included (Table 2). Multivariate analyses were performed for GLUT-1 and GLUT-14 markers, respectively, and as a combined factor.
In the GLUT-1 analysis, UICC stages (p < 0.001), R category (p = 0.006), and GLUT-1 expression (p = 0.011) kept their prognostic independence. The same factors, including GLUT-1 expression (p = 0.006) and Lauren classification (p = 0.007), kept their prognostic independence when the patients with neoadjuvant chemotherapy (n = 28) were excluded from the analysis.
In the multivariate analysis for GLUT-14 expression, UICC stages (p < 0.001), R category (p = 0.044), and GLUT-14 status (p = 0.025) kept their prognostic independence. When the patients with neoadjuvant therapy were excluded, UICC stages (p = 0.006), R category (p = 0.001), Lauren classification (p = 0.023), and GLUT-14 (p = 0.025) kept their prognostic independence.
Multivariate analysis for double-GLUT marker expression showed UICC stages (p < 0.001), R category (p = 0.010), surgery alone versus neoadjuvant/perioperative chemotherapy (p = 0.040), and double-GLUT marker expression (p = 0.001) to be independent prognostic markers. The double-GLUT marker kept its prognostic independence when patients with neoadjuvant chemotherapy were excluded (p = 0.001).
Discussion
In gastric cancer, the conventional prognostic parameters (UICC stages and pT, pN, pM, and R categories) are known to be associated with decreased survival at higher stages of disease. However, establishing new prognostic markers has failed in many studies, with most multivariate analyses and meta-analyses showing no effect on prognosis.24
The current study focused on GLUT-1, GLUT-14, and HIF1-α expression and identified positive GLUT-1 and GLUT-14 expression as a potential predictor of decreased survival. Immunohistochemical evaluation of cancer tissue is a feasible, accurate, cost-effective, and fast method for completing the patient`s risk profile. In the current study, GLUT-1 expression had a prognostic power comparable with that of the pT category and superior to that of the pN and pM categories. The patients with GLUT-1 expression had a median overall survival time about 40 months shorter than the patients without GLUT-1 expression (Fig. 2). A comparable prognostic impact could be shown for GLUT-14 expression, which was a powerful predictor in the multivariate analysis (Table 2). However, in contrast to other studies, HIF1-α did not predict outcome and did not correlate with GLUT-1 expression.25
One important characteristic of many cancer cells is the switch from mitochondrial to anaerobic glycolysis (Warburg effect), which may partly explain why malignant cells overexpress GLUT transporters, allowing them to increase their glucose consumption.26 This may explain why positive GLUT-1 expression is associated with a more aggressive type of cancer, resulting in decreased survival time.
Elevated GLUT-1 expression has been described in hepatic, pancreatic, breast, esophageal, brain, renal, cutaneous, colorectal, endometrial, ovarian, and cervical carcinoma, mostly indicating an inferior prognosis for patients with high expression.27–36 In accordance with these findings, the current study suggests that detectable GLUT-1 expression is associated with greater tumor aggressiveness in gastric cancer, resulting in higher pT, pN, and pM categories. Furthermore, our study suggests a significant association between GLUT-1 expression and WHO and Lauren classifications. The intestinal type was positively associated with GLUT-1 expression, and signet-ring cell carcinoma showed significant lower GLUT-1 expression than other subtypes according to the WHO classification. These results are confirmed by several other studies.14,15,37
An association of GLUT-1 expression with the intestinal type of gastric cancer was first shown by Kim et al.14 and a negative correlation with survival was first described by Kawamura et al.15 Our study confirmed those findings for a Caucasian cohort of patients, and more importantly, it showed that these findings also are applicable for patients who received neoadjuvant treatment. Pretreated patients were not included in previous studies although those patients represent a substantial part in Western countries.
The impact of GLUT-14 in tumor biology, especially in gastric adenocarcinoma, has never been analyzed previously. The majority of our patients (77.4 %) showed a GLUT-14-positive staining pattern with a significant coexpression of GLUT-1 and GLUT-14. Remarkably, similar to GLUT-1, a positive GLUT-14 status seemed to be associated with lower survival rates, giving further evidence that both markers reflect tumor aggressiveness. Thus, the combination of both GLUT markers may be a powerful tool for survival prediction, with the worst survival rates seen among patients with double-positive GLUT expression (Fig. 2d) in this study. Similar to hormone status in breast cancer, after prospective confirmation studies, GLUT status could be used to identify high-risk patients, especially because determining GLUT-expression is feasible and can be performed easily even on biopsies.38 In prospective studies, those high-risk patients could be identified as candidates for neoadjuvant chemotherapy. Although not attempted with humans, GLUT-1 as a medicamentous target has been tested in vitro and in vivo (mouse) on colorectal cancer cells with promising results.39
In conclusion, our results suggest that detection of GLUT-1 and GLUT-14 expression in the surgical resection specimen, especially a combination of the two, has high prognostic value in gastric cancer. This is the first study to investigate the relevance of GLUT-14 in tumor biology, with results encouraging for further investigations. Because GLUT status gives additional information to the UICC categories, it is helpful for identifying patients with inferior prognosis. Confirmation of our results in further ideally prospective studies certainly is necessary before these markers are included in future classification systems for gastric adenocarcinoma. In a prospective setting, GLUT status also could be used as an additional tool for indication of divergent therapeutic regimens.
References
Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107–16.
Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–61.
Kim JP, Kwon OJ, Oh ST, Yang HK. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann Surg. 1992;216:269–78 (discussion 278–269).
Hansson LE, Sparen P, Nyren O. Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230:162–9.
Yasui W, Oue N, Aung PP, Matsumura S, Shutoh M, Nakayama H. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86–94.
Alakus H, Holscher AH, Grass G, et al. Extracapsular lymph node spread: a new prognostic factor in gastric cancer. Cancer. 2010;116:309–15.
Alakus H, Grass G, Hennecken JK, et al. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–23.
Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. WJG World J Gastroenterol. 2014;20:5679–84.
Barrett MP, Walmsley AR, Gould GW. Structure and function of facilitative sugar transporters. Curr Opin Cell Biol. 1999;11:496–502.
Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–38.
Montel-Hagen A, Kinet S, Manel N, et al. Erythrocyte GLU-1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–48.
Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–25.
Birnbaum MJ, Haspel HC, Rosen OM. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987;235:1495–8.
Kim WS, Kim YY, Jang SJ, Kimm K, Jung MH. Glucose transporter 1 (GLUT1) expression is associated with intestinal type of gastric carcinoma. J Korean Med Sci. 2000;15:420–4.
Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–41.
Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics. 2002;80:553–7.
The Human Protein Atlas. Retrieved 2 February 2015. http://www.proteinatlas.org/ENSG00000173262-SLC2A14/cancer.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32.
Griffiths EA, Pritchard SA, Welch IM, Price PM, West CM. Is the hypoxia-inducible factor pathway important in gastric cancer? Eur J Cancer. 2005;41:2792–805.
Urano N, Fujiwara Y, Doki Y, et al. Overexpression of hypoxia-inducible factor-1 alpha in gastric adenocarcinoma. Gastric Cancer. 2006;9:44–9.
Jung JH, Im S, Jung ES, Kang CS. Clinicopathological implications of the expression of hypoxia-related proteins in gastric cancer. Int J Med Sci. 2013;10:1217–23.
Knight G, Earle CC, Cosby R, et al. Neoadjuvant or adjuvant therapy for resectable gastric cancer: a systematic review and practice guideline for North America. Gastric Cancer. 2013;16:28–40.
A Japanese Gastric Cancer. Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer. 1998;1:10–24.
Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Critical reviews in oncology/hematology. Gastric Cancer. 2009;71:127–64.
Marin-Hernandez A, Gallardo-Perez JC, Ralph SJ, Rodriguez-Enriquez S, Moreno-Sanchez R. HIF-1alpha modulates energy metabolism in cancer cells by inducing overexpression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9:1084–101.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Yamamoto T, Seino Y, Fukumoto H, et al. Overexpression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223–30.
Wang BY, Kalir T, Sabo E, Sherman DE, Cohen C, Burstein DE. Immunohistochemical staining of GLUT1 in benign, hyperplastic, and malignant endometrial epithelia. Cancer. 2000;88:2774–81.
Rudlowski C, Moser M, Becker AJ, et al. GLUT1 mRNA and protein expression in ovarian borderline tumors and cancer. Oncology. 2004;66:404–10.
Rudlowski C, Becker AJ, Schroder W, Rath W, Buttner R, Moser M. GLUT1 messenger RNA and protein induction relates to the malignant transformation of cervical cancer. Am J Clin Pathol. 2003;120:691–8.
Ogawa J, Inoue H, Koide S. Glucose-transporter-type-I-gene amplification correlates with sialyl-Lewis-X synthesis and proliferation in lung cancer. Int J Cancer. 1997;74:189–92.
Nishioka T, Oda Y, Seino Y, et al. Distribution of the glucose transporters in human brain tumors. Cancer Res. 1992;52:3972–9.
Nagase Y, Takata K, Moriyama N, Aso Y, Murakami T, Hirano H. Immunohistochemical localization of glucose transporters in human renal cell carcinoma. J Urol. 1995;153(3 Pt 1):798–801.
Haber RS, Rathan A, Weiser KR, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34–40.
Cantuaria G, Fagotti A, Ferrandina G, et al. GLUT-1 expression in ovarian carcinoma: association with survival and response to chemotherapy. Cancer. 2001;92:1144–50.
Brown RS, Wahl RL. Overexpression of GLUT-1 glucose transporter in human breast cancer: an immunohistochemical study. Cancer. 1993;72:2979–85.
Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20:597–604.
Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014;10:2293–301.
Abouzeid AH, Patel NR, Rachman IM, Senn S, Torchilin VP. Anti-cancer activity of anti-GLUT1 antibody-targeted polymeric micelles co-loaded with curcumin and doxorubicin. J Drug Target. 2013;21:994–1000.
Acknowledgment
This study did not receive financial or other support from any organizations or other people not listed as authors.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berlth, F., Mönig, S., Pinther, B. et al. Both GLUT-1 and GLUT-14 are Independent Prognostic Factors in Gastric Adenocarcinoma. Ann Surg Oncol 22 (Suppl 3), 822–831 (2015). https://doi.org/10.1245/s10434-015-4730-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4730-x