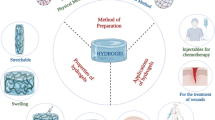
Abstract
Hydrogels are a network of crosslinked polymers which can hold a huge amount of water in their matrix. These might be soft, flexible, and porous resembling living tissues. The incorporation of different biocompatible materials and nanostructures into the hydrogels has led to emergence of multifunctional hydrogels with advanced properties. There are broad applications of hydrogels such as tissue culture, drug delivery, tissue engineering, implantation, water purification, and dressings. Besides these, it can be utilized in the field of medical surgery, in biosensors, targeted drug delivery, and drug release. Similarly, hyaluronic acid hydrogels have vast applications in biomedicines such as cell delivery, drug delivery, molecule delivery, micropatterning in cellular biology for tissue engineering, diagnosis and screening of diseases, tissue repair and stem cell microencapsulation in case of inflammation, angiogenesis, and other biological developmental processes. The properties like swellability, de-swellability, biodegradability, biocompatibility, and inert nature of the hydrogels in contact with body fluids, blood, and tissues make its tremendous application in the field of modern biomedicines nowadays. Various modifications in hydrogel formulations have widened their therapeutic applicability. These include 3D printing, conjugation, thiolation, multiple anchoring, and reduction. Various hydrogel formulations are also capable of dual drug delivery, dental surgery, medicinal implants, bone diseases, and gene and stem cells delivery. The presented review summarizes the unique properties of hydrogels along with their methods of preparation and significant biomedical applications as well as different types of commercial products available in the market and the regulatory guidance.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
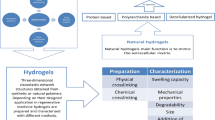
Hydrogels are the system of complex network of crosslinked polymers which can hold a huge amount of fluid in their matrix [1,2,3]. These can be prepared by physical or chemical crosslinking of either natural or synthetic polymers and sometimes by crosslinked clusters of colloids [4,5,6,7,8,9,10,11,12,13]. Due to ability of water absorption, these might be soft, flexible, and porous resembling like living tissue and behave like the extracellular matrix components providing a favorable environment for the survival as well as growth of cells [2, 14, 15]. The concentration of polymer in the hydrogel can be directly related with the mechanical characteristics of it and lowers the biocompatibility and biodegradability which limits the biomedical application of hydrogels [16,17,18]. The properties like swellability, de-swellability, biodegradability, biocompatibility, and inert nature of the hydrogels in contact with body fluids, blood, and tissues make its tremendous application in the field of modern biomedicines nowadays [2, 15, 19,20,21,22,23,24,25,26,27,28]. The hydrogels have numerous applications such as 3D cell culture, drug delivery, wound dressings, tissue engineering, medical surgery, biosensing [2, 15, 29,30,31], and likewise as cell delivery, molecule delivery, micropatterning in cellular biology for tissue engineering, diagnosis and screening of diseases, tissue repair and stem cell microencapsulation in case of inflammation, angiogenesis and other biological developmental process [32,33,34,35,36,37], prevention of medical device–related pressure ulcers [38] as well as in the construction of sensors [39,40,41,42,43,44]. These are widely used for the purification of water [45, 46]. Hydrogels that have broad applications in biomedical field incorporate natural, synthetic, and natural/synthetic polymers composite hydrogels [47,48,49,50,51].
Chemical Modifications in Hydrogels
Natural polysaccharide’s poor mechanical strength, low elasticity, quick breakability, and brittleness prevent natural hydrogels from being used biologically. Chitosan is widely employed in the creation of hydrogels for the engineering of biomaterials since it naturally possesses biological qualities like antioxidant, antibacterial, and anti-inflammatory activity [52,53,54]. Jafari et al. (2022) developed chitosan-based hydrogels via a method of enzyme (horseradish peroxidase)–mediated crosslinking and phenolated polyelectrolyte complexation between chitosan and alginate so as to increase 3D printing ability. The phenolic compounds were introduced for conjugation in the chitosan and alginate using 3-(4-hydroxyphenyl) propionic acid and tyramine respectively. Then, the hydrogels were prepared firstly by enzyme-mediated crosslinking using hydrogen peroxide to activate enzyme horseradish peroxidase and secondly by phenolated polyelectrolyte complexation of different concentrations of phenolated chitosan and tyramine conjugated alginate as shown in Fig. 1. The developed hydrogels showed the increase in toughness, loss modulus, moldability, flexibility, and dynamic viscosity as well as excellent 3D printing property [55].
Hewawasam et al. (2022) modify the human dECM (decellularized extracellular matrix) chemically via thiolation, i.e., reacting traut’s reagent (2-iminothiolane hydrochloride) with free amines of human dECM in the 3 mM EDTA for 1 h at room temperature. Then, the PEGαMA (poly(ethylene glycol)-alpha methacrylate) synthesized by reaction between poly-(ethylene glycol)-hydroxyl and sodium hydride following stirring for 30 min at room temperature followed by dropwise addition of ethyl 2-(bromomethyl)acrylate, stirring for 48 h at room temperature, quenching with 1 N acetic acid, filtration through celite-545, and purification by precipitation with diethyl ether and dialysis was reacted with thiolated human dECM and dithiothreitol (DTT) crosslinker in a base-catalyzed Micheal addition reaction to construct hybrid hydrogels. They demonstrated that the developed hydrogel containing human dECM promotes investigation of dynamic mechanosensing and allows researchers to examine the dynamic cell-matrix interactions that sustain fibrotic disorders by controlling and decoupling the biochemical changes that take place during fibrotic pathogenesis from the consequent increases in stiffness [56].
For cartilage tissue engineering, stable host tissue integration of hydrogel implants is crucial. One of the biggest hurdles is creating hydrogels with high adhesive strength, stability, and regeneration capacity. Chen et al. (2021) chemically modified the hyaluronic acid hydrogels with aldehyde groups and methacrylate on the polysaccharide backbone via the mechanisms of multiple anchoring. At first, hyaluronic acid (HA) was reacted with sodium periodate for specified time period and inactivated the unreacted periodate for 1 h with the addition of ethylene glycol followed by dialysis to prepare aldehyde-modified hyaluronic acid (AHA). Then, HA or AHA (1 g in 100 mL) was introduced for reaction with 1 mL methacrylate for 12 h at a pH of 8–8.5 using ice bath followed by dialysis for 2 days and freeze drying to synthesize methacrylated hyaluronic acid (HAMA) and methacrylated aldehyde-modified hyaluronic acid (AHAMA). Now the HAMA or AHAMA dissolved in 3% phosphate buffer saline solution with 0.1% 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (photoinitiator) was exposed with 365 nm UV light for 5 min to develop hydrogel. They demonstrated that the developed hydrogel significantly improved cartilage regeneration after 4 and 12 weeks of post-implantation in AHAMA groups respectively and hence AHAMA hydrogel is a favorable adhesive biomaterial for clinical cartilage regeneration [57].
Chemical modification of graphene (Gr) has been demonstrated as a potential approach to address the low gas sensitivity of virgin graphene (Gr). However, the current approach of chemical functionalization requires the use of hazardous substances, raising the risk to public safety (Table I). Wu et al. (2020) developed a grapheme hydrogel by chemical modification for the detection of NH3 and NO2. The vitamin C (0.2 g) was mixed with 20 mL aqueous solution of grapheme oxide of concentration 2 mg/mL by stirring for 10 min and heating for 1 h at 95°C without stirring followed by washing with deionized water to prepare vitamin C modified reduced graphene hydrogel as a biosensor for NH3 and NO2. They described that the developed sensor displays exceptional selectivity, linearity, and a broad detection range [58]. The chemical modification is shown in Fig. 2.
Composite for Bone Defects
The clinical treatment of bone defects is a tedious and complex task. Zhang et al. (2023) developed a scaffold of 3D-printed polycaprolactone incorporating biodegradable mesoporous silica nanoparticles containing the small molecular drug fingolimod. They prepared the vancomycin-loaded hydrogel from aldehyde hyaluronic acid and carboxymethyl chitosan using the Schiff base reaction, which filled the pores of the 3D-printed scaffold and produced bifunctional composite scaffold. The author demonstrated that the composite scaffold had vancomycin concentration–dependent antimicrobial activity and the fingolimod-loaded composite scaffold had significant angiogenic and osteogenic activity in in vitro study. The rat femoral defect model infected with bacteria showed that the dual drug composite had better effect in both bone regeneration and infection control [59].
Treatment of bone defect in osteoporosis is considered a complicated challenge since the site of injury has inflammation and reactive oxygen species such as hydrogen peroxide. Chen et al. (2022) developed a composite hydrogel with manganese dioxide–coated calcium phosphate microsphere loaded with fibroblast activating protein inhibitor in which the continual release of fibroblast activating protein inhibitor is employed to control the immunological response and bone development, and MnO2 is intended to function as an advanced army to remove H2O2 and produce oxygen. The author described that the hydrogel effectively reduced the reactive oxygen species and healed the inflammation. Further, the hydrogel increased the osteogenesis and inhibited osteoclast genesis and ultimately treated the osteoporotic bone defects [60].
Moderate hyperthermia has beneficial healing effect for the bone defects. So, Wang et al. (2022) prepared a favorable hydrogel composite to generate hyperthermia at the site of bone defects via embedding arginine-glycine-aspartate-coated, core-shell-structured magnetic iron oxide nanoparticles in the agarose. This hydrogel composite showed significant tissue penetration and produced hyperthermic temperature of 41–42°C promoting the osteogenic differentiation and biomineralization of pre-osteoblasts [61].
Biomaterials are necessary to localize cells to the defect and promote osteogenic differentiation, which is essential for treatments based on cells to be effective as an alternative to autologous bone grafts. Ingavle et al. (2019) developed an advanced hydrogel composite via entrapping mesenchymal stromal cells (MSCs) based on natural polymers, alginate and hyaluronate containing biomineralized polymeric microspheres (Table II). The author used repair in sheep bone defect model. In comparison to acellular gels or untreated defects, defects treated with MSCs implanted in composite gels showed a considerable increase in blood vessel density, osteoid formation, and bone formation after 12 weeks of implantation [62].
Bone Materials (Calcium Phosphate)
In orthopedics, bone abnormalities brought on by trauma, tumors, congenital malformations, or inflammations are quite prevalent. Xu et al. (2023) prepared a biphasic calcium phosphate–acrylated methacrylate gelatin composite gel and demonstrated that in vitro study for osteogenic activity showed enhanced osteogenesis of bone marrow mesenchymal stem cells and the in vivo study by rat skull defect model for bone repair activity showed new bone formation in rat skull defect model [63].
One of the most incapacitating effects of aging is osteoporosis, and osteoporotic fractures and a higher risk of recurrent fractures result in significant impairment and fatalities, demonstrating the importance of both local fracture healing and early anti-osteoporosis therapy. Wang et al. (2023) developed a tough hydrogel loaded with calcium phosphate cement and injectable through the interactions between inorganic biological scaffolds (calcium phosphate cement) and organic osteogenic molecules (gelatin methacryloyl and N-Hydroxyethyl acrylamide) called one-pot process. The author demonstrated that the developed composite gel showed enhanced osteogenesis activity [64].
Osteosarcoma is considered the most common primary malignant tumor of the bone and clinical treatment of it is a complex task. Marti et al. (2023) developed a composite from robocasted calcium phosphate cement infiltrated with plasma‐treated gelatin-alginate hydrogel and demonstrated that the in vitro study showed the significant selective osteosarcoma cells’ killing ability and the in vivo study showed excellent anticancer and bone regenerating activity [65].
Chronopoulou et al. (2020) developed an injectable biomimetic composite material which was based on peptidic hydrogel (tripeptides) and calcium phosphates via reverse hydrolysis in which cell adhesion was promoted by Arg-Gly-Asp-grafted chitosan. The author demonstrated that the developed composite hydrogel had mimicking effect on the chemical composition of natural bone tissue (Table III) [66].
Bioglass
The development material of side effect free and complete recovery for the treatment of oral submucous fibrosis is a major subject of concern nowadays. Considering this problem, Guo et al. (2023) developed an injectable sodium hyaluronate/45S5 bioglass composite hydrogel which showed that the composite hydrogel significantly relieved mucosal pallor and restricted mouth opening in oral submucous fibrosis induced with arecoline in rats involving the mechanism of inhibition of collagen deposition and inflammation as well as promotion of angiogenesis and epithelial regeneration with very least side effects [67].
To overcome the problems of tedious and complex clinical treatment, Sadeghian et al. (2023) prepared a dentin extracellular matrix–loaded gelatin methacrylate-5% bioactive glass hydrogel and found that in vitro study showed improved dental pulp regeneration activity [68].
To overcome the problems of repairing large bone injuries, Manoochhri et al. (2022) prepared chitosan/alginate/strontium-doped bioglass composite scaffolds using freeze drying method and found the scaffold with more cell differentiation efficiency for repairing major bone injuries [69].
The implanted biomaterials following surgery should combine the functions of both tumor therapy and bone regeneration to address tumor-related irregular bone deformities. To achieve this requirement, Yang et al. (2021) developed double crosslinking injectable composite hydrogels on the basis of furan-sodium alginate/bis-maleimide-polyethylene glycol/copper-doped bioactive glass-ceramic microspheres using Diels-Alder reaction and ionic crosslinking. The author demonstrated that the composite hydrogel showed outstanding photothermal effects and destroyed most tumor cells after in vitro study and in mice (Table IV); it had inhibited tumor growth [70].
3D Printing
Pea protein hydrogel’s enhanced mechanical and 3D printing capabilities aid in the creation of novel plant-based gel products. Wang et al. (2023) developed a method forming pea protein–hydroxypropyl starch interpenetrating network hydrogels by changing pH to regulate the structure, 3D printing characteristics, and strength of the hydrogels. They demonstrated that the pH affected the gelation process of pea protein–hydroxypropyl starch hydrogels and found that gel inks at pH 3 produced 3D-printed items with outstanding structural fidelity and integrity at 60°C which could be applied in food industry [71].
For three-dimensional cell culture, hydrogel is an appropriate material since it has high water content and can more closely resemble a natural extracellular matrix. Hao et al. (2023) carried out the study to test the impact of 3D-printed porous structures on the osteogenic differentiation of BMSCs (bone marrow mesenchymal stem cells) within hydrogel scaffolds via creating porous hydrogel scaffolds (gelatin-sodium alginate-laponite/BMSCs) with medium pore sizes (100–1000 µm) using 3D printing technology and using porous hydrogel scaffolds without pores and non-printed hydrogel scaffolds as controls. They demonstrated that when compared to the non-porous groups, the osteogenic differentiation of BMSCs in the structured porous hydrogels was significantly greater in in vitro study and following ectopic implantation in the posterior gluteal muscle punch, the structured porous hydrogel showed ectopic osteogenesis and reasonably excellent mineralization. They concluded that the method based on 3D printing offers an easy method for making hydrogels with intermediate pore shapes [72].
Tailored nutrition and medicine have recently gained popularity as a way to alter target agent dosage and enable tailored treatment by altering the geometries of printed gels. Emir et al. (2022) firstly used photoinitiator and riboflavin in precursor resin for the stereolithogaphy printing of poly (ethylene glycol) dimethacrylate–based hydrogels embedded with oleuropein via the use of UV-induced polymerization with varying shapes. The author demonstrated that the printed 3D gels had good printability, physical properties, physicochemical properties, and drug release property and concluded that it could contribute to the food and pharmaceutical industries in order to design personalized oleuropein dosages utilizing the stereolithography printing with controlled release through the manipulation of geometry only [73].
Tough hydrogels must be transformed into complex topologies in order to be used as structural components. Dong et al. (2022) used the digital light processing printing technique to develop tough hydrogel architectures via the use of aqueous precursor; commercial photoinitiator, acrylic acid, and zirconium ion readily formed tough metallosupramolecular hydrogel in the influence of digital light due to the formation of complexes of carboxyl-zirconium ion in situ. The author described that encoding structure gradients with grayscale digital light while printing allowed for the gel to change shape due to swelling and the mechanical properties of printed hydrogel were enhanced by incubation in water because of pH variation and co-ordination complex rearrangement (Table V). They demonstrated that the printed gels were used for devising an impact absorption element or highly sensitive pressure gels [74].
Gels for Dental Surgeries and Implants
Various dental conditions that cannot be treated through regular measures alone are addressed with dental surgeries by the dental professionals. Modern dentistry, which aims to address complicated dental diseases and restore oral health, includes dental surgery and implants as essential components [75]. Extraction of teeth, installation of dental implants, corrective jaw surgery, bone grafting, and the management of oral diseases are all procedures included in dental surgery. Dental implants, on the other hand, are artificial tooth roots that are surgically inserted into the jawbone to act as a secure base for dental restorations [76]. They are manufactured of biocompatible materials. Evaluation, implant insertion, healing and integration, abutment placement, and crown implantation are all steps in the dental implant process. Dental implants have many advantages, including as better dental health, improved looks, and long-term durability. To choose the best course of treatment and guarantee positive results, speaking with a dental expert is essential. Hydrogel polymers have utilizations in various regenerative medicines [77].
Struillou et al. (2013) employed hydrogels and biphasic calcium phosphate to address peri-implant problems of the dehiscence kind in a dog model. They investigated three biomaterials, i.e., BCP alone, HPMC/BCP material in a putty, and BCP covered by Si-HPMC, a polymer barrier. At 3 months, defects filled with HPMC/BCP or Si HPMC/BCP considerably outperformed spontaneous healing in the control (P = 0.032 and P = 0.046, respectively) and outperformed BCP alone in terms of new bone production. Fresh bone formation was additionally observed in indirect contact with the implant site in each of the three groups who received BCP. The inclusion of HPMC to the BCP granules may have enhanced the initial stability of the material within the blood clot in these significant and complex osseous abnormalities. In periodontology and implantology, the composite MBCP/putty appears to be an effective bone-graft material for complex defects, but the Si-HPMC hydrogel can also act as an occlusive film that covers the BCP, potentially improving the longevity of the granules in the defective area [78].
Another study supported the idea that, rather than using ginger extract directly, implant coating can successfully prolong the release of ginger fraction by loading the ginger fraction in the gelatin methacryloyl (GelMA) hydrogel. The outer surface was altered by pre-calcification and anodization prior to covering the ginger-loaded hydrogel to create a super-hydrophilic surface with the formation of titanium dioxide (TiO2) and hydroxyapatite. The functional ginger hydrogel’s coating properties (adhesion and homogeneity) were improved, and the Ti surface’s bioactivity in simulated body fluid (SBF) was increased thanks to the changed surface. The ginger hydrogel increased the effectiveness of ginger loading and continually released the essential components in ginger, which had a synergistic effect on the reduction of bacterial adhesion and a rise in osteogenesis surrounding the Ti surface. [79].
It was also shown that acellular self-assembling peptide hydrogels may produce extracellular matrix mimicking designs that direct in vivo tissue deposition and neo vasculature growth. In a large animal (canine) orthotopic model, the therapeutic potential of an angiogenic hydrogel to restore vascularized pulp-like soft tissue was investigated. Neural filaments, blood vessels, and an odontoblast-like layer close to dentinal tubules are only a few examples of the major characteristics of native pulp that the regenerated soft tissue replicates. In a canine pulpectomy model, the hydrogel’s material properties are similar to those of natural tooth pulp, and the material promotes excellent bio-integration and soft tissue regeneration (Table VI). As useful acellular biomaterials, supramolecular peptide hydrogels hold considerable potential for enhancing our tissue engineering tools [80].
Drug Delivery in Hydrogels
Polymeric nanoparticles are nanosized particles composed of biodegradable polymers that can be used for drug delivery, imaging, diagnosis, and therapy of various diseases [81, 82]. One of the applications of polymeric nanoparticles is targeting bone cancer, which is a malignant growth of bone tissue that can be primary or metastatic [82]. Bone cancer is a challenging disease to treat due to its complex microenvironment, poor vascularization, drug resistance, and metastasis [83].
One of the strategies to improve the efficacy of bone cancer therapy is to use polymeric nanoparticles that can specifically target the bone tissue and deliver the anticancer agents to the tumor site. This can be achieved by modifying the surface of the nanoparticles with bone-targeting ligands, such as bisphosphonates, tetracyclines, hydroxyapatite-binding peptides, bone morphogenetic proteins, and integrin-binding peptides [81, 84]. These ligands can bind to the bone matrix or the receptors on the bone cells and increase the accumulation and retention of the nanoparticles in the bone tissue [84].
Another strategy is to use polymeric nanoparticles that can target the mitochondria of the cancer cells, which are the organelles responsible for energy production, apoptosis regulation, and oxidative stress generation [85, 86]. Mitochondria-targeted nanoparticles can induce mitochondrial dysfunction, oxidative stress, apoptosis, and necrosis in the cancer cells, leading to their death. Mitochondria-targeted nanoparticles can be designed by incorporating mitochondria-penetrating peptides, lipophilic cations, or photosensitizers into the nanoparticles [87,88,89].
Polymeric nanoparticles can be incorporated into gel formulations for topical or transdermal delivery of drugs to treat bone cancer. Gel formulations are semisolid systems that can adhere to the skin surface and provide sustained release of drugs. Gel formulations can also enhance the permeability of drugs through the skin layers and improve their bioavailability [90]. Polymeric nanoparticles can be incorporated into gel formulations by using gel-forming excipients, such as Carbopol, alginate, chitosan, or cellulose derivatives. Polymeric nanoparticle gel formulations can offer advantages such as improved stability, reduced irritation, controlled release, and targeted delivery of drugs for bone cancer therapy [90, 91]. The strategy for dual drug delivery using hydrogels is shown in Fig. 3.
Some examples of polymeric nanoparticle gel formulations for bone cancer therapy are:
A Gel-Mps composite system was created by Cao et al. for the in situ treatment of osteosarcoma. Gel@Col-Mps@Dox/Pio serves a number of purposes, including the continuous inhibition of tumor growth and recurrence by stable drug release from microspheres, targeted penetration of the disintegrating tumor extracellular matrix, and synergistic drug delivery. The sequential administration of collagenase and therapeutic medicines (doxorubicin and pioglitazone) to osteosarcoma tumors creates a pathway for drug penetration by breaking down the extracellular matrix. Doxorubicin, a cancer treatment medicine, causes DNA damage and tumor cell death, and pioglitazone, a cancer treatment drug, works synergistically to treat cancer by diminishing the stemness of osteosarcoma stem cells, overriding P-gp-mediated doxorubicin resistance, regaining the cancer’s sensitivity to chemotherapy, and lessening metastasis and invasiveness [92].
In order to achieve local chemodynamic therapy (CDT)/photothermal therapy (PTT) with doxorubicin-encapsulated iron-gallic acid (FeGA-DOX) nanoparticles (NPs) and enable osteosarcoma tumor suppression in mice, Ying et al. created a unique method employing injectable agarose (AG) hydrogels. The FeGA-DOX NPs release a lot of heat when exposed to a near-infrared (NIR) wavelength laser, which causes cell apoptosis by hyperthermia. While this is happening, a local temperature increase may encourage the release of FeGA-DOX into the tumor. It is widely known that doxorubicin can encourage the production of H2O2, which FeGA can then use in a Fenton reaction in an acidic environment to produce reactive oxygen species (ROS). With the help of FeGA-DOX + AG therapy, the synergistic effect of CDT/PTT was thus realized. The approach overcomes the drawback of a single CDT or PTT and demonstrated exceptional therapeutic effects in mice with osteosarcoma tumors with acceptable biocompatibility results. Since this H2O2 self-sufficient AG-encapsulated FeGA-DOX can combine the benefit of CDT/PTT, this unique technique has the potential to be used in clinical settings [93].
Wu et al. (2018) used a gelatin methacryloyl (GelMA) hydrogel to photo crosslink gemcitabine (GEM) hydrochloride–loaded liposomes to assess its efficacy for osteosarcoma ablation. The hydrogel released GEM over the course of 4 days in vitro, according to the investigators, in a controlled and sustained manner (Table VII). In addition, the hydrogel showed in vivo osteosarcoma inhibition in BALB/c MG-63 carrying mice [94].
Hydrogels for Cutaneous Applications
A three-dimensional network of hydrophilic polymers known as a hydrogel is capable of absorbing and holding huge volumes of water or biological fluids. They have a long history of usage in cutaneous applications such cosmetics, medication administration, tissue engineering, and wound dressing [95, 96].
Liposomes (LS) are nanocarriers that are largely made of cholesterol and phospholipids [97]. Sexually transmitted infections are brought on by Chlamydia trachomatis. Oral azithromycin and doxycycline, both of which have possible side effects, are the available treatments. Jraholmen et al. [98] employed a natural chitosan (CHI) hydrogel infused with the polyphenol Resveratrol (RVT) LS to successfully cure C. trachomatis while minimizing side effects. To maximize the potential therapeutic benefit of RVT, they employed LS as the main release media and CHI hydrogel as an additional media. Since LS do not interact with vaginal flora and RVT reduces biofilm formation, these nanocarriers are favored for topical therapy. Chitosan hydrogel also successfully prevents the growth of vaginal biofilms. RVT was discovered to increase in solubility in LS preparation, offer sustained action, and improve chemical stability, allowing for medical applications. Additionally, RVT’s ability to bind to microorganisms is enhanced in the LS formulation, leading to a more effective antimicrobial effect even at low dosages. Nitric oxide, which is the main free radical responsible for inflammation, was shown in the study to be inhibited by RVT-LS in CHI hydrogel. The addition of RVT-LS to the CHI hydrogel delivery system enhanced RVT’s anti-chlamydial performance at lower doses and brought attention to the need for an efficient delivery system.
A vitamin C–loaded self-double-emulsifying drug delivery system (SDEDDS) was made by Wang Q et al. [99] before being mixed with a xanthan gum (XG) hydrogel. After being incorporated into hydrogels, vitamin C–loaded SDEDDS demonstrated higher physical potency, indicating that the shell is better able to preserve vitamin C against deterioration, particularly from ionization solution and oxygen exposure. Due to XG’s bio adhesive properties, adding vitamin C to SEDDS-based hydrogels enhances the amount of vitamin C that permeates into the skin. Even better vitamin C–controlled release from the SDEDDS formulation may be possible thanks to the oil vesicle’s protective layer. The distribution and permeability of vitamin C within the skin may be significantly increased by encapsulating it in hydrogels, SDEDDS, or perhaps both. Overall, skin penetration will be significantly improved by SDEDDS coupled with XG hydrogels that have vitamin C added [99].
Research on polymeric microparticles (MP) as a helpful and new carrier for the prolonged and controlled release of powerful pharmaceuticals has been extensive. In order to treat chronic wounds, medications must typically be given over a longer period of time at regular intervals. The frequency of injection may be reduced while maintaining medication concentration at the site of a wound with long-term sustained release therapy. Yasasvini S et al. [100] developed simvastatin (SIM) CHI-MP and added it to polyvinyl alcohol (PVA) hydrogels to enhance wound healing activity considering this information. Ninety-two percent of the SIM in the 2.5 mg dosage of the 5% PVA hydrogel was released after 7 days. This SIM release from 5% PVA was related to the swelling index. Low dosages (2.5 mg) had a larger swelling index value when compared to SIM concentrations of 5 and 10. The in vivo wound healing experiment demonstrated that SIM delivered in a regulated way causes ongoing wound healing. According to the results above, the combination of APIs in the MP formulation combined with hydrogels may be the optimum for releasing the APIs over time and successfully promoting topical wound healing.
Hydrogels for Gene Delivery
Gene delivery is the process of introducing foreign genetic material into cells for therapeutic purposes. It can be used to treat various diseases such as cancer, genetic disorders, or infections by modulating the expression of specific genes. Incorporating cyclodextrins or other supramolecular hosts to form inclusion complexes with DNA vectors and enhance their loading and release [101].
Cyclodextrin-based hydrogels for gene delivery. Cyclodextrins are cyclic oligosaccharides that can form inclusion complexes with various guest molecules, such as drugs, proteins, and nucleic acids. Cyclodextrin-based hydrogels are formed by crosslinking cyclodextrins with guest molecules via supramolecular interactions. Cyclodextrin-based hydrogels can encapsulate or conjugate various genes, such as plasmid DNA (pDNA), small interfering RNA (siRNA), and messenger RNA (mRNA), within their network. Cyclodextrin-based hydrogels can protect the genes from degradation and release them in a controlled manner. Cyclodextrin-based hydrogels can also enhance the gene transfection efficiency by facilitating the endosomal escape and nuclear entry of genes [102].
Weiss et al. used an alginate-based hydrogel loaded with pDNA encoding for BMP-2 (pDNA-BMP-2) to study the effectiveness of bone formation. Goat multipotent stromal cells (gMSCs) and ceramic granules were mixed and injected intramuscularly into goats. This DNA delivery system’s transfection of cells resulted in sustained BMP-2 expression for 16 weeks, encouraging osteogenic differentiation and subsequent bone production. When pDNA-BMP-2 was administered via gelatin-based hydrogels to treat a mouse calvarial bone deficiency, a similar pattern was shown [103].
Local DNA delivery via a hydrogel scaffold would expand the uses of gene therapy for cancer treatment and tissue regeneration. Lei Y et al. (2011) developed hyaluronic acid and fibrin hydrogels with concentrated and unaggregated polyplexes (DNA/cationic polymer nanoparticles) through caged nanoparticle encapsulation (CnE) and found that the developed hydrogels showed ability to deliver genes in vivo (Table VIII) [104].
Hydrogels for Stem Cell Delivery
Undifferentiated cells called stem cells have the capacity to differentiate into a variety of cell types and repair damaged tissues. Stem cell therapy is a promising strategy for the treatment of various diseases, such as cardiovascular diseases, diabetes, and neurodegenerative disorders. However, stem cell therapy faces several challenges, such as low survival rate, poor engraftment, immune rejection, and ethical issues [105, 106].
Alginate hydrogels for stem cell microencapsulation. Alginate is a polymer generated naturally from brown algae that may crosslink with divalent cations, such as calcium, to form hydrogels. Alginate hydrogels are biocompatible, biodegradable, and easy to prepare. Alginate hydrogels have been used to encapsulate various types of stem cells, such as embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and neural stem cells (NSCs). Alginate hydrogels can protect the encapsulated stem cells from mechanical stress, immune attack, and oxidative stress. Alginate hydrogels can also modulate the stem cell differentiation by incorporating different bioactive molecules or by varying the gel stiffness [107].
Gelatin hydrogels for stem cell microencapsulation. Gelatin is a natural protein derived from collagen that can form hydrogels by physical or chemical crosslinking methods. Gelatin hydrogels are biocompatible, biodegradable, and have similar amino acid composition to the ECM. Gelatin hydrogels have been used to encapsulate various types of stem cells, such as MSCs, ESCs, iPSCs, and hematopoietic stem cells (HSCs). Gelatin hydrogels can enhance the stem cell attachment, proliferation, and differentiation by providing integrin-binding sites and growth factor–binding sites. Gelatin hydrogels can also be modified with various functional groups or nanoparticles to improve their mechanical strength, stability, and responsiveness [108].
Polyethylene glycol (PEG) hydrogels for stem cell microencapsulation. PEG is a synthetic polymer that can form hydrogels by photo-polymerization or chemical crosslinking methods. PEG hydrogels are biocompatible, non-immunogenic, and non-degradable. PEG hydrogels have been used to encapsulate various types of stem cells, such as ESCs, MSCs, iPSCs, and HSCs. PEG hydrogels can provide a three-dimensional (3D) environment for the encapsulated stem cells and prevent their aggregation or leakage. PEG hydrogels can also be tailored to have different mechanical properties, degradation rates, and stimuli responsiveness by varying the molecular weight, crosslinking density, and functionalization of PEG [109]. Strategy for stem cell delivery utilizing hydrogel is presented in Fig. 4.
Commercial products of hydrogels are tabulated in Table IX.
Regulatory Aspects of Hydrogels
The variety of basic components used to create hydrogel scaffolds makes regulatory approval and arrangement difficult. According to Sect. 201(g) of the FD&C Act, hydrogels are categorized as “devices” as opposed to “drugs,” which have more specific classifications. In addition, the majority of hydrogel-based products, with a few notable exceptions, must go through further FDA assessment of a 510(k) pre-market notification filing in order to get legal marketing rights in the USA, which requires lengthy regulatory approval. However, hydrogels are classified as medical device class III under the new European regulations, and as such, they must be taken into account at every stage of the hydrogel’s lifetime, from material and machine certification to scale up. The Council Directives 90/385/EEC and 93/42/EEC have specific obligations, as stated in Commission Regulation (EU) No. 722/2012 of August 8, 2012. In order to maintain a high level of safety and health protection against the possibility of transmitting animal spongiform encephalopathies, the regulation governing active implantable medical devices and medical devices made with animal tissues was adopted. The regulation also takes into account the fact that Class III active implantable medical devices and other medical devices are subject to conformity assessment procedures prior to being marketed or put into service, which necessitates the adoption of more specific requirements pertaining to risk management. On May 26, 2017, two new European Health Products Regulations went into effect. The first one, the Medical Devices Regulation (EU) 2017/745, which amends Directive 2001/83/EC and its derived rules and repeals Council Directives 90/385/EEC and 93/42/EEC, is in effect as of May 26, 2020. The second one, Regulation (EU) 2017/746 of in vitro medical devices, which takes effect on May 26, 2022, repeals both Directive 98/78/EC and Commission Decision 2010/227/EU. These new rules represent a necessary shift in the medical device industry and will require strict requirements from all market participants, which increases product transparency and traceability assurances and further promotes safety and dependability [114,115,116].
Conclusion
Hydrogels are the crosslinked polymer network system widely applied in the field of biomedicine with very least side effects and least cost. These have been used for the treatment of bone cancer, bone fracture, and tissue defects as well as for tissue culture, drug delivery, stem cell delivery, etc. In summary, hydrogels offer a versatile platform for drug delivery with unique features such as biocompatibility, sustained release, and stimuli-responsive behavior. These characteristics make them valuable in various biomedical applications, ranging from localized drug delivery to tissue engineering and diagnostic imaging. Ongoing research continues to explore new ways to optimize hydrogel formulations for specific therapeutic purposes, paving the way for innovative drug delivery strategies in the future. The improvement in the hydrogels development is required so as to minimize rejection due to immune sensitivity, problem in biodegradability and stability issues, etc.
References
Tanaka T. Gels. Scientific American. 1981;244(1):124-S-17.
Ho T-C, Chang C-C, Chan H-P, Chung T-W, Shu C-W, Chuang K-P, et al. Hydrogels: properties and applications in biomedicine. Molecules. 2022;27(9):2902.
Eelkema R, Pich A. Pros and cons: supramolecular or macromolecular: what is best for functional hydrogels with advanced properties? Adv Mater. 2020;32(20):1906012.
Hahn SK, Park JK, Tomimatsu T, Shimoboji T. Synthesis and degradation test of hyaluronic acid hydrogels. Int J Biol Macromol. 2007;40(4):374–80.
Ranjha NM, Mudassir J, Akhtar N. Methyl methacrylate-co-itaconic acid (MMA-co-IA) hydrogels for controlled drug delivery. J Sol-Gel Sci Technol. 2008;47:23–30.
Eagland D, Crowther N, Butler C. Complexation between polyoxyethylene and polymethacrylic acid—the importance of the molar mass of polyoxyethylene. Eur Polymer J. 1994;30(7):767–73.
Yokoyama F, Masada I, Shimamura K, Ikawa T, Monobe K. Morphology and structure of highly elastic poly (vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym Sci. 1986;264:595–601.
Cerchiara T, Luppi B, Bigucci F, Orienti I, Zecchi V. Physically cross-linked chitosan hydrogels as topical vehicles for hydrophilic drugs. J Pharm Pharmacol. 2002;54(11):1453–9.
Peppas NA, Merrill EW. Development of semicrystalline poly (vinyl alcohol) hydrogels for biomedical applications. J Biomed Mater Res. 1977;11(3):423–34.
Dror M, Elsabee M, Berry G. Interpenetrating polymer networks for biological applications. Biomater Med Devices Artif Organs. 1979;7(1):31–9.
Peppas NA, Moynihan HJ, Lucht LM. The structure of highly crosslinked poly (2-hydroxyethyl methacrylate) hydrogels. J Biomed Mater Res. 1985;19(4):397–411.
Douglas AM, Fragkopoulos AA, Gaines MK, Lyon LA, Fernandez-Nieves A, Barker TH. Dynamic assembly of ultrasoft colloidal networks enables cell invasion within restrictive fibrillar polymers. Proc Natl Acad Sci. 2017;114(5):885–90.
Nair SK, Basu S, Sen B, Lin M-H, Kumar AN, Yuan Y, et al. Colloidal gels with tunable mechanomorphology regulate endothelial morphogenesis. Sci Rep. 2019;9(1):1072.
Zhang Y, Liu Y, Liu J, Guo P, Heng L. Super water absorbency OMMT/PAA hydrogel materials with excellent mechanical properties. RSC Adv. 2017;7(24):14504–10.
Chen G, Tang W, Wang X, Zhao X, Chen C, Zhu Z. Applications of hydrogels with special physical properties in biomedicine. Polymers. 2019;11(9):1420.
Sabzi M, Samadi N, Abbasi F, Mahdavinia GR, Babaahmadi M. Bioinspired fully physically cross-linked double network hydrogels with a robust, tough and self-healing structure. Mater Sci Eng C. 2017;74:374–81.
Thakur A, Jaiswal MK, Peak CW, Carrow JK, Gentry J, Dolatshahi-Pirouz A, et al. Injectable shear-thinning nanoengineered hydrogels for stem cell delivery. Nanoscale. 2016;8(24):12362–72.
Ciccone G, Dobre O, Gibson GM, Rey JM, Gonzalez-Garcia C, Vassalli M, et al. what caging force cells feel in 3D hydrogels: a rheological perspective. Adv Healthcare Mater. 2020;9(17):2000517.
Cabrera-Munguia DA, Claudio-Rizo JA, Becerra-Rodríguez JJ, Flores-Guia TE, Rico JL, Vásquez-García SR. Enhanced biocompatibility and bactericidal properties of hydrogels based on collagen–polyurethane–aluminium MOFs for biomedical applications. Bull Mater Sci. 2023;46(2):1–14.
Zhu H, Zheng J, Oh XY, Chan CY, Low BQL, Tor JQ, et al. Nanoarchitecture-integrated hydrogel systems toward therapeutic applications. ACS Nano. 2023;17(9):7953–78.
Gan X, Li C, Sun J, Zhang X, Zhou M, Deng Y, et al. GelMA/κ-carrageenan double-network hydrogels with superior mechanics and biocompatibility. RSC Adv. 2023;13(3):1558–66.
Song C, Liu R, Kong B, Gu Z, Chen G. Functional hydrogels for treatment of dental caries. Biomed Technol. 2024;5:73–81.
Suneetha M, Zo S, Choi SM, Han SS. Antibacterial, biocompatible, hemostatic, and tissue adhesive hydrogels based on fungal-derived carboxymethyl chitosan-reduced graphene oxide-polydopamine for wound healing applications. Int J Biol Macromol. 2023;241: 124641.
Solanki D, Vinchhi P, Patel MM. Design considerations, formulation approaches, and strategic advances of hydrogel dressings for chronic wound management. ACS Omega. 2023;8(9):8172–89.
Liu Y, Dong T, Chen Y, Sun N, Liu Q, Huang Z, et al. Biodegradable and cytocompatible hydrogel coating with antibacterial activity for the prevention of implant-associated infection. ACS Appl Mater Interfaces. 2023;15(9):11507–19.
Liu L, Liu Y-x, Zhong H, Li X-r, Jun Y-l, Wang Q-l, et al. Folic acid conjugated palygorskite/Au hybrid microgels: temperature, pH and light triple-responsive and its application in drug delivery. Colloids Surf B Biointerfaces. 2023:113432.
Moura D, Pereira AT, Ferreira HP, Barrias CC, Magalhães FD, Bergmeister H, et al. Poly (2-hydroxyethyl methacrylate) hydrogels containing graphene-based materials for blood-contacting applications: From soft inert to strong degradable material. Acta Biomater. 2023;164:253–68.
Fan Y, Luchow M, Badria A, Hutchinson DJ, Malkoch M. Placenta powder-infused thiol-ene PEG hydrogels as potential tissue engineering scaffolds. Biomacromolecules. 2023;24(4):1617–26.
Morello G, De Iaco G, Gigli G, Polini A, Gervaso F. Chitosan and pectin hydrogels for tissue engineering and in vitro modeling. Gels. 2023;9(2):132.
Yu W, Gong E, Liu B, Zhou L, Che C, Hu S, et al. Hydrogel-mediated drug delivery for treating stroke. Chin Chem Lett. 2023;10:108205.
Wang G, Zhang X, Bu X, An Y, Bi H, Zhao Z. The application of cartilage tissue engineering with cell-laden hydrogel in plastic surgery: a systematic review. Tissue Eng Regen Med. 2022;1:1–9.
Ahmadian E, Dizaj SM, Eftekhari A, Dalir E, Vahedi P, Hasanzadeh A, et al. The potential applications of hyaluronic acid hydrogels in biomedicine. Drug Res. 2020;70(01):6–11.
Fang Y, Shi L, Duan Z, Rohani S. Hyaluronic acid hydrogels, as a biological macromolecule-based platform for stem cells delivery and their fate control: a review. Int J Biol Macromol. 2021;189:554–66.
Bonhome-Espinosa AB, Campos F, Durand-Herrera D, Sánchez-López JD, Schaub S, Durán JD, et al. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J Mech Behav Biomed Mater. 2020;104: 103619.
Li J, Lv Y, Zhu D, Mei X, Huang K, Wang X, et al. Intrapericardial hydrogel injection generates high cell retention and augments therapeutic effects of mesenchymal stem cells in myocardial infarction. Chem Eng J. 2022;427: 131581.
Zhao X, Huang Y-f, Tian X, Luo J, Wang H, Wang J, et al. Polysaccharide-based adhesive antibacterial and self-healing hydrogel for sealing hemostasis. Biomacromolecules. 2022;23(12):5106–15.
Sharma AD, Jarman EH, Fox PM. Scoping review of hydrogel therapies in the treatment of diabetic chronic wounds. Plast Reconstr Surg Glob Open. 2023;11(5):e4984.
Grigatti A, Gefen A. What makes a hydrogel-based dressing advantageous for the prevention of medical device-related pressure ulcers. Int Wound J. 2022;19(3):515–30.
Zhang Y, Chen K, Li Y, Lan J, Yan B, Shi L, et al. High-strength, self-healable, temperature-sensitive, MXene-containing composite hydrogel as a smart compression sensor. ACS Appl Mater Interfaces. 2019;11(50):47350–7.
Zhao L, Zhao J, Zhang F, Xu Z, Chen F, Shi Y, et al. Highly stretchable, adhesive, and self-healing silk fibroin-dopted hydrogels for wearable sensors. Adv Healthcare Mater. 2021;10(10):2002083.
Zhang D, Zhang M, Wang J, Sun H, Liu H, Mi L, et al. Impedance response behavior and mechanism study of axon-like ionic conductive cellulose-based hydrogel strain sensor. Adv Compos Hybrid Mater. 2022;5(3):1812–20.
Li H, Zou R, Su C, Zhang N, Wang Q, Zhang Y, et al. Ratiometric fluorescent hydrogel for point-of-care monitoring of organophosphorus pesticide degradation. J Hazard Mater. 2022;432: 128660.
Zou Q, Zhang S, Su Q, Xue T, Lan K. Flexible multimodal sensor based on double-network hydrogel for human and robotic applications. ChemistrySelect. 2023;8(11): e202204319.
Lu Y, Yue Y, Ding Q, Mei C, Xu X, Jiang S, et al. Environment-tolerant ionic hydrogel–elastomer hybrids with robust interfaces, high transparence, and biocompatibility for a mechanical–thermal multimode sensor. InfoMat. 2023;5(4): e12409.
Ni A, Fu D, Lin P, Xia Y, Pei D, Han X, et al. Rapid fabrication of porous photothermal hydrogel coating for efficient solar-driven water purification. ACS Appl Mater Interfaces. 2022;14(39):44809–20.
Cai W, Zhao S, Zhang K, Guo K, Wang Y, Chen Q, et al. Synergy of light trapping and water management in interconnected porous PEDOT: PSS hydrogels for efficient solar-driven water purification. Ind Eng Chem Res. 2023;62:10175–83.
Zhou L, Wang Y, Liu Z, Huang Q. Characteristics of equilibrium, kinetics studies for adsorption of Hg (II), Cu (II), and Ni (II) ions by thiourea-modified magnetic chitosan microspheres. J Hazard Mater. 2009;161(2–3):995–1002.
Zhou Y-T, Nie H-L, Branford-White C, He Z-Y, Zhu L-M. Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J Colloid Interface Sci. 2009;330(1):29–37.
Hernández R, Mijangos C. In situ synthesis of magnetic iron oxide nanoparticles in thermally responsive alginate-poly (N-isopropylacrylamide) semi-interpenetrating polymer networks. Macromol Rapid Commun. 2009;30(3):176–81.
Place ES, George JH, Williams CK, Stevens MM. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev. 2009;38(4):1139–51.
Patenaude M, Hoare T. Injectable, mixed natural-synthetic polymer hydrogels with modular properties. Biomacromolecules. 2012;13(2):369–78.
Kim I-Y, Seo S-J, Moon H-S, Yoo M-K, Park I-Y, Kim B-C, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26(1):1–21.
Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polymer J. 2013;49(4):780–92.
Rajabi M, McConnell M, Cabral J, Ali MA. Chitosan hydrogels in 3D printing for biomedical applications. Carbohyd Polym. 2021;260: 117768.
Jafari H, Delporte C, Bernaerts KV, Alimoradi H, Nie L, Podstawczyk D, et al. Synergistic complexation of phenol functionalized polymer induced in situ microfiber formation for 3D printing of marine-based hydrogels. Green Chem. 2022;24(6):2409–22.
Hewawasam RS, Blomberg R, Šerbedžija P, Magin CM. Chemical modification of human decellularized extracellular matrix for incorporation into phototunable hybrid-hydrogel models of tissue fibrosis. ACS Appl Mater Interfaces. 2023;15(12):15071–83.
Chen J, Yang J, Wang L, Zhang X, Heng BC, Wang D-A, et al. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioact Mater. 2021;6(6):1689–98.
Wu J, Wei Y, Ding H, Wu Z, Yang X, Li Z, et al. Green synthesis of 3D chemically functionalized graphene hydrogel for high-performance NH3 and NO2 detection at room temperature. ACS Appl Mater Interfaces. 2020;12(18):20623–32.
Zhang Q, Zhou X, Du H, Ha Y, Xu Y, Ao R, et al. Bifunctional hydrogel-integrated 3D printed scaffold for repairing infected bone defects. ACS Biomater Sci Eng. 2023;9(8):4583–93.
Chen Q, Li J, Han F, Meng Q, Wang H, Wei Q, et al. A multifunctional composite hydrogel that rescues the ROS microenvironment and guides the immune response for repair of osteoporotic bone defects. Adv Func Mater. 2022;32(27):2201067.
Wang L, Hu P, Jiang H, Zhao J, Tang J, Jiang D, et al. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today. 2022;43: 101401.
Ingavle GC, Gionet-Gonzales M, Vorwald CE, Bohannon LK, Clark K, Galuppo LD, et al. Injectable mineralized microsphere-loaded composite hydrogels for bone repair in a sheep bone defect model. Biomaterials. 2019;197:119–28.
Xu R-J, Ma J-J, Yu X, Zhou X-Q, Zhang J-Y, Li Y-D, et al. A biphasic calcium phosphate/acylated methacrylate gelatin composite hydrogel promotes osteogenesis and bone repair. Connect Tissue Res. 2023;64(5):45–456.
Wang Y, Peng Z, Zhang D, Song D. Tough, Injectable calcium phosphate cement based composite hydrogels to promote osteogenesis. Gels. 2023;9(4):302.
Solé-Martí X, Labay C, Raymond Y, Franch J, Benitez R, Ginebra MP, et al. Ceramic-hydrogel composite as carrier for cold-plasma reactive-species: safety and osteogenic capacity in vivo. Plasma Process Polym. 2023;20(1):2200155.
Chronopoulou L, Cacciotti I, Amalfitano A, Di Nitto A, D’Arienzo V, Nocca G, et al. Biosynthesis of innovative calcium phosphate/hydrogel composites: Physicochemical and biological characterisation. Nanotechnology. 2020;32(9): 095102.
Guo Z-X, Zhang Z, Yan J-F, Xu H-Q, Wang S-Y, Ye T, et al. A biomaterial-based therapy using a sodium hyaluronate/bioglass composite hydrogel for the treatment of oral submucous fibrosis. Acta Biomater. 2023;157:639–54.
Sadeghian A, Kharaziha M, Khoroushi M. Dentin extracellular matrix loaded bioactive glass/GelMA support rapid bone mineralization for potential pulp regeneration. Int J Biol Macromol. 2023;234: 123771.
Manoochehri H, Ghorbani M, MoosazadehMoghaddam M, Nourani MR, Makvandi P, Sharifi E. Strontium doped bioglass incorporated hydrogel-based scaffold for amplified bone tissue regeneration. Sci Rep. 2022;12(1):10160.
Yang Z, Zhao F, Zhang W, Yang Z, Luo M, Liu L, et al. Degradable photothermal bioactive glass composite hydrogel for the sequential treatment of tumor-related bone defects: From anti-tumor to repairing bone defects. Chem Eng J. 2021;419: 129520.
Wang Y, Liu Q, Yang Y, Qiu C, Jiao A, Jin Z. Impact of pH on pea protein–hydroxypropyl starch hydrogel based on interpenetrating network and its application in 3D-printing. Food Res Int. 2023;170: 112966.
Hao X, Miao S, Li Z, Wang T, Xue B, Chen J, et al. 3D printed structured porous hydrogel promotes osteogenic differentiation of BMSCs. Mater Des. 2023;227: 111729.
Emir AA, Erunsal SC. Impact of oleuropein on LCD-based stereolithography-assisted fabrication of 3D printed PEGDMA hydrogels. Eur Polymer J. 2022;180: 111592.
Dong M, Han Y, Hao XP, Yu HC, Yin J, Du M, et al. Digital light processing 3D printing of tough supramolecular hydrogels with sophisticated architectures as impact-absorption elements. Adv Mater. 2022;34(34):2204333.
Duong HY, Roccuzzo A, Stähli A, Salvi GE, Lang NP, Sculean A. Oral health‐related quality of life of patients rehabilitated with fixed and removable implant‐supported dental prostheses. Periodontology 2000. 2022;88(1):201–37.
Xia P, Lopes AM, Restivo MT. Virtual reality and haptics for dental surgery: a personal review. Vis Comput. 2013;29:433–47.
Luo L, He Y, Jin L, Zhang Y, Guastaldi FP, Albashari AA, et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact Mater. 2021;6(3):638–54.
Struillou X, Rakic M, Badran Z, Macquigneau L, Colombeix C, Pilet P, et al. The association of hydrogel and biphasic calcium phosphate in the treatment of dehiscence-type peri-implant defects: an experimental study in dogs. J Mater Sci Mater Med. 2013;24:2749–60.
Kim S-y, Choi A-j, Park J-E, Jang Y-s, Lee M-h. Antibacterial activity and biocompatibility with the concentration of ginger fraction in biodegradable gelatin methacryloyl (GelMA) hydrogel coating for medical implants. Polymers. 2022;14(23):5317.
Siddiqui Z, Sarkar B, Kim K-K, Kadincesme N, Paul R, Kumar A, et al. Angiogenic hydrogels for dental pulp revascularization. Acta Biomater. 2021;126:109–18.
Zhou X, Cornel EJ, He S, Du J. Recent advances in bone-targeting nanoparticles for biomedical applications. Mater Chem Front. 2021;5(18):6735–59.
Nafo W. Polymer-based nanosystems and their applications in bone anticancer therapy. Front Chem. 2023;11:1218511.
Siddiqui L, Mahtab A, Rabbani SA, Verma A, Talegaonkar S. Polymeric nanoparticles-assisted macrophage targeting: basic concepts and therapeutic goals. Macrophage Targeted Delivery Systems: Basic Concepts and Therapeutic Applications: Springer; 2022. p. 123–43.
Sabir F, Asad MI, Qindeel M, Afzal I, Dar MJ, Shah KU, et al. Polymeric nanogels as versatile nanoplatforms for biomedical applications. J Nanomater. 2019;2019:1526186.
Guo X, Yang N, Ji W, Zhang H, Dong X, Zhou Z, et al. Mito-bomb: targeting mitochondria for cancer therapy. Adv Mater. 2021;33(43):2007778.
Chaturvedi M, Kumar M, Pathak K. A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res. 2011;2(4):215.
Liew SS, Qin X, Zhou J, Li L, Huang W, Yao SQ. Smart design of nanomaterials for mitochondria-targeted nanotherapeutics. Angew Chem Int Ed. 2021;60(5):2232–56.
Tabish TA, Hamblin MR. Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer. Biomater Biosyst. 2021;3: 100023.
Huang L, Sun Z, Shen Q, Huang Z, Wang S, Yang N, et al. Rational design of nanocarriers for mitochondria-targeted drug delivery. Chin Chem Lett. 2022;33(9):4146–56.
Wang M, Wang M, Zhang S, Chen J. Pickering gel emulsion stabilized by enzyme immobilized polymeric nanoparticles: a robust and recyclable biocatalyst system for biphasic catalysis. React Chem Eng. 2019;4(8):1459–65.
Amir Z, Saaid IM, MohdJunaidi MU, Wan Bakar WZ. Weakened PAM/PEI polymer gel for oilfield water control: Remedy with silica nanoparticles. Gels. 2022;8(5):265.
Cao J, Du X, Zhao H, Zhu C, Li C, Zhang X, et al. Sequentially degradable hydrogel-microsphere loaded with doxorubicin and pioglitazone synergistically inhibits cancer stemness of osteosarcoma. Biomed Pharmacother. 2023;165: 115096.
Ying H, Wang H, Jiang G, Tang H, Li L, Zhang J. Injectable agarose hydrogels and doxorubicin-encapsulated iron-gallic acid nanoparticles for chemodynamic-photothermal synergistic therapy against osteosarcoma. Front Chem. 2022;10:1045612.
Wu W, Dai Y, Liu H, Cheng R, Ni Q, Ye T, et al. Local release of gemcitabine via in situ UV-crosslinked lipid-strengthened hydrogel for inhibiting osteosarcoma. Drug Deliv. 2018;25(1):1642–51.
Wang Q, Zuo Z, Cheung CKC, Leung SSY. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int J Pharm. 2019;559:86–101.
Abdelkader DH, Tambuwala MM, Mitchell CA, Osman MA, El-Gizawy SA, Faheem AM, et al. Enhanced cutaneous wound healing in rats following topical delivery of insulin-loaded nanoparticles embedded in poly (vinyl alcohol)-borate hydrogels. Drug Deliv Transl Res. 2018;8:1053–65.
Furlani F, Rossi A, Grimaudo MA, Bassi G, Giusto E, Molinari F, et al. Controlled liposome delivery from chitosan-based thermosensitive hydrogel for regenerative medicine. Int J Mol Sci. 2022;23(2):894.
Jøraholmen MW, Johannessen M, Gravningen K, Puolakkainen M, Acharya G, Basnet P, et al. Liposomes-in-hydrogel delivery system enhances the potential of resveratrol in combating vaginal chlamydia infection. Pharmaceutics. 2020;12(12):1203.
Wang Q, Zhang H, Huang J, Xia N, Li T, Xia Q. Self-double-emulsifying drug delivery system incorporated in natural hydrogels: a new way for topical application of vitamin C. J Microencapsul. 2018;35(1):90–101.
Yasasvini S, Anusa R, VedhaHari B, Prabhu P, RamyaDevi D. Topical hydrogel matrix loaded with Simvastatin microparticles for enhanced wound healing activity. Mater Sci Eng, C. 2017;72:160–7.
Fliervoet LA, Engbersen JF, Schiffelers RM, Hennink WE, Vermonden T. Polymers and hydrogels for local nucleic acid delivery. J Mater Chem B. 2018;6(36):5651–70.
Rey-Rico A, Cucchiarini M. Supramolecular cyclodextrin-based hydrogels for controlled gene delivery. Polymers. 2019;11(3):514.
Weiss DJ, Bates JH, Gilbert T, Liles WC, Lutzko C, Rajagopal J, et al. Stem cells and cell therapies in lung biology and diseases: conference report. Ann Am Thorac Soc. 2013;10(5):S25–44.
Lei Y, Rahim M, Ng Q, Segura T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J Control Release. 2011;153(3):255–61.
Carballo-Pedrares N, Fuentes-Boquete I, Díaz-Prado S, Rey-Rico A. Hydrogel-based localized nonviral gene delivery in regenerative medicine approaches—an overview. Pharmaceutics. 2020;12(8):752.
Li Z, Ning W, Wang J, Choi A, Lee P-Y, Tyagi P, et al. Controlled gene delivery system based on thermosensitive biodegradable hydrogel. Pharm Res. 2003;20:884–8.
Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014;10(4):1646–62.
Shin H, Olsen BD, Khademhosseini A. Gellan gum microgel-reinforced cell-laden gelatin hydrogels. J Mater Chem B. 2014;2(17):2508–16.
Qayyum AS, Jain E, Kolar G, Kim Y, Sell SA, Zustiak SP. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication. 2017;9(2): 025019.
Al-Tabbaa O, Ankrah S. Social capital to facilitate ‘engineered’university–industry collaboration for technology transfer: a dynamic perspective. Technol Forecast Soc Chang. 2016;104:1–15.
Aswathy S, Narendrakumar U, Manjubala I. Commercial hydrogels for biomedical applications. Heliyon. 2020;6(4):e03719.
Juric D, Rohner NA, von Recum HA. Molecular imprinting of cyclodextrin supramolecular hydrogels improves drug loading and delivery. Macromol Biosci. 2019;19(1):1800246.
Trattnig S, Ohel K, Mlynarik V, Juras V, Zbyn S, Korner A. Morphological and compositional monitoring of a new cell-free cartilage repair hydrogel technology–GelrinC by MR using semi-quantitative MOCART scoring and quantitative T2 index and new zonal T2 index calculation. Osteoarthr Cartil. 2015;23(12):2224–32.
Catoira MC, González-Payo J, Fusaro L, Ramella M, Boccafoschi F. Natural hydrogels R&D process: technical and regulatory aspects for industrial implementation. J Mater Sci Mater Med. 2020;31:1–16.
Donawa M. Regulation of novel biomedical hydrogel products. Biomedical Hydrogels: Elsevier; 2011. p. 81–100.
Mohapatra S, Mirza MA, Hilles AR, Zakir F, Gomes AC, Ansari MJ, et al. Biomedical application, patent repository, clinical trial and regulatory updates on hydrogel: an extensive review. Gels. 2021;7(4):207.
Author information
Authors and Affiliations
Contributions
R. K. and M. G. have written the manuscript. J. A. S. and A. S. have helped in drawing the diagrams. N. R. and R. B. have validated the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kohar, R., Ghosh, M., Sawale, J.A. et al. Insights into Translational and Biomedical Applications of Hydrogels as Versatile Drug Delivery Systems. AAPS PharmSciTech 25, 17 (2024). https://doi.org/10.1208/s12249-024-02731-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02731-y