Abstract
Background
This study aimed to examine the prognostic relevance of glucose transporter type 1 (GLUT-1), which is a key regulator of the glucose metabolism. In particular, the study aimed to examine the association between GLUT-1 expression and the therapeutic effect of chemoradiotherapy (CRT) in pancreatic ductal adenocarcinoma (PDAC).
Methods
Patients with PDAC were enrolled in the study. Patients with distant metastases and those who received only chemotherapy as treatment were excluded from the study. Specimens for immunohistochemical evaluations were obtained through surgical resection and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of the primary tumor before any treatment.
Results
This study included 197 patients. Of these 197 patients, 100 underwent upfront surgery, and 97 received neoadjuvant CRT (NACRT), which was performed mainly for patients with locally advanced tumors. Of the 97 patients who received NACRT, 21 later underwent surgical resection. For the patients who underwent upfront surgery, low GLUT-1 expression was an independent factor for a better prognosis. For the patients who underwent NACRT, low GLUT-1 expression was significantly associated with greater tumor size reduction, a higher resection rate, and a better prognosis. Additionally, GLUT-1 expression was significantly increased after NACRT treatment.
Conclusions
Among the patients with PDAC, those with low GLUT-1 expression in the primary tumor had a better prognosis those with high GLUT-1 expression. Moreover, the patients with low GLUT-1 expression displayed a better therapeutic response to NACRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic ductal adenocarcinoma (PDAC), one of the most lethal malignancies, is associated with a very poor prognosis and a 5-year survival rate of only 6%.1 Surgical resection is the only potentially curative therapy for PDAC. However, most patients have advanced tumors at diagnosis 2 and experience local or distant metastases even after resection.3
To improve the curative resection rate and prognosis, neoadjuvant chemoradiotherapy (NACRT) has been adopted in cases of borderline resectable and unresectable diseases without distant metastasis.4,5,6 Tumor radiosensitivity is thought to be a critical factor in the use of NACRT.
A change in the energy metabolism is a hallmark of tumor cells and a key contributor to tumor progression.7,8 Malignant cells take advantage of the physiologic preferential use of glycolysis over oxidative phosphorylation, resulting in higher rates of glucose uptake and lactate production. This is known as the Warburg effect.9
Tumor radioresistance may result from the interaction of multiple tumor-intrinsic and tumor-extrinsic factors. The metabolic shift to glycolysis is apparently involved in radioresistance.10,11 The transmembrane glucose transporter type 1 (GLUT-1) mediates the first rate-limiting step in glucose transport across the plasma membrane.12 Hypoxia-inducible factor 1 (HIF-1) upregulates GLUT-1,10 whereas mutant p53 promotes translocation of GLUT-1 to the plasma membrane.13 Both factors stimulate the Warburg effect. Hypoxia, which drives tumor progression, is an important characteristic of the microenvironment of malignant tumors, including PDAC.14 Moreover, mutations in TP53 are frequently observed in PDAC.15,16
Overexpression of GLUT-1 has been shown to correlate with poor prognosis in several malignancies.17,18,19 Reported findings show GLUT-1 expression to be significantly higher in PDAC lesions than in adjacent non-cancerous tissue.20 Expression of GLUT-1 progressively increases from low- to high-grade dysplastic lesions in both pancreatic intraepithelial neoplasms and intraductal papillary mucinous neoplasms.21 A recent meta-analysis has shown that high GLUT-1 expression is associated with shorter overall survival (OS) for patients with PDAC.22 However, the association between GLUT-1 expression and radioresistance in PDAC was not examined.
The current study examined the association between GLUT-1 expression and prognosis for patients with PDAC who underwent upfront surgery. In addition, we explored the significance of GLUT-1 expression for patients who underwent NACRT using specimens obtained through endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) biopsy.
Materials and Methods
Patient and Tumor Sampling
Patients who underwent upfront surgery or NACRT for PDAC between January 2000 and December 2015 at the Department of Digestive Surgery, Breast and Thyroid Surgery, Kagoshima University were enrolled in the study. Since 2006, NACRT has been performed mainly for patients with a diagnosis of locally advanced tumors. Patients who had distant metastases or received chemotherapy were excluded from the study. This study was approved by the institutional ethics review board of our hospital, and written informed consent for data analysis and publication was obtained from all patients.
Specimens for immunohistochemical evaluation of GLUT-1 expression were obtained by surgical resection and EUS-FNA of the primary tumor, and EUS-FNA was performed before any treatment. All resected specimens were examined histologically according to the seventh tumor-node-metastasis (TNM) classification system.23 Imaging examinations, including computed tomography (CT) and magnetic resonance imaging (MRI), were performed every 4 months for the first 2 years, then every 6 months during the following 3 years. The CRT regimens included hyperfractionated accelerated radiotherapy administered with S-1 (TS-1; Taiho Pharmaceutical Co, Ltd, Tokyo, Japan) at a dose of 80 mg/m2 for the first 21 days.24 A total of 50–58 Gy was administered in 40 fractions during 4 weeks. Then, 1 month after CRT completion, S-1 was administered for 2 weeks, followed by a 2-week rest period. Either CT or MRI was performed 1 month after CRT and every 3 months afterward. Tumor response after NACRT was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST).25
Immunohistochemistry of GLUT-1
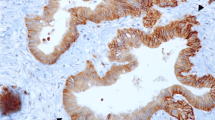
Paraffin blocks were sliced into 3-μm-thick sections. After deparaffinization and rehydration, endogenous peroxidase activity was blocked by immersing the slides in an absolute methanol solution containing 0.3% hydrogen peroxide for 30 min. The sections then were treated with 1% bovine serum albumin for 30 min to block nonspecific reactions. Heat-induced antigen retrieval was performed by autoclave pretreatment (120 °C for 5 min) in citrate buffer solution (pH 6.0). The sections then were incubated overnight at 4 °C with rabbit polyclonal anti-GLUT-1 antibody (ab15309; Abcam, Cambridge, UK) at a dilution of 1:400.
After incubation with the appropriate secondary antibodies, staining was performed using an avidin–biotin complex detection kit (Vector Laboratory, Burlingame, CA, USA) and a diaminobenzidine substrate system according to the manufacturer’s instructions. The slides were counterstained with hematoxylin before mounting. Positive controls for GLUT-1 expression consisted of red blood cells in each section. Negative controls were obtained by omitting the primary antibody.
Evaluation of GLUT-1 Expression
All tissue sections were simultaneously assessed by two investigators (H.K. and K.M.) who were blinded to the patient’s clinicopathologic details. Levels of GLUT-1 expression were evaluated by measuring the intensity and frequency of tumor cell membrane staining. The expression of GLUT-1 was considered high when more than 30% of the tumor cells were intensely stained and low when 30% or less of the tumor cells were intensely stained. For evaluation of GLUT-1 expression, we used only EUS-FNA specimens that contained more than 500 tumor cells.
Statistical Analysis
Associations between different categorical variables were assessed using the Chi square test or Fisher’s exact test. The Wilcoxon signed-rank test was used to compare continuous variables before and after NACRT for the same patients. Survival curves were plotted using the Kaplan–Meier method and analyzed using the log-rank test. The unstratified Cox proportional hazard model was used to estimate the hazard ratio (HR) and its corresponding 95% confidence interval (CI).
Overall survival was calculated as the time between initial treatment and death by any cause. Recurrence-free survival (RFS) was calculated as the time between surgical resection and initial recurrence. Progression-free survival (PFS) was calculated as the time between initial treatment and tumor progression for patients who underwent NACRT.
The multivariate Cox proportional hazards model was used to estimate adjusted HR and to determine independent factors associated with survival using significant factors from the univariate analysis as covariates. All p values lower than 0.05 were considered to indicate statistical significance. Statistical evaluation was performed using SigmaPlot version 12.5 for Windows (HULINKS Inc., Tokyo, Japan).
Results
Patient Characteristics
Among the 197 patients involved in this study, 100 received upfront surgery and 97 received NACRT, 21 of whom also were eligible for surgery. The patients who underwent NACRT had more advanced T factor (Table S1). Of the 100 patients who underwent upfront surgery, 65 received postoperative adjuvant therapy, with 14 receiving 5-fluorouracil, 27 receiving gemcitabine, and 24 receiving S-1. The study excluded 78 patients who underwent NACRT without EUS-FNA (n = 70) or without EUS-FNA specimens feasible for evaluation of GLUT-1 expression (n = 8). The median follow-up period was 20.5 months (mean, 29.4 months).
GLUT-1 Expression and Prognosis for Patients Who Underwent Upfront Surgery
We evaluated GLUT-1 expression using immunohistochemistry of resected specimens from patients who underwent upfront surgery. Acinar and ductal cells of the adjacent noncancerous tissue showed no GLUT-1 staining. The findings showed GLUT-1 expressed primarily in tumor cell membranes (Fig. 1a–d). Expression of GLUT-1 was classified as low in 40 patients (40%) and high in 60 patients (60%).
Table 1 shows the analyses of OS for patients who underwent upfront surgery. Univariate analysis found male gender, tumor larger than 30 mm, lymph node metastasis, moderate or poor differentiation, serum carbohydrate antigen 19-9 level higher than 37 U/mL, R1 resection, no adjuvant therapy, and high expression of GLUT-1 to be significant factors for a poor prognosis. Multivariate analysis indicated that all these factors, except for adjuvant therapy, remain as independent prognostic factors.
Figure S1 shows OS curves according to the GLUT-1 expression. The median survival time (MST) was 19 months (95% CI 14.2–23.8 months) for the patients with high GLUT-1 expression and 35.1 months (95% CI 18.5–51.7 months) for the patients with high low GLUT-1 expression (p = 0.008 log-rank; adjusted HR, 0.328; 95% CI 0.184–0.585). Furthermore, high GLUT-1 expression was an independent factor of shorter RFS (Table S2).
GLUT-1 Expression Using EUS-FNA Specimens and the Effect of NACRT
We evaluated GLUT-1 expression in specimens obtained through EUS-FNA according to the same criteria used for surgically resected specimens (Fig. 2). Tumor size and serum CA 19-9 were significantly reduced after NACRT (Table S3).
The findings showed 54 patients with CR/PR and 43 patients with SD/PD. One patient showed CR. We then examined the association between clinical factors and the effect of NACRT (Table 2). Low GLUT-1 expression was significantly associated with the rate of CR/PR.
Figure 3a shows the OS of patients who underwent NACRT. The MST was 17 months (95% CI 13.0–21.0 months) for the patients with high GLUT-1 expression and 26.6 months (95% CI 16.4–36.8 months) for the patients with low GLUT-1 expression (p = 0.008, log-rank; adjusted HR, 0.640; 95% CI 0.380–1.078; Table S4).
Kaplan–Meier survival curves of overall survival (OS) from the initial treatment. a OS of all patients who underwent neoadjuvant chemoradiotherapy (NACRT). Patients with low glucose transporter type 1 (GLUT-1) expression showing better OS than those with high GLUT-1 expression. b Resection rate after NACRT according to GLUT-1 expression. The resection rate for low GLUT-1-expressing tumors was significantly higher than for high GLUT-1-expressing tumors (p = 0.028)
Figure 3b shows the resection rates after NACRT according to GLUT-1 expression. Of the 42 patients with low GLUT-1 expression, 14 underwent surgical resection after NACRT. By contrast, only 7 of the 55 patients with high GLUT-1 expression underwent surgical resection. The resection rate for low GLUT-1-expressing tumors (33.3%) was significantly higher than for high GLUT-1-expressing tumors (12.7%).
We examined the prognostic impact of GLUT-1 expression using EUS-FNA specimens according to surgical resection after NACRT (Fig. S2). For the patients who underwent surgical resection after NACRT, high GLUT-1 expression was a significant factor for a shorter RFS (p = 0.043, log-rank; HR, 0.260; 95% CI 0.063–1.076). For those who did not undergo surgical resection after NACRT, the high GLUT-1 expression group tended to have a shorter PFS than the low GLUT-1 expression group (p = 0.057, log-rank; HR, 0.629; 95% CI 0.383–1.035).
Comparison of GLUT-1 Expression Between EUS-FNA Specimens and Surgically Resected Specimens
Expression of GLUT-1 was evaluated in surgically resected and EUS-FNA specimens from 20 patients who underwent upfront surgery. The findings of GLUT-1 expression in the surgically resected specimens were concordant with those in the EUS-FNA specimens from 18 patients (90%). We then evaluated GLUT-1 expression using surgically resected and EUS-FNA specimens from 20 patients who underwent surgical resection after NACRT (excluding one patient with a pathologic complete response). Among the 20 patients, 8 (40%) experienced a low-to-high change in GLUT-1 expression due to NACRT. However, none of the patients experienced a high-to-low change in GLUT-1 expression. Nonetheless, prognosis did not differ significantly according to changes in GLUT-1 expression (Fig. S3).
Discussion
Reports show that CRT improves the resection rate and prognosis for patients with locally advanced PDAC. However, its therapeutic effect on the primary tumor varies considerably across individuals.4,5,6 Predicting the therapeutic effect of CRT is pivotal in selecting the optimal therapy for locally advanced PDAC. The metabolic shift to glycolysis, a hallmark of tumor cells, seems to be an important factor in radioresistance.10,11 For this process, GLUT-1 is the key molecule.12 Chikamoto et al.26 demonstrated that GLUT-1 expression is associated with the maximum standardized uptake value of18 F-fluorodeoxyglucose positron emission tomography and the prognosis for patients with pancreatic cancer.
In the current study, GLUT-1 expression was an independent prognostic factor for the patients with PDAC who underwent upfront surgery: Those with low GLUT-1 expression showed a significantly better prognosis. Furthermore, evaluation of GLUT-1 expression using EUS-FNA specimens before treatment was significantly associated with the therapeutic effect of NACRT. Low GLUT-1 expression was significantly associated with greater reduction in tumor size, higher resection rates, and a better prognosis.
The effects of radiotherapy, due partly to radiation-induced radical and oxidative stress, can be reduced in tumor cells via the upregulation of their endogenous antioxidant capacity through the accumulation of pyruvate and lactate caused by increased glycolysis in tumor cells.10 Expression and localization of GLUT-1 are regulated by HIF-1 and mutant p53.10,13 The current study demonstrated that GLUT-1 expression evaluated in EUS-FNA specimens is associated with the effectiveness of NACRT. The role of EUS-FNA in the diagnosis of pancreatic masses is increasing.27 Furthermore, recent studies have demonstrated that immunohistochemical evaluations using EUS-FNA specimens can predict distant metastasis and prognosis for patients with PDAC.28,29 Yamada et al.30 used EUS-FNA specimens to demonstrate that human equilibrative nucleoside transporter expression can predict responses to gemcitabine-based CRT.
Immunohistochemical evaluation using EUS-FNA specimens is thought to be affected by intratumoral heterogeneity due to the small amount of tissue obtained from this procedure. It remains unclear how many tumor cells are required for adequate immunohistochemical evaluation. The current study used only EUS-FNA specimens that included more than 500 tumor cells. The concordance of the evaluation between EUS-FNA and surgically resected specimens of patients who underwent upfront surgery was 90%. For evaluation in EUS-FNA specimens, GLUT-1 appears to be a suitable candidate given its absence in the noncancerous pancreatic ducts and acinar cells. Furthermore, erythrocytes, which usually are included in EUS-FNA specimens, can be used as positive controls.
Eight (40%) of the patients who underwent surgical resection after NACRT showed a low-to-high change in GLUT-1 expression due to NACRT. However, none of the patients experienced a high-to-low change in GLUT-1 expression. One possible reason should be that subclones of high GLUT-1-expressing tumor cells had survived and proliferated during NACRT.
The current study showed no significant difference in prognosis according to changes in GLUT-1 expression. However, analysis of a larger sample is required to show the true prognostic impact of changes in GLUT-1 expression. A recent study demonstrated that inhibition of GLUT-1 expression by antisense oligodeoxynucleotides improved radiosensitivity in laryngeal carcinoma.11 Therefore, GLUT-1 expression may be not only an important factor associated with the effect of NACRT but also a potent target for improving the therapeutic effect of NACRT in PDAC.
The current study had several limitations. First, given its retrospective nature, it had no defined criteria for resection after NACRT. Second, the number of patients who underwent surgical resection after NACRT was relatively small. A prospective study including a larger sample is required for further confirmation. Despite these limitations, the current study demonstrated that GLUT-1 expression was an independent prognostic factor for patients with PDAC who underwent upfront surgery. Furthermore, our findings suggest that the evaluation of GLUT-1 expression in EUS-FNA specimens before treatment can help to predict the effects of NACRT.
In conclusion, our findings demonstrated that among patients with PDAC, those with low GLUT-1 expression in the primary tumor have a better prognosis than those with high GLUT-1 expression. Moreover, patients with low GLUT-1 expression before treatment show better therapeutic responses to NACRT.
References
Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267.
Ying JE, Zhu LM, Liu BX. Developments in metastatic pancreatic cancer: is gemcitabine still the standard? World J Gastroenterol. 2012;18:736–45.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Small W Jr, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008; 26:942–47.
Shinchi H, Maemura K, Mataki Y, et al. A phase II study of oral S-1 with concurrent radiotherapy followed by chemotherapy with S-1 alone for locally advanced pancreatic cancer. J Hepatobiliary Pancreat Sci. 2012;19:152–58.
Takahashi H, Akita H, Tomokuni A, et al. Preoperative gemcitabine-based chemoradiation therapy for borderline resectable pancreatic cancer: impact of venous and arterial involvement status on surgical outcome and pattern of recurrence. Ann Surg. 2016;264:1091–97.
Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–44.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res. 2012;18:5585–94.
Luo XM, Xu B, Zhou ML, Bao YY, Zhou SH, Fan J, Lu ZJ. Co-inhibition of GLUT-1 expression and the PI3K/Akt signaling pathway to enhance the radiosensitivity of laryngeal carcinoma xenografts in vivo. PloS One. 2015;10:e0143306.
Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–62.
Zhang C, Liu J, Liang Y, et al. Tumour-associated mutant p53 drives the Warburg effect. Nat Commun. 2013;4:2935.
Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–54.
Shin SH, Kim SC, Hong SM, Kim YH, Song KB, Park KM, Lee YJ. Genetic alterations of K-ras, p53, c-erbB-2, and DPC4 in pancreatic ductal adenocarcinoma and their correlation with patient survival. Pancreas. 2013;42:216–22.
Kim H, Saka B, Knight S, et al. Having pancreatic cancer with tumoral loss of ATM and normal TP53 protein expression is associated with a poorer prognosis. Clin Cancer Res, 2014;20:1865–72.
Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895–8.
Haber RS, Rathan A, Weiser KR, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34–40.
Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–41.
Melstrom LG, Salabat MR, Ding XZ, et al. Apigenin down-regulates the hypoxia response genes: HIF-1alpha, GLUT-1, and VEGF in human pancreatic cancer cells. J Surg Res. 2011;167:173–81.
Basturk O, Singh R, Kaygusuz E, Kaygusuz E, Balci S, Dursun N, Adsay NV. GLUT-1 expression in pancreatic neoplasia: implications in pathogenesis, diagnosis, and prognosis. Pancreas. 2011;40:187–92.
Sharen G, Peng Y, Cheng H, Liu Y, Shi Y, Zhao J. Prognostic value of GLUT-1 expression in pancreatic cancer: results from 538 patients. Oncotarget. 2017;8:19760–7.
Sobin LH, Gospodarowicz MK, Wittekind C, International Union against Cancer (2010) TNM Classification of Malignant Tumours. 7th ed.Wiley-Blackwell, Chichester.
Maemura K, Shinchi H, Noma H, et al. Comparison of hyper-fractionated accelerated and standard fractionated radiotherapy with concomitant low-dose gemcitabine for unresectable pancreatic cancer. Anticancer Res. 2008;28:2369–72.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Chikamoto A, Inoue R, Komohara Y, et al. Preoperative high maximum standardized uptake value in association with glucose transporter 1 predicts poor prognosis in pancreatic cancer. Ann Surg Concol. 2017;24:2040–6.
Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267–79.
Higashi M, Yokoyama S, Yamamoto T, et al. Mucin expression in endoscopic ultrasound-guided fine-needle aspiration specimens is a useful prognostic factor in pancreatic ductal adenocarcinoma. Pancreas. 2015;44:728–34.
Kurahara H, Maemura K, Mataki Y, Sakoda M, Shinchi H, Natsugoe S. Impact of p53 and PDGFR-beta expression on metastasis and prognosis of patients with pancreatic cancer. World J Surg. 2016;40:1977–84.
Yamada R, Mizuno S, Uchida K, et al. Human equilibrative nucleoside transporter 1 expression in endoscopic ultrasonography-guided fine-needle aspiration biopsy samples is a strong predictor of clinical response and survival in the patients with pancreatic ductal adenocarcinoma undergoing gemcitabine-based chemoradiotherapy. Pancreas. 2016;45:761–71.
Acknowledgment
This study was funded by Grants-in-Aid for Scientific Research (26462067) from the Japan Society for the Promotion of Science, Ministry of Health, Labour and Welfare, Japan.
Disclosure
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2018_6357_MOESM5_ESM.jpg
Fig. S1 Kaplan–Meier survival curves from the initial treatment (surgery) for patients who underwent upfront surgery.. Supplementary material 5 (JPEG 690 kb)
10434_2018_6357_MOESM6_ESM.jpg
Fig. S2 Kaplan–Meier survival curves from the initial treatment for patients who underwent neoadjuvant chemoradiotherapy (NACRT). a Overall survival (OS) of patients who underwent surgical resection after NACRT. b Recurrence-free survival of patients who underwent surgical resection after NACRT. c OS of patients who did not undergo surgical resection after NACRT. d Progression-free survival of patients who did not undergo surgical resection after NACRT. Supplementary material 6 (JPEG 1027 kb)
10434_2018_6357_MOESM7_ESM.jpg
Fig. S3 Kaplan–Meier survival curves from the initial treatment for patients who underwent neoadjuvant chemoradiotherapy (NACRT). Eight patients experienced a low-to-high change in glucose transporter type 1 (GLUT-1) expression due to NACRT. Supplementary material 7 (JPEG 712 kb)
Rights and permissions
About this article
Cite this article
Kurahara, H., Maemura, K., Mataki, Y. et al. Significance of Glucose Transporter Type 1 (GLUT-1) Expression in the Therapeutic Strategy for Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 25, 1432–1439 (2018). https://doi.org/10.1245/s10434-018-6357-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6357-1