Abstract
Khellin, a furanochromone isolated from fruits and seeds of Ammi visnaga, is traditionally used in many eastern Mediterranean countries. The plant decoction and the crystalline substance khellin have many pharmacological activities. For instance, it acts as a bronchodilator and also relieves renal colic and urethral stones, etc. However, the low water solubility (~ 120 µg/mL) and low bioavailability limit its therapeutic application. Thus, the present research explores the development of its binary and ternary solid dispersion formulations to improve its solubility and dissolution behavior. A 24-well plate miniaturized protocol was established to identify the optimal hydrophilic polymer to prepare its solid dispersions. PEG-4000 was recognized as the favorable hydrophilic carrier in preparation of solid dispersion, SSB17. The formulation displayed ~ five-fold enhancement in the aqueous solubility of khellin. The binary solid dispersion SSB17 was manufactured at a gram scale and evaluated using 1H-NMR, 13C-NMR, FT-IR, p-XRD, SEM, DSC, in vitro dissolution, and predicted pharmacokinetics. The quantitative dissolution data of SSB17 demonstrated ~ 2–3-fold improvement in AUC at physiological pH conditions. These conclusions highlight the basis for further preclinical studies on solid dispersions of khellin with improved biopharmaceutical properties.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Khellin is a furanochromone class of compound and a principal constituent of Amma visnaga Linn (1). It was first isolated from the fruits of Ammi visnaga by Mustapha in 1879. The fruits and seeds of Amma visnaga consist of ~ 1% w/w of khellin. Egyptians traditionally use the preparations made from this plant for relieving renal colic, ureteral spasm, ureteral stones, etc. (2). Additionally, the decoction and tincture of Ammi visnaga are part of Egyptian Pharmacopoeia 1934 and recommended as antispasmodic for renal colic. Khellin was dispensed commercially in Egypt and the USA as tablets or injectable solutions (3,4,5).
Khellin exhibits numerous pharmacological activities including analgesic (6), anti-inflammatory (6), antimutagenic (7), antineoplastic (8), and cancer chemopreventive (9). The research on khellin led to the discovery of first-in-class drugs, namely amiodarone (10, 11) and cromolyn sodium (12), approved by the FDA in 1985 and 2001. However, despite being a potent phytomolecule, khellin has never advanced to clinical trials due to its low bioavailability (13). One of the plausible reasons for its low plasma exposure is low aqueous solubility (~ 120 µg/mL) (14). The physicochemical properties of khellin and its chemical are mentioned in Fig. 1.
Traditionally, khellin is used as a decoction of Ammi visnaga, and conventional tablets are also reported. However, the conventional formulations have limitations in delivering desired therapeutic efficacy of khellin due to the aforementioned biopharmaceutical hiccups. The topical delivery was studied owing to the poor aqueous solubility of khellin. Novel topical gel formulations of khellin are explored for treating vitiligo. Furthermore, ascosome vesicles (15), hydrogels (16), gel-in-oil emulsions (17), and nanotubes (18) were attempted to improve the bioavailability of khellin. The amorphous solid dispersions are scalable from the lab to the pilot scale and thus have clinical potential. However, no formulation strategy is explored to improve the solubility of khellin, thereby achieving its oral delivery. The present research work is conceived due to its further application in the drug discovery and development program. This scaffold has already provided two first-in-class drugs, and currently it is being explored to identify more lead compounds in various drug discovery programs. Since the developed formulation approach is novel and scalable, it has a potential for converting into the bench-to-bedside technology. This work will serve as the background for further formulation work around this scaffold or lead molecules.
Developing solid dispersions (SDs) of drugs has a successful track record of modulating biopharmaceutical properties of poorly water-soluble molecules. SD formulations improve the solubility and dissolution performance of the molecule, thereby enhance its bioavailability (19,20,21,22). The added advantage of SDs is their scalability, which results in the development of “bench to bedside” formulations, as evidenced by numerous commercial SD technologies. This trajectory began in 1985 with the approval of the first SD formulation of the BCS class II drug nabilone. It is an anticancer molecule for which SD formulation was developed using PVP as a polymer by solvent evaporation technique (23). In the 2010s-decade, the FDA approved the ritonavir SD formulation prepared by melt extrusion using HPMCAS and PEG as the polymer (24).
In 1966, Mayersohn and Gibaldi (25) defined solid-state dispersions, later renamed “solid dispersions” by Chiou and Riegelman in 1971 (26). They classified solid dispersions into six groups, namely (i) simple eutectic mixtures, (ii) solid solutions, (iii) glass solutions and glass suspensions, (iv) amorphous precipitates in a crystalline carrier, (v) compound or complex formation, and (vi) combinations of the five types mentioned above. Additionally, based on the crystalline and amorphous nature of the drug and carrier, SDs are classified into five generations. The first-generation SDs comprise eutectic mixtures wherein API and carrier are crystalline, leading to low solubility and dissolution rate. In the second-generation SDs, the drug and carrier are in the amorphous state wherein the drug is supersaturated in a molten matrix (27). The third-generation SDs possess multiple carriers with self-emulsifying properties resulting in solubility improvement (28). Fourth-generation SDs are prepared using polymers, either water-insoluble or swellable polymers (28). The fifth-generation SDs comprise drug dispersed in carriers capable of enhancing the drug delivery system (29).
The formation of dissolved amorphous SD is a crucial step in improving the dissolution profile of the drug and is directly linked with bioavailability. The mechanism of drug dissolution and uptake from SD is discussed previously. Briefly, API’s enhanced solubility is attributed to the formation of colloidal SD, which induces intestinal absorption of dissolved API (24, 30). The intestinal absorption of the drug from SDs involves the dissolution of SD in dissolution media, uptake of dissolved API, and equilibration of the drug in dissolved solution (31). However, the dissolution of SD is a crucial step directly linked to the enhanced bioavailability of the drug. There are three mechanisms of dissolution of SD formulations: carrier-controlled release, dissolution-controlled release, and drug-controlled release (32). The carrier-controlled release involves forming a viscous gel of polymer matrix due to entrapment of dissolution media in SD. The drug diffuses slowly in a controlled manner and is dependent on the dissolution media volume. Dissolution-controlled release mechanism involves the dissolution of API-polymer matrix in dissolution media and depends on supersaturation concentration of drug in SD formulation. The drug-controlled release comprises of dissolution of the drug and polymer in dissolution media. However, the drug in SD formulation dissolves in a controlled manner and is likely to provide a spring and parachute effect (33, 34).
Solid dispersions consist of drugs dispersed in an inert carrier medium such as sugars, carriers, and hydrophilic polymers. In addition, there exists binary (drug mixed with one polymer) and ternary (drug mixed with two polymers) SDs prepared to enhance the supersaturation of poorly water-soluble APIs (35,36,37,38,39,40). Based on these strong literature reports, it was assumed that khellin, when formulated using hydrophilic polymer, could enhance its aqueous solubility, dissolution, and thus oral pharmacokinetics.
The polymers suitable for formulation work were identified using our published solubility-guided miniaturized protocol (41, 42). The miniaturized protocol helped us in carrying out the screening investigations in milligram quantities. The present work describes the identification of appropriate binary and or ternary SDs of khellin. The effect of varying ratio and polymer combinations on solubility and thereby dissolution of khellin was also investigated. Finally, the optimized amorphous SD formulations were manufactured at a gram quantity and evaluated for their physicochemical properties, in vitro dissolution performance, and predicted pharmacokinetics by the in vitro-in vivo correlation (IVIVC) approach.
Materials and Methods
Materials and Equipment
The natural product khellin used in the present study was procured from a commercial supplier, Sigma-Aldrich (60,730-10G). All the excipients/carriers/polymers such as synperonic F-108, PEG-PPG-PEG (MW. 5800), and kolliphor HS15 were also procured from Sigma-Aldrich India. Different grades of PEG (1500 and 4000) were purchased from S. D. Fine Chemicals, India. HP-β-CD and poloxamer-188 were bought from Alfa Aesar, India. Two grades of PVP (K30 and K90) were obtained from Research-Lab Fine Chem Industries, India. Water and methanol for HPLC were purchased from Fisher Scientific, Mumbai. The HPLC analysis was carried out on the Shimadzu HPLC system (LC-6AD) connected to the reverse phase C18 column (25 cm × 4.6 mm, 5 µm; Ascentis). Other routine equipments used were microcentrifuge 5430R (Eppendorf), vortex mixer (IKA vortex Genius 3), micropipettes (Eppendorf), microplate shaker-incubator (Eppendorf, ThermoMixer, Germany), USP dissolution apparatus (Make: Lab-India, Model: DS 8000; Type 2—Paddle), etc.
1H/13C NMR, FTIR, and DSC spectra were recorded on 400 MHz NMR (Bruker), IR spectrophotometer (Perkin-Elmer), and TA Instruments Q10 DSC. In addition, powder X-ray diffractions of the drug and formulations were recorded on PANalytical’s X-ray diffractometer. The in vitro dissolution studies of optimized formulation(s) were conducted using dissolution apparatus (model: Lab India DS 8000) by paddle method as per the Indian Pharmacopoeia, 2007.
Analytical Method for Khellin
Using the HPLC method, the detection and quantification of khellin during solubility determinations and in vitro dissolution studies were carried out. The analysis was conducted using Shimadzu HPLC system fitted with Ascentis® C18 (25 cm × 4.6 mm, 5 µm) column, photodiode detector (SPD˗M20A, Prominence, Shimadzu), LC-6AD Shimadzu pump, and SIL˗20A HT Prominence auto-sampler. Throughout the study, the temperature of the column was maintained at 37 °C using a column oven (CTO˗10ASVP). The column was eluted with the isocratic mobile phase comprising 90 parts of methanol and 10 parts aqueous solution of formic acid (0.1% v/v) at a 1 mL/min flow rate. Then, the samples (injection volume, 5.0 µL) were injected into the column using an autosampler. The khellin eluted at 3.1 min, and thus, the analysis run time was 10 min.
Preliminary Screening of Hydrophilic Carrier(s) for SD Formulations
The 0.5% w/v aqueous solutions of various hydrophilic carriers/polymers, viz. polyethylene glycols PEG-4000, PEG-1500, PEG-PPG-PEG copolymer, HP-β-CD, PVP-K90, PVP-K30, poloxamer-188, synperonic F-108, and kolliphor HS15 were prepared. The thermodynamic equilibrium solubility of khellin was determined in these aqueous solutions using a shake-flask method as described earlier (41,42,43,44). The solubility experiment was performed in 1.5-mL Eppendorf tubes. In each experiment set, the excess quantity of the compound (khellin) was added to the 0.5 mL of 0.5% v/v aqueous solutions of different polymers/carriers as described above. All tubes were placed in the thermomixer maintained at 25 °C and shaken continuously for 24 h at 300 rpm. All samples were then centrifuged (16,000 rpm, 15 min) to separate the undissolved solid particles of khellin. The supernatant solution of each tube was analyzed to determine the amount of khellin dissolved in each experiment. The concentration of khellin was determined as microgram per milliliter. Each experiment was carried out in triplicates, and the data were analyzed statistically by a one-way ANOVA test using GraphPad Prism 6.01 software. Based on the solubility values obtained in this experiment, additional optimization of respective binary SDs was carried out.
Binary Solid Dispersions (Binary SDs)
The polymers that showed improved aqueous solubility of khellin in the preliminary screening experiment were chosen for binary SDs. These include PEG-1500, PEG-4000, poloxamer-188, and PVP-K90. The binary SDs were prepared using khellin and selected polymers in different ratios using the solvent casting and evaporation method. The experiment design consisted of using a 24-well plate assay platform, as depicted in Fig. 2a. Three different ratios of khellin: polymer, viz. 1:4, 1:8, and 1:16 were investigated. The stock solution of khellin (2 mg/mL) and polymers/carriers (10 mg/mL) was prepared in a suitable solvent (water or methanol). The 500 µL of khellin solution and the required volume of carrier solution (as per selected ratios) were thoroughly mixed in the Eppendorf tube using a vortex mixer. The resulting drug-polymer mixture was transferred to the 24-well plate. The plate was continuously shaken on a thermoshaker at 300 rpm at 50 °C to allow the solvent to evaporate. Post-evaporation of the solvent, the microtiter plate was further stored in a vacuum desiccator to remove the solvent completely. These binary SD formulations were assessed for saturation solubility of khellin in water. In this analysis, to each well of the microplate, 400 µL of water was added, and the plate was sealed tightly with aluminum foil and parafilm. The plate was then shaken on a thermoshaker at 300 rpm and 25 °C for 24 h. The aliquots were collected from each well and loaded to the HPLC autosampler for HPLC analysis. The concentration of khellin was estimated in each well and is reported as µg/mL. Based on the solubility values, the formulations were chosen for scale-up.
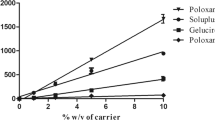
a Miniaturized 24-well plate procedure to prepare binary SDs of khellin; b experimental design of ternary SDs; c average solubility of khellin in 0.5% w/v aqueous solution of carriers; d aqueous solubility of binary SDs of khellin. Statistical analysis: ****P < 0.0001; ***0.0005 < P > 0.0001; ns: P > 0.05
Ternary Solid Dispersions (Ternary SDs)
The polymers PEG-4000, PVP-K90, and poloxamer-188 were considered further to prepare ternary SDs from experimental conclusions of binary SDs. Their combined effect on the solubility of khellin was studied. The 1:8:8 ratio of khellin:polymer 1:polymer 2 was tried, resulting in 3 formulation batches of khellin (Fig. 2b and Table I). Ternary SDs were prepared according to the 24-well plate protocol. The water solubility of khellin was determined in these ternary SD formulations using the HPLC method as described above.
Scale-up and Analysis of Optimized SD Formulations
From all the above-mentioned formulation experiments, binary SD formulations of khellin were considered for scale-up studies based on its solubility improvement. The optimized SD formulations were prepared at a 1-g scale. Briefly, methanolic solution of khellin, PEG-1500, and PEG-4000 was prepared (section “Binary Solid Dispersions (Binary SDs)”), and the formulations were labeled as SSB16 and SSB17, respectively. These ingredients were mixed thoroughly by mechanical stirring in the desired ratio to obtain a solution. The solvent was evaporated at 50 °C and 50 rpm under reduced pressure using a rotary evaporator. The dry solid formulation was removed and ground using mortar and pestle to get uniform powder. The obtained powder was further passed through sieve #30 mesh and stored in a vacuum desiccator. Both batches were assessed for khellin content using the HPLC method. Accurately weighed formulation was mixed with 10 mL of methanol. The supernatant was diluted further and was subjected to HPLC analysis.
1H NMR, 13C NMR, DSC, FT-IR, SEM, and p-XRD Studies
The optimized SD formulation SSB17 was characterized using various spectroscopic/thermal/crystallography techniques to assess drug stability and understand the formulation’s physical nature. The solution-state NMR analysis of khellin, polymer, and the formulation was carried out in DMSO-d6. The NMR data were assessed for changes in the chemical shift values of the khellin in the formulation. Next, the FT-IR analysis was carried out to determine any interaction between the functional groups of khellin and the polymer. The absorbance frequencies of the khellin and polymer were compared to that of peaks in the formulation spectrum. Other key solid-state characterization studies of the formulation include differential scanning calorimetry (DSC), SEM, and p-XRD. The DSC, SEM, and p-XRD studies were carried out under similar conditions and protocols described earlier in our previous publication (41, 42, 45). The thermal behavior of khellin, PEG-4000, and SSB17 was determined using the DSC instrument, Mettler Toledo. Briefly, the analysis was conducted using 3 − 5 mg of the sample sealed in an aluminum pan. Furthermore, it was scanned from 30 to 300 °C with an incremental heating rate of 10 °C/min. The SEM images of khellin and SSB17 were recorded on a scanning electron microscope, JEOL JSM-IT300. The gold-coated particles were affixed using double-sided carbon tape on an aluminum sample holder. The p-XRD studies were performed on PANalytical’s X’Pert Pro X-ray diffractometer. A Cu Kα (λ = 45 kV, 40 mA) anode was used as the X-ray radiation source. The angle 2θ was recorded between 5 and 50° with an incremental scan rate of 0.017° at 0.5 s/step.
pH-Solubility Profile of Khellin and In Vitro Dissolution Studies of SD Formulations
The solubility of khellin at different pH was determined as per our published protocol (45). Hydrochloric acid and phosphate buffers were used for the study. Briefly, eight buffers were prepared, namely HCl buffers, pH 1.2 and 2.0, and phosphate buffers, pH 3.0, 4.0, 5.0, 6.0, 7.0, and 8.0, respectively. The graph of pH vs. solubility of khellin was obtained for the determination of the pHmax.
The in vitro dissolution of SD formulation was performed using USP dissolution apparatus (paddle method) containing 250 mL of dissolution medium at 37 ± 0.5 °C and 50 rpm. The dissolution study of khellin and SSB17 was conducted in physiological solutions comprising water, HCl buffer (pH 1.2), and phosphate buffer (pH 6.8) under non-sink conditions. To compare the dissolution performance of khellin and SSB17, the experiments were carried out with ~ 10 mg of khellin or SSB17 formulation containing 10 mg khellin. The aliquots were collected manually at 5, 15, 30, 60, 120, and 180 min. At each time point, 1 mL of dissolution medium was withdrawn and replaced with the fresh one. The prefiltered aliquot was analyzed for khellin content by the HPLC method. Each experiment was performed in triplicate. The dissolution results for the formulation and parent compound, khellin, were compared (45, 46).
In Vitro Parallel Artificial Membrane Permeability Assay (PAMPA) and Predicted Pharmacokinetics of SD Formulation
The membrane permeability of plain drug and SD formulation was determined. The 10 mM concentration of khellin and equivalent amount of SSB17 were dissolved in DMSO. Both solutions were further diluted to 100 µM with PBS buffer pH 7.4. The porcine polar brain lipid (PBL) was dissolved in dodecane to get a 20 mg/mL concentration. The membrane filter of the donor plate was coated with 4 µL of porcine polar brain lipid (PBL), and 200 µL of drug solution was added to it. Furthermore, the acceptor plate was filled with 200 µL of PBS pH 7.4 buffer and was allowed to equilibrate for 18 h at 25 °C. The drug concentration in the acceptor and donor compartment was determined at 247 nm using SpectraMax Plus 384 UV plate reader (47). The permeability of the molecule was calculated using the following equation.
where C = (VD × VA) / (VD + VA) × area × time.
VD and VA represent the volume in donor and acceptor plates, respectively.
From in vitro dissolution data, the in vivo plasma concentration of khellin in the formulation was predicted mathematically. The FDA has released regulatory guidance for relating in vitro and in vivo release profiles, commonly referred to as in vitro-in vivo correlation (IVIVC) (48, 49). The method involves as follows: (a) computation of discrete amount (mg) released during each sampling time obtained from in vitro dissolution data; (b) considering first-order kinetics, the drug elimination rate was calculated using the following equation:
where C1 and C2 are the predicted concentrations of drug in plasma at a given time (t1 and t2) and Ke is the first-order elimination rate constant.
The graph of predicted blood amount vs. time (h) was plotted (50).
Results and Discussion
Natural products have always provided new drugs for modern medicine. Khellin is one of the privileged natural products that yielded two first-in-class drugs. As a part of drug development, the modulation of physicochemical properties is to be carried out to address the ADME properties. Furthermore, for better plasma exposure, the modulation of the aqueous solubility of khellin is desirable. Thus, herein we investigated the SD approach to modulate dissolution performance and the oral absorption under in vivo conditions. The formulation development was carried out here in a step-wise manner, first identifying the suitable polymers that help to dissolve the compound in water. Next, the detailed optimization of the SD, such as the binary or ternary SDs and the drug:polymer ratio, was also optimized. Each step is discussed below.
Identification of Polymers or Carriers by Solubility-Guided Multiwell Protocol
The preliminary screening experiments were performed to identify polymers/carriers demonstrating solubility-improvement of khellin. The results are shown in Fig. 2c, which indicated that except for PEG-1500, PEG-4000, poloxamer-188, and PVP-K90, other carriers did not significantly improve the aqueous solubility of khellin. Hence, these four hydrophilic carriers were selected for preparing binary SDs using the 24-well plate microtiter protocol. On the other hand, there was no significant enhancement in solubility of khellin with PVP K-30, HP-β-CD, kolliphor HS15, and synperonic F-108 and hence were eliminated in the preparation of binary SDs.
The drug:polymer ratio plays a crucial role in refining the desired physiochemical outcome in formulation optimization. Thus, the drug:polymer ratio (1:4, 1:8, and 1:16) was varied to identify the best polymer concentration that provides superior aqueous solubility. Various binary SDs were prepared using a 24-well plate protocol and were assessed for aqueous solubility. The 24-well microtiter plate protocol used a minimal amount of compound. Thus, a total of 12-binary SD formulations were prepared with varying concentrations of the carrier. The criteria for selecting a suitable formulation were as follows: (a) the physical nature of formulation and (b) improvement in the aqueous solubility of khellin. The multiwell screening showed that PEG-1500 (SSB1, SSB2, and SSB3; ~ 360–500 µg/mL), PEG-4000 (SSB4, SSB5, SSB6; ~ 325–425 µg/mL), and poloxamer-188 (SSB7; ~ 325 µg/mL) significantly enhanced solubility of khellin in comparison to its original solubility (122.98 ± 10.19 µg/mL). Formulation SSB7 comprises of khellin:poloxamer-188 (1:4). Only one concentration was of poloxamer-188 was selected because it was the lowest concentration or ratio at which significant improvement in solubility of khellin was observed compared to the other formulations containing different concentrations of poloxamer-188. The solubility results are depicted in Fig. 2d and Table I, which indicated no proportionate increase in solubility of khellin with respect to the carrier concentration. Hence, the lowest carrier concentration of PEG-1500 and PEG-4000 was considered as it showed a 3 to 5-fold solubility improvement. Thus, based on solubility results from binary SDs, the formulations containing PEG-1500 (SSB1) and PEG-4000 (SSB4) were chosen for scale-up. Both the selected formulations consist of a drug:polymer ratio of 1:4. Binary SDs of poloxamer-188 and PVP-K90 (1:4, 1:8, and 1:16) did not improve the solubility of khellin.
Additionally, the ternary SDs of khellin were also prepared to explore the possibility of further improvement in aqueous solubility. Different combinations of PEG-4000, poloxamer-188, and PVP K90 were tried. The solubility results of these formulations indicated no significant enhancement in solubility of khellin (~ 218–284 µg/mL) compared to the binary SD formulations (Table I). Therefore, two binary SD formulations (SSB1 and SSB4) were considered for scale-up and characterization studies.
The optimized formulations, i.e., binary SDs, SSB1, and SSB4, were prepared to 1-g scale. The scale-up formulations were labeled as SSB16 and SSB17. The % khellin content in SSB16 and SSB17 was 101.12 ± 6.49 and 95.02 ± 1.43, respectively. The aqueous solubility of khellin in SSB16 and SSB17 was also determined and found to be 443.82 ± 67.68 and 482.29 ± 24.88 µg/mL, respectively. The physical nature of SSB16 was sticky lumps that were difficult to triturate. On the contrary, SSB17 was a free-flowing powder formulation, and hence it was considered for further characterization as the final optimized SD formulation.
Characterization of SDs Using 1H and 13C-NMR, DSC, FTIR, p-XRD, and SEM
The characterization techniques such as NMR, FTIR, p-XRD, and DSC were used for analyzing physical forms of APIs. Additionally, these techniques provide valuable information on the compatibility of the drug with the excipients in the formulation. It also provides information on chemical interaction between drug and excipients, if any.
The possible interactions between khellin and PEG4000 are hydrogen bonding between the oxygen atom of PEG with -C = O or -OMe groups of khellin. The 1H and 13C NMR of khellin and SSB17 were recorded to assess the presence of H-bonding interaction. The objective of recording NMR spectrums was to locate the chemical shift value change because of the intermolecular H-bonding. The OMe signals in 1H NMR and 13C NMR should shift if its oxygen atom is involved in the H-bonding with other molecules. Similarly, the chemical shift value of -C = O should have a chemical shift if it involves an H-bonding interaction (51). The recorded 1H and 13C NMR spectra (Fig. 3a–b) do not show a change in the chemical shift values of any of these groups. All the peaks corresponding to protons and carbons of khellin were unaffected in terms of their chemical shift values and relative integration. This indicates that the khellin and PEG-4000 do not undergo any chemical interaction.
The thermograph of khellin, PEG-4000, and SSB17 is depicted in Fig. 4. The DSC spectrum of khellin shows a sharp melting endotherm at 150.86 °C, indicative of the crystalline nature. Furthermore, single glass transition temperature (Tg = 58.23 °C) in the SSB17 formulation was found to be above room temperature. This was matching with the Tg value of the carrier, PEG-4000 (61.43 °C). This confirmed the uniform miscibility of khellin in the carrier, PEG-4000 imparting storage stability of the formulation (52). However, the absence of melting endotherm of khellin in the formulation has indicated the amorphous transformation of khellin from its original crystalline nature.
FT-IR analysis of SSB17 showed all the absorbance frequency peaks for khellin (C = O stretch, C-O stretch, C-H stretch) and PEG-4000 (C-O stretch, C-H stretch). The characteristics C = O stretch of khellin were observed at the absorbance frequencies of 1649, 1631, and 1618 cm−1. Furthermore, absorbance peaks of PEG-4000, C-H stretch, and C-O stretch were present at 2860 and 1465/1340, respectively. The FT-IR spectra indicated the absence of a new peak or no shift in the absorption frequency, confirming the compatibility of PEG-4000 with khellin in the formulations. The crystallinity of khellin and SSB17 was studied using powered X-ray diffraction. The results are shown in Fig. 5. The p-XRD spectrum of khellin has shown sharp and distinct peaks, indicative of its crystalline nature. In contrast, the XRD-spectrum of SSB17 comprises low-intensity peaks indicating loss of crystallinity in the formulation. The plausible reason for the reduced crystallinity of khellin in SD formulation is attributed to PEG-4000 (53). Since NMR (1H and 13C) spectral analysis indicated no chemical interaction between khellin and polymer, thus PEG-4000 may act as a precipitation inhibitor and, therefore, prevent crystallization of khellin in the SD formulation in gastrointestinal fluids.
The SEM results have clearly shown the differences in morphology of khellin and SSB17 formulation (Fig. 6). The micrograph in Fig. 6a and b revealed clustered rod-shaped crystalline morphology (2–100 μm) of khellin. On the other hand, a photomicrograph of SSB17 shows a distinct particle morphology with uniformly dispersed flakes indicating the absence of free drug particles (Fig. 6c and d). This observation suggests the formation of homogeneous dispersion of khellin with PEG-4000.
pH-Solubility Profile of Khellin, In Vitro Dissolution Studies, and Predicted Pharmacokinetic Parameters of SSB17
The solubility of khellin at different pH conditions is depicted in Fig. 7a. The pH-dependent solubility of drug candidates provides valuable information about the dissolution of molecules in the gastrointestinal tract (GIT). The pH-dependent dissolution profile of the drug relies on its pH-dependent solubility. Khellin has relatively low solubility at the pH of the stomach, which is reflected by in vitro dissolution studies. This is a plausible indication of higher absorption of khellin in SSB17 at intestinal pH.
In vitro dissolution study of khellin and SSB17 was performed in three physiological media, including water, HCl buffer (pH 1.2), and phosphate buffer (pH 6.8) (45). The time vs. percent drug release profiles of khellin and SSB17 are depicted in Fig. 6. The dissolution of khellin in SD formulation (SSB17) in water, pH 1.2 HCl buffer, and phosphate buffer (pH 6.8) was superior to the plain molecule. SSB17 showed instant and superior dissolution of khellin at physiological pH conditions, which did not decline, confirming the absence of a parachute and spring effect. Furthermore, the improved dissolution of khellin over an extended time offers a broad absorption window upon its oral administration. Thus, the SD formulation offers superior release and exposure of khellin in a biological medium.
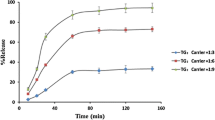
The dissolution profiles of SSB17 were further critically assessed, and various parameters such as Cmax and AUC were computed for khellin in different media (Table II). As a result, the formulation SSB17 showed enhanced dissolution and AUC0−t compared to khellin in water, HCl buffer (pH 1.2), and phosphate buffer (pH 6.8) (Fig. 7b, c, and d). For instance, % drug dissolved at the end of 30 min in water for SSB17 was ~ 63 compared to the parent compound (~ 27%). A similar trend was observed for SSB17 in pH 1.2 HCl buffer and pH 6.8 phosphate buffer wherein drug dissolved at 30 min was 51.71 and 51.94% (~ 38.68 and 22.57% respectively for parent compound, khellin).
The dissolution performance of khellin and its SD formulation, SSB17, was quantitatively compared using AUC obtained from the concentration–time profiles. The dissolution results in water, pH 1.2 HCl buffer, and pH 6.8 phosphate buffer are given in Table II. The enhanced dissolution and thus AUC0−t of SSB17 was observed compared to the molecule. In water, the improvement in the AUC of khellin in the formulation was found to be 2.2-fold. Furthermore, in pH 6.8 phosphate buffer, the AUC of khellin in SSB17 was 2.3-fold higher than the molecule. The fold dissolution improvement in pH 1.2 HCl buffer was 1.3 due to limited khellin solubility at acidic pH. Thus, the quantitative dissolution data were in accordance with the pH-solubility profile of khellin. The results confirm that the khellin has a relatively higher absorption at intestinal pH. Moreover, the tmax of khellin and SSB17 in the dissolution media indicate that SD of khellin (SSB17) provided a reasonably higher drug concentration in a short period than pure khellin molecule.
The PAMPA permeability was for khellin, alone and in the formulation, SSB17 was found to be 9.22 × 10−6 cm/s vs. 8.63 × 10−6 cm/s, respectively. These results support that the formulation process and the excipients do not affect the permeability of the molecule. Additionally, PEG-4000 does not allow khellin to crystallize in the solution state and thus inhibits the “spring and parachute” effect. Moreover, the convolution technique was used to predict the plasma concentration of khellin in formulation from the dissolution data (54). The IVIVC data of SD formulation SSB17 correlated very well and indicated a 2.3-fold higher AUC than the molecule (Fig. 7e).
Conclusion
In every drug development program, the oral route is always the preferred route of administration. However, each efficacious candidate does not possess desirable physicochemical properties that are essential for effective oral delivery. For adjusting the physicochemical parameters, the chemical modulation approach often results in a certain level of compromise with efficacy. Thus, formulation approaches frequently assist in resolving such absorption barriers associated with the low solubility of APIs. The present work improved the aqueous solubility of a unique natural product, khellin, exhibiting diverse biological activities. The systematic and step-wise solubility-guided approach employed herein has successfully identified an optimized SD formulation with the optimum drug: polymer ratio. The identified formulation was characterized thoroughly for its chemical interaction analysis (NMR and FTIR), solid-state properties (SEM, p-XRD, and DSC), and dissolution performance.
The characterization by 1H/13C-NMR and FT-IR revealed no chemical interaction between khellin and polymer in the formulation. Furthermore, the solid-state methods indicated the amorphous transformation of khellin in the formulation. The final binary SD formulation, SSB17, displayed high aqueous solubility and superior dissolution performance compared to the parent molecule. The developed formulation also allowed khellin to remain in the solution-state for more amount of time. The dissolution parameters and predicted pharmacokinetics proved enhanced drug exposure in the formulation. In a nutshell, the present study has again revealed that SD is a powerful technique to address the solubility barrier of lead candidates. The current research will provide valuable inputs to the drug discovery and development teams working on a similar scaffold.
Abbreviations
- AUC:
-
Area under the curve
- BCS:
-
Biopharmaceutical Classification System
- C max :
-
Maximum concentration
- DMSO:
-
Dimethyl sulfoxide
- DSC:
-
Differential scanning calorimetry
- FDA:
-
Food and Drug Administration
- FT-IR:
-
Fourier-transform infrared spectroscopy
- HPMCAS:
-
Hydroxypropylmethylcellulose acetate succinate
- HP-β-CD:
-
2-Hydroxypropyl-β-cyclodextrin
- HPLC:
-
High-performance liquid chromatography
- IVIVC:
-
in vitro-in vivo Correlation
- KBr:
-
Potassium bromide
- NMR:
-
Nuclear magnetic resonance
- p-XRD:
-
Powder X-ray diffraction
- PEG:
-
Polyethylene glycol
- PEG-PPG-PEG:
-
Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol)
- PVP-K30:
-
Polyvinylpyrrolidone K30
- PVP-K90:
-
Polyvinylpyrrolidone K90
- SD:
-
Solid dispersion
- SEM:
-
Scanning electron microscopy
- T max :
-
Time to reach the maximum concentration
References
Huttrer CP, Dale E. The chemistry and physiological action of khellin and related products. Chem Rev. 1951;48(3):543–79. https://doi.org/10.1021/cr60151a003.
Anrep GV, Barsoum GS, Kenawy MR. The pharmacological actions of the crystalline principles of Ammi Visnaga Linn. J Pharm Pharmacol. 1949;1(3):164–76. https://doi.org/10.1111/j.2042-7158.1949.tb12395.x.
Quimby MW. Ammi Visnaga Lam.—a medicinal plant. Econ Bot. 1953;7:89–92.
Scott RC, Iglauer A, Green RS, Kaufman JW, Berman B, Mc GJ. Studies on the effect of oral and parenteral administration of visammin (khellin) in patients with angina pectoris. Circulation. 1951;3(1):80–8. https://doi.org/10.1161/01.cir.3.1.80.
Kennedy MC, Stock JP. The bronchodilator action of khellin. Thorax. 1952;7(1):43–65. https://doi.org/10.1136/thx.7.1.43.
Abu-Hashem AA, Youssef MM. Synthesis of new visnagen and khellin furochromone pyrimidine derivatives and their anti-inflammatory and analgesic activity. Molecules. 2011;16(3):1956–72. https://doi.org/10.3390/molecules16031956.
Edenharder R, Speth C, Decker M, Kolodziej H, Kayser O, Platt KL. Inhibition of mutagenesis of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) by coumarins and furanocoumarins, chromanones and furanochromanones. Mutat Res. 1995;345(1–2):57–71. https://doi.org/10.1016/0165-1218(95)90070-5.
Atteya R, Ashour ME, Ibrahim EE, Farag MA, El-Khamisy SF. Chemical screening identifies the beta-Carboline alkaloid harmine to be synergistically lethal with doxorubicin. Mech Ageing Dev. 2017;161(Pt A):141–8. https://doi.org/10.1016/j.mad.2016.04.012.
Sharma R, Williams IS, Gatchie L, Sonawane VR, Chaudhuri B, Bharate SB. Khellinoflavanone, a semisynthetic derivative of khellin, overcomes benzo[a]pyrene toxicity in human normal and cancer cells that express CYP1A1. ACS Omega. 2018;3(8):8553–66. https://doi.org/10.1021/acsomega.8b01088.
Singh BN, Venkatesh N, Nademanee K, Josephson MA, Kannan R. The historical development, cellular electrophysiology and pharmacology of amiodarone. Prog Cardiovasc Dis. 1989;31(4):249–80. https://doi.org/10.1016/0033-0620(89)90033-9.
Singh BN. Amiodarone: historical development and pharmacologic profile. Am Heart J. 1983;106(4 Pt 2):788–97. https://doi.org/10.1016/0002-8703(83)90002-9.
Kuzemko JA. Twenty years of sodium cromoglycate treatment: a short review. Respir Med. 1989;83 Suppl A:11–4; discussion 5–6. https://doi.org/10.1016/s0954-6111(89)80245-8.
Hasa D, Perissutti B, Dall’Acqua S, Chierotti MR, Gobetto R, Grabnar I, et al. Rationale of using Vinca minor Linne dry extract phytocomplex as a vincamine’s oral bioavailability enhancer. Eur J Pharm Biopharm. 2013;84(1):138–44. doi: https://doi.org/10.1016/j.ejpb.2012.11.025.
Bharate SS, Kumar V, Vishwakarma RA. Determining partition coefficient (Log P), distribution coefficient (Log D) and ionization constant (pKa) in early drug discovery. Comb Chem High Throughput Screen. 2016;19(6):461–9. https://doi.org/10.2174/1386207319666160502123917.
Risaliti L, Ambrosi M, Calamante M, Bergonzi MC, Lo Nostro P, Bilia AR. Preparation and characterization of ascosome vesicles loaded with khellin. J Pharm Sci. 2020;109(10):3114–24. https://doi.org/10.1016/j.xphs.2020.06.017.
Risaliti L, Yu X, Vanti G, Bergonzi MC, Wang M, Bilia AR. Hydroxyethyl cellulose hydrogel for skin delivery of khellin loaded in ascosomes: characterization, in vitro/in vivo performance and acute toxicity. Int J Biol Macromol. 2021;179:217–29. https://doi.org/10.1016/j.ijbiomac.2021.02.206.
Pereira J, Goncalves R, Barreto M, Dias C, Carvalho F, Almeida AJ, et al. Development of gel-in-oil emulsions for khellin topical delivery. Pharmaceutics. 2020;12(5). https://doi.org/10.3390/pharmaceutics12050398.
Lisuzzo L, Cavallaro G, Milioto S, Lazzara G. Halloysite nanotubes coated by chitosan for the controlled release of khellin. Polymers (Basel). 2020;12(8). https://doi.org/10.3390/polym12081766.
Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315–499.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86(1):1–12. https://doi.org/10.1021/js9601896.
Serajuddin AT. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88(10):1058–66.
Herman TS, Jones SE, Dean J, Leigh S, Dorr R, Moon TE, et al. Nabilone: a potent antiemetic cannabinol with minimal euphoria. Biomedicine. 1977;27(9–10):331–4.
Pandi P, Bulusu R, Kommineni N, Khan W, Singh M. Amorphous solid dispersions: an update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int J Pharm. 2020;586: 119560. https://doi.org/10.1016/j.ijpharm.2020.119560.
Mayersohn M, Gibaldi M. New method of solid-state dispersion for increasing dissolution rates. J Pharm Sci. 1966;55(11):1323–4. https://doi.org/10.1002/jps.2600551138.
Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60(9):1281–302. https://doi.org/10.1002/jps.2600600902.
Hallouard F, Mehenni L, Lahiani-Skiba M, Anouar Y, Skiba M. Solid dispersions for oral administration: an overview of the methods for their preparation. Curr Pharm Des. 2016;22(32):4942–58. https://doi.org/10.2174/1381612822666160726095916.
Jadav NB, Paradkar A. Solid dispersions: technologies used and future outlook. In: Shegokar R, editor. Nanopharmaceuticals: Elsevier; 2020. p. 91–120.
Yu DG, Li JJ, Williams GR, Zhao M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: a review. J Control Release. 2018;292:91–110. https://doi.org/10.1016/j.jconrel.2018.08.016.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20. https://doi.org/10.1023/a:1016212804288.
Arca HC, Mosquera-Giraldo LI, Dahal D, Taylor LS, Edgar KJ. Multidrug, Anti-HIV amorphous solid dispersions: nature and mechanisms of impacts of drugs on each other’s solution concentrations. Mol Pharm. 2017;14(11):3617–27. https://doi.org/10.1021/acs.molpharmaceut.7b00203.
Schittny A, Huwyler J, Puchkov M. Mechanisms of increased bioavailability through amorphous solid dispersions: a review. Drug Deliv. 2020;27(1):110–27. https://doi.org/10.1080/10717544.2019.1704940.
Dahlberg C, Millqvist-Fureby A, Schuleit M, Furo I. Relationships between solid dispersion preparation process, particle size and drug release–an NMR and NMR microimaging study. Eur J Pharm Biopharm. 2010;76(2):311–9. https://doi.org/10.1016/j.ejpb.2010.06.006.
Puncochova K, Ewing AV, Gajdosova M, Sarvasova N, Kazarian SG, Beranek J, et al. Identifying the mechanisms of drug release from amorphous solid dispersions using MRI and ATR-FTIR spectroscopic imaging. Int J Pharm. 2015;483(1–2):256–67. https://doi.org/10.1016/j.ijpharm.2015.02.035.
Vasconcelos T, Marques S, das Neves J, Sarmento B. Amorphous solid dispersions: rational selection of a manufacturing process. Adv Drug Deliv Rev. 2016;100:85–101. https://doi.org/10.1016/j.addr.2016.01.012.
Vo CL, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85:799–813. https://doi.org/10.1016/j.ejpb.2013.09.007.
Baghel S, Cathcart H, O’Reilly NJ. Investigation into the solid-state properties and dissolution profile of spray-dried ternary amorphous solid dispersions: a rational step toward the design and development of a multicomponent amorphous system. Mol Pharm. 2018;15:3796–812. https://doi.org/10.1021/acs.molpharmaceut.8b00306.
Hanada M, Jermain SV, Williams RO 3rd. Enhanced dissolution of a porous carrier-containing ternary amorphous solid dispersion system prepared by a hot melt method. J Pharm Sci. 2018;107:362–71. https://doi.org/10.1016/j.xphs.2017.09.025.
Metre S, Mukesh S, Samal SK, Chand M, Sangamwar AT. Enhanced biopharmaceutical performance of rivaroxaban through polymeric amorphous solid dispersion. Mol Pharm. 2018;15(2):652–68. https://doi.org/10.1021/acs.molpharmaceut.7b01027.
Prasad D, Chauhan H, Atef E. Role of molecular interactions for synergistic precipitation inhibition of poorly soluble drug in supersaturated drug-polymer-polymer ternary solution. Mol Pharm. 2016;13(3):756–65. https://doi.org/10.1021/acs.molpharmaceut.5b00655.
Kumar V, Mintoo MJ, Mondhe DM, Bharate SB, Vishwakarma RA, Bharate SS. Binary and ternary solid dispersions of an anticancer preclinical lead, IIIM-290: In vitro and in vivo studies. Int J Pharm. 2019;570: 118683. https://doi.org/10.1016/j.ijpharm.2019.118683.
Kumar V, Vishwakarma RA, Bharate SS. Engineering solid dispersions of anticancer preclinical lead, IIIM-985: physicochemical characterization and in vivo pharmacokinetics. J Drug Deliv Sci Technol. 2019;49:594–602.
Bharate SS, Vishwakarma RA. Thermodynamic equilibrium solubility measurements in simulated fluids by 96-well plate method in early drug discovery. Bioorg Med Chem Lett. 2015;25:1561–7.
Kumar V, Bharate SS, Vishwakarma RA. Modulating lipophilicity of rohitukine via prodrug approach: preparation, characterization, and in vitro enzymatic hydrolysis in biorelevant media. Eur J Pharm Sci. 2016;92:203–11. https://doi.org/10.1016/j.ejps.2016.07.010.
Kumar V, Bharate SB, Vishwakarma RA, Bharate SS. Selection of a water-soluble salt form of a preclinical candidate, IIIM-290: multiwell-plate salt screening and characterization. ACS Omega. 2018;3(7):8365–77. https://doi.org/10.1021/acsomega.8b00801.
Fung M, Be Rzins KR, Suryanarayanan R. Physical stability and dissolution behavior of ketoconazole-organic acid coamorphous systems. Mol Pharm. 2018;15(5):1862–9. https://doi.org/10.1021/acs.molpharmaceut.8b00035.
Augustin N, Nuthakki VK, Abdullaha M, Hassan QP, Gandhi SG, Bharate SB. Discovery of helminthosporin, an anthraquinone isolated from Rumex abyssinicus Jacq as a dual cholinesterase inhibitor. ACS Omega. 2020;5(3):1616–24. https://doi.org/10.1021/acsomega.9b03693.
Vogelpoel H, Welink J, Amidon GL, Junginger HE, Midha KK, Moller H, et al. Biowaiver monographs for immediate release solid oral dosage forms based on biopharmaceutics classification system (BCS) literature data: verapamil hydrochloride, propranolol hydrochloride, and atenolol. J Pharm Sci. 2004;93(8):1945–56. https://doi.org/10.1002/jps.20131.
Anonymous. Guidance for industry immediate release solid oral dosage forms scale-up and postapproval changes: chemistry, manufacturing, and controls, in vitro dissolution testing, and in vivo bioequivalence documentation. Center for Drug Evaluation and Research (CDER). 1995:1–30.
Rastogi V, Yadav P, Lal N, Rastogi P, Singh BK, Verma N, et al. Mathematical prediction of pharmacokinetic parameters-an in-vitro approach for investigating pharmaceutical products for IVIVC. Future J Pharm Sci. 2018;4:175–84.
Van den Mooter G, Wuyts M, Blaton N, Busson R, Grobet P, Augustijns P, et al. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur J Pharm Sci. 2001;12(3):261–9.
Gupta P, Thilagavathi R, Chakraborti AK, Bansal AK. Role of molecular interaction in stability of celecoxib-PVP amorphous systems. Mol Pharm. 2005;2(5):384–91. https://doi.org/10.1021/mp050004g.
Xu S, Dai WG. Drug precipitation inhibitors in supersaturable formulations. Int J Pharm. 2013;453(1):36–43. https://doi.org/10.1016/j.ijpharm.2013.05.013.
Bonlokke L, Hovgaard L, Kristensen HG, Knutson L, Lindahl A, Lennernas H. A comparison between direct determination of in vivo dissolution and the deconvolution technique in humans. Eur J Pharm Sci. 1999;8(1):19–27. https://doi.org/10.1016/s0928-0987(98)00055-4.
Acknowledgements
The author thanks the central instrumentation laboratory (CIL) of Shobhaben Pratapbhai Patel School of Pharmacy & Technology Management, SVKM’s NMIMS, Mumbai, and sophisticated analytical instrumentation facility (SAIF) of Punjab University, Chandigarh, India, for DSC analysis and pXRD studies of formulations.
Author information
Authors and Affiliations
Contributions
Conceptualization, experimental design, experimental execution, data analysis and interpretation, writing — original draft, review, and editing: Sonali S. Bharate.
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bharate, S.S. Enhancing Biopharmaceutical Attributes of Khellin by Amorphous Binary Solid Dispersions. AAPS PharmSciTech 22, 260 (2021). https://doi.org/10.1208/s12249-021-02126-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02126-3