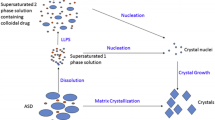
Abstract
An ideal dissolution test for amorphous solid dispersions (ASDs) should reflect physicochemical, physiological, and hydrodynamic conditions which accurately represent in vivo dissolution. However, this is confounded by the evolution of different molecular and colloidal species during dissolution, generating a supersaturated state of the drug. The supersaturated state of a drug is thermodynamically unstable which drives the process of precipitation resulting in a loss of solubility advantage. Maintaining a supersaturated state of the drug with the help of precipitation inhibiting excipients is a key component in the design of ASDs. Therefore, a biopredictive dissolution test is critical for proper risk assessment during the development of an optimal ASD formulation. One of the overlooked components of biopredictive dissolution is the role of drug permeability. The kinetic changes in the phase behavior of a drug during dissolution of ASDs are influenced by drug permeability across a membrane. Conventionally, drug dissolution and permeation are analyzed separately although they occur simultaneously in vivo. The kinetic phase changes occurring during dissolution of ASDs can influence the thermodynamic activity and membrane flux of a drug. The present review evaluates the feasibility, predictability, and practicability of permeability/dissolution for the optimal development and risk assessment of ASD formulations.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
A large number of drugs in the drug discovery pipeline suffer from poor solubility which demands solubility enabling techniques to improve their biopharmaceutical properties. Current estimates suggest that almost 70–90% of drugs in the discovery pipeline belong to BCS class II (1). Drugs in DCS class IIb (which shows solubility-limited dissolution) according to the developability classification system (DCS) and BCS class IV belongs to the high-risk category in terms of developability and presents a unique challenge to formulators (2). Among different methods available for solubility enhancement, amorphous solid dispersions (ASDs) have received much attention in recent times (2,3,4,5,6) (Fig. 1).
Comparative statistics of different solubility enhancement technologies published in scientific journals. Data were obtained from PubMed with the keyword “solubility enhancement” along with the name of the respective technologies. ASD, amorphous solid dispersions; SEDDS, self-emulsifying drug delivery systems
ASD is an attractive formulation strategy in which a drug is amorphized in a polymeric matrix with the advantage that there is no energy expenditure in crystal lattice disruption during dissolution in comparison to its crystalline counterpart. Dissolution studies, by and large, have not been able to comprehensively predict the in vivo outcome of enabling formulations from supersaturation data (7). For a paradigm shift in the evaluation of biopharmaceutical property of enabling formulations, the role of permeability is pivotal during in vitro dissolution studies (8, 9). Dissolution methods with dual chamber representing intestinal lumen and serosal compartments by separating them with a membrane representing intestinal epithelium can simultaneously analyze dissolution/permeability.
Factors affecting dissolution and passive drug absorption are summarized in Table I. In the current review, we focus our discussion on the factors which influence the interplay between drug supersaturation (SS) and membrane permeability during dissolution of ASDs.
Excipients play multiple roles to stabilize the SS state. It can have a role on three important aspects of formulation performance, viz., SS, solubilization, and permeability during dissolution. The ability of an excipient to maintain SS is referred as the “spring and parachute effect” in which spring refers to rapid dissolution and SS of a drug and excipients act like a parachute to slow down the rate of crystallization from a supersaturated drug solution (10). For the major part of ASD research, excipients were judged based on their precipitation inhibiting property. Different screening methods based on a solvent shift method have been devised to rank-order the excipients on the basis of its crystallization inhibiting property (11, 12). This method based on a plate assay is able to predict maximum achievable concentration and precipitation inhibition during amorphous dissolution (13). However, lately, it has been realized that excipients can also influence permeability and the overall performance of the ASD. Provided their influence on SS, precipitation, and permeability, the choice of an excipient is the most important decision to be made during the formulation development of ASDs.
One of the impediments in the successful formulation of ASDs is the complexity to decipher the dissolution phase behavior which influences the SS stability as well as the permeability of the drug. Permeation of a drug is linearly correlated (r = 0.959) with drug absorption (14, 15). Solubility and permeability form the basis of the BCS classification, and despite the critical role of permeability in drug absorption and bioavailability, its significance has been overlooked in dissolution studies. The influence of permeability on the interplay of SS and precipitation is critical for a biopredictive dissolution assay. This interplay of factors in an absorptive environment was explicitly demonstrated by comparison with a non-absorptive setup for the first time by Bevernage et al. (16). The one-compartment dissolution methods lack predictive capacity because of the oversimplified design. Predictive dissolution studies should include a permeability component, and a combined dissolution/permeability study is gaining attention for the evaluation of ASDs. Most of these dissolution systems are not important from a regulatory perspective but help us to gain insight into the mechanistic aspect of dissolution and help us to rank the performance efficiency of a drug-polymer system (17). A summary of dissolution setup for simultaneous evaluation of dissolution/absorption is summarized in Table II.
Therefore solubility-enabling techniques must be seen in the context of its concomitant role in the solubility-permeability interplay (29).
UNSTIRRED WATER LAYER AND ITS ROLE IN DRUG PERMEABILITY
The absorptive flux of a drug may be limited by diffusion across the intestinal membrane or the hydrodynamics of the unstirred water layer (UWL) (Fig. 2). UWL is a hydrodynamic barrier in the vicinity of a membrane where drug diffusional movement exceeds convection (30). For some lipophilic drugs, diffusion across the UWL can be a slow process compared to its membrane partitioning and permeability. A material sparing method for analyzing the rate-limiting steps in mass transport has been reported (31). Drugs in the form of colloidal species, bound and unbound can diffuse through the UWL to promote absorption (Fig. 2). Based on this study, drugs are classified as having membrane limited (e.g., ketoconazole), UWL-limited (e.g., itraconazole), and dissolution rate-limited flux (e.g., ketoconazole under certain conditions). Classifying drugs based on their rate-limiting step for membrane transport can aid formulation development and for predicting food effect, regiospecific drug absorption, dose linearity, etc. (32). ASDs on dissolution can generate colloidal species which may alter oral absorption. To explain the perceived improvement in oral absorption, these colloidal species are thought to act as a shuttle across the UWL for an unbound drug by reducing diffusional resistance (33). A key assumption is that the colloidal species formed during dissolution improves the diffusivity across the UWL based on their size and concentration (34). This is a useful approach for a highly lipophilic drug with good epithelial cell permeability for which UWL is a limiting factor and the formation of colloidal species can enhance absorption (35,36,37). The mechanism of formation of the array of colloidal species on dissolution of ASD is critical for predicting oral absorption. The diffusion of different colloidal and molecular species through the UWL is proportional to its diffusion coefficient and drug loading in the formulation.
SIGNIFICANCE OF SOLUBILITY-PERMEABILITY INTERPLAY
It is often observed that conventional techniques used to increase apparent solubility results in a trade-off with permeability resulting in the loss of solubility-advantage gained by these techniques (38). This is attributed to a low distribution coefficient of the drug between the intestinal milieu and the absorption membrane. The drug-complex or the micellar structure formed by the solubility enabling techniques like complexation and surfactant solubilization is responsible for a change in distribution coefficient which is directly proportional to the permeability and passive diffusion of the drug across the membrane (39, 40).
This can be explained by the modified Noyes-Whitney equation for mass transport per unit time (dM/dt) across a membrane under a steady state.
where A the surface area, Cs the saturation solubility, D is the membrane diffusivity of a drug, and K is the distribution coefficient of the drug between intestinal fluid and the intestinal membrane of thickness h. The flux of a drug across a membrane is limited by the diffusion across the unstirred water layer (UWL) and the absorption membrane which can be explained by a steady-state dissolution and diffusion model. The permeability across the two diffusion barriers, i.e., the UWL and the absorptions membrane, depends on the diffusion coefficient of the diffusing species (molecule, micellar, colloidal form, etc.) according to Fick’s first law. Permeability across the UWL is given by the equation where P is the permeability, D is the diffusion coefficient of the diffusing species, and h is the thickness of the diffusion barrier.
Equation 1 is used for the permeability across a membrane. The product of permeability and concentration gives the value of flux across a diffusional barrier. The rate limiting step in absorption depends on the properties of the drug and its propensity to form colloidal and micellar structures as highlighted above in this section (31).
Techniques like the cosolvancy and hydrotrophy have also been associated with a trade-off between solubility and permeability (40,41,42). Therefore, treating solubility and permeability in isolation may result in misleading information on drug absorption and availability. One of the advantages of using an ASD formulation is that in most cases, an increase in apparent solubility is translated into improved passive absorption, unlike other solubility enabling techniques (10, 43,44,45).
ROLE OF SPECIATION AND DEGREE OF SUPERSATURATION (DS)
Speciation refers to the free drug that is available for absorption relative to the bound drug (26). During dissolution, the released drug may be available in different molecular and bound species, viz. ionized, unionized, and bound forms (micellar aggregates, liposomes, mixed micellar form, etc.) in complex biological media (46, 47) (Table III).
Micelle forming polymer has been used to stabilize SS by sequestering the drugs in the micellar corona by a non-covalent interaction between the polymer and the drug (48, 49). Speciation results in different forms of the drug (with varying thermodynamic activity) in a supersaturated state with a consequent effect on the absorptive flux (50). The apparent permeability of a drug is contingent on the thermodynamic activity of the molecular species. The drug concentration is related to its thermodynamic activity (α) by the equation
where γ is the activity coefficient of the drug which is equal to one, at or below equilibrium crystalline solubility. The thermodynamic activity of the drug is the driving force for the absorptive flux (J) across a membrane given by the equation
where M is the mass, A is the surface area for absorption, h is the thickness of the membrane, D is the diffusivity, and γm is the activity coefficient of the drug in the membrane. Here D, h, and γm are constant, and therefore flux is directly proportional to the thermodynamic activity. In a supersaturated state, the flux across an absorptive membrane depends on the thermodynamic activity of the drug and not on the concentration (26, 51). Speciation determines the thermodynamic activity with the free form of a drug showing maximum thermodynamic activity and therefore the maximum flux.
Supersaturated state of a drug is often defined by the DS. Both solubilization and SS increase apparent solubility, yet demonstrate different thermodynamic consequences in terms of flux across a membrane. The DS in the presence of a solubilizing agent must be defined on thermodynamic terms because of its impact on membrane transport. The DS is a critical determinant of the amount of free drug available for absorption and drug precipitation.
DS is fundamentally expressed as follows (52).
obtained from Eq. (3) i.e., α = γC
In most practical purposes, DS is expressed by the equation
assuming that γ/ γ* ~ 1 which is reasonable for dilute solutions. DS is directly proportional to the flux because of the direct relationship between flux and activity as shown in Eq. 4 assuming that γ/ γ* ~ 1.
The assumption that DS = C*/C and γ/ γ* ~ 1, however, breaks down in the presence of solubilizing agent at a supersaturated state and underpredicts the DS (53). A solubilizing agent although can increase the saturation concentration (C) of the drug, but its DS is reduced by a factor depending on the solubilizing effect. The low DS results in a consequent reduction in flux, as a result of which the presence of solubilizing agent shows lower flux as compared to a solution with the same concentration without a solubilizing additive. The flux data is therefore the most reliable metric for the prediction of the DS (54). Solubilization capacity varies as a function of the rise in concentration during SS which confounds the determination of DS (53).
A high DS can increase the drug available for absorption; however, at the same time, it can result in a consequent high de-supersaturation by crystallization. The relationship between DS and induction time (tind) for precipitation according to classical nucleation theory is as shown in Eq. 5 (55).
where α and β describe tind at high and low DS. Drugs showing a high β value can show stable SS over a longer duration if DS is kept sufficiently low. Drugs have been ranked based on their propensity to supersaturate based on β value (13).
THE INTERPLAY OF SOLUBILIZATION, SUPERSATURATION, AND PERMEABILITY
A surfactant can have a variable effect on permeability across a membrane. Multiple mechanisms by which surfactant can influence SS and permeability are shown in Table IV.
The molecular state of a drug during dissolution influences its permeability across a membrane. The surfactant, poloxamer 407 has been shown to retard the permeability of carbamazepine due to the size of surfactant micelles formed during dissolution which has been attributed to the large size of the polymer molecule (65). It has also been reported that poloxamer enhances permeability below its critical micelle concentration (CMC) (66). It has been suggested that supersaturation by using polymeric precipitation inhibitors is more likely to improve permeation than surfactant stabilization (67). Colloidal stability of the micelles during dissolution is critical as in contact with biorelevant media, anionic micelles tend to destabilize resulting in drug precipitation due to the formation of mixed micelle. Polysorbate micelles however were found to be stable without the forming mixed micelle (68). Surfactants can form micelles above the CMC and surfactants with low CMC value tends to form stable micelles upon dilution (CMC ≪ 1 mM) (69).
Different colloidal and micellar species of a drug will have a different impact on the diffusive transport that can be explained by the Stokes–Einstein equation according to which diffusivity of the species (Dm for micelles, Dc for colloidal species) is inversely proportional to the particle diameter (dc and dm) (32).
Diffusivity of colloidal species can be calculated using the Stokes–Einstein equation based on the hydrodynamic diameter measured by dynamic light scattering (DLS). The flux across a membrane can be calculated from the concentration gradient across the membrane following steady-state diffusion with the assumption that the rate of dissolution/generation of micelles is faster than its diffusion in the UWL (32).
D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) on the other hand has been shown to improve supersaturation stability by reducing DS and prevent drug crystallization due to steric hindrance (70). A 40% increase in drug absorption was observed with TPGS in combination with HPMCAS. Surfactants such as TPGS can improve absorption by blocking the efflux pump pg-p glycoprotein (44, 71) and also by altering the biophysical property of the absorption membrane (72).
Surfactant solubilization on the one hand can increase the concentration of the dissolved drug, but on the other hand, it may negatively influence drug permeability, whereas polymers can stabilize drug SS with negligible effect on permeability (65). Different permeability behavior may be attributed to a different association between the drug and the excipients. The colloidal species formed during the dissolution due to the presence of solubilizing components either as excipients in the ASD formulation or the intestinal physiological media can influence the supersaturated state and thermodynamic activity of a drug with a consequent effect on the permeability of a drug. The difference in molecular interaction between surfactant, polymer, and the drug can influence permeability due to a change in the thermodynamic activity of the drug. Formulation sensitive to nanospeciation can be utilized for improving the permeability of drugs (73). This has been demonstrated by a nearly twofold increase in permeability of 17β-Estradiol, a non-ionizable poorly soluble drug in the presence of polysorbate 80 (1).
However, another experimental/computational study shows that surfactant and cyclodextrins can retard molecular diffusion across the UWL by decreasing the relative diffusivity and concentration gradient of drug molecules (64). Drugs for which membrane permeability is a limiting factor, on the other hand, show no effect of surfactants. It has been reported that nanoformulation strategy can have little or no benefit on bioavailability for drugs with low membrane permeability (74). Contrary to this report, a recent study on the effect of in situ formed colloidal species on oral bioavailability shows that the nanospecies formed during dissolution can also increase the bioavailability of molecules with low membrane permeability (75). It was hypothesized that apart from the particle drifting property of the colloidal species across the UWL, other yet unknown mechanisms are thought to play a role in oral absorption. Solubilization can have a profound impact on the SS and absorptive flux by influencing the amorphous solubility, LLPS, and membrane transport of drug molecules. During dissolution of ASDs, several different drug species may coexist in media, and its effect on membrane transport gives key insights into oral drug absorption (31).
Supersaturation and solubilization, although are solubility enabling techniques but both have a different effect on the membrane transport. In the presence of solubilizing additives, the increase in solubility is a poor predictor of the absorptive flux (53). Instead, the thermodynamic activity of the drug is a good predictor of absorptive flux. The solubilization technique results in a dynamic equilibrium between free drug and the micellar drug, and the solute activity is determined by the free drug. Factors that influence the solute activity include the solubilizing capacity of the media, formulation additives, the property of the drug, and the drug concentration. Micellar partitioning (Km/w) has been used as a parameter to study the effect of concentration on solubilization where it has been observed that the value of Km/w is much lower at amorphous solubility when compared to crystalline solubility (54). The concentration-based DS grossly underpredicts the membrane transport, whereas activity-based SS is a good predictor of membrane flux in surfactant-containing media. It is hypothesized that in a supersaturated state at higher DS, the micelles lose the capacity to solubilize the same fraction of the drug that is solubilized at the saturated state. This results in a larger fraction of the molecular form of the drug with higher solution thermodynamics at a supersaturated state.
It has been demonstrated that flux measurement and kinetic solubility determination can be used to predict food effect and bioequivalence among different itraconazole formulations (sporanox solution vs sporanox capsule) (76). The flux measurement was able to predict even a slight change in food effect and was found to be a very sensitive method to a change in the microenvironment. The results of flux measurement were found to agree with in vivo results.
COMPETITIVE KINETIC PROCESSES INFLUENCE A DYNAMIC SUPERSATURATION AND ABSORPTIVE FLUX
Methods that can analyze polymer dissolution, crystallization, and permeability across a membrane can provide a complete picture of the drug available for absorption. SS stability and phase separation are a consequence of the interplay between different competing kinetic processes (Fig. 3).
Flux measurement is influenced by dissolution, precipitation, and permeability, events responsible for the evolution of a supersaturated state of the drug. Permeation across the membrane relieves the supersaturated state of the thermodynamic drive to precipitate by reducing the DS. The mass transport across a membrane depends on the initial DS (16). Crystallization inhibition during SS is considered to be the most important step in maintaining drug SS. Other properties of a polymer are also important notably the rate of dissolution of the polymer. In a recent study, it was reported that the most efficient crystallization inhibitor used in the study was outperformed by a less efficient inhibitor in terms of increasing the in vivo rate and amount of drug absorbed (77). The interplay between crystallization inhibition and the rate of polymer dissolution was found to be responsible for sustained SS. Fast polymer dissolution allows drug release from ASDs, and sufficient polymer is made available in the dissolution media for crystallization inhibition. Polymer dissolution is also influenced by the physicochemical property of a drug (1). Phase separation occurs due to competitive kinetics between a polymer-controlled dissolution and drug-controlled dissolution with the former leading to LLPS (78). Drug loading in ASDs was found to be a determining factor with a low drug loading resulting in polymer-controlled drug release. There is a threshold value of drug loading below which LLPS occurs where drug release due to polymer dissolution dominates, resulting in high flux. At higher drug loading, the kinetics of matrix phase separation and crystallization is dominant with a resultant low flux. Different techniques used for studying these kinetic processes and drug-polymer interaction are highlighted in Table V.
The variability in solubility advantage among different approaches in ASDs has been ascribed to the rate of generation of SS (83). Methods to control the sudden surge of SS during dissolution can be a method to prevent rapid de-supersaturation by nucleation and crystallization (84). There exists a critical SS below which SS can be sustained without sudden de-supersaturation. The critical SS corresponds to a particular dose that can sustain the SS effect (85). Therefore, maintaining DS below a threshold level can sustain SS. The use of water-insoluble polymer to reduce the rate of supersaturation by a matrix diffusion method is an alternate strategy to maintain prolonged SS without the need for crystallization inhibitors (86, 87). The influence of drug permeability can have a profound influence on all the kinetic processes mentioned above since the DS and the molecular species formed during dissolution is determined by the drug, excipient, and the technology employed for the formulation of ASDs.
LIQUID–LIQUID PHASE SEPARATION (LLPS) AND ITS ROLE IN MEMBRANE TRANSPORT
Drugs that are slow crystallizers undergo LLPS to form a drug-rich nanodroplet phase which exists in metastable equilibrium with a drug lean solution phase (25, 88). For drugs that are fast crystallizers, the presence of polymer can retard crystallization and induce LLPS. The phase separation is predicted to occur near the amorphous solubility and depends on the pKa of the drug and the pH of the dissolution media which changes progressively in the GI tract (59). LLPS during the dissolution of ASDs generates constant flux across a membrane which has been attributed to the reservoir effect of the drug-rich nanodroplets formed during LLPS (25, 26). The nanodroplets formed by LLPS act as a reservoir to replenish the drug removed by mass transport across a membrane into the receptor compartment, thereby maintaining a constant flux. The duration of constant flux depends on the number of nanodroplet and the amount of drug in the nanodroplet phase (24). This is critical for the in vivo performance of ASDs because the formation of LLPS has shown to improve plasma exposure of drugs in animal studies, and recently LLPS has also been observed in the aspirated human intestinal fluid (57). The rank order of oral absorption of ASD formulations correlates with in vitro flux in the presence of biorelevant concentration of bile salts (32). Diffusion of a drug across the membrane stabilizes the nanodroplet phase by preventing crystallization from occurring. Excipients that can stabilize these drug-rich nanodroplets are thought to improve drug absorption without precipitation (89).
The polymer dissolution rate and the formation and stability of the nanodroplet phase have been suggested to be critical for the formulation optimization of ASDs (90). The size and the stability of the nanodroplet phase with respect to time depend on the excipient used and are important for sustained SS and constant flux (25). The polymer, depending on its property, is incorporated in the nanodroplet phase which correlates with the nanodroplet stability and SS stability (91). However, in a recent study, it has been reported that the extent to which polymer is incorporated in the nanodroplet phase is not the sole predictor of SS stability (62).
The DS has a major influence on the predictive power of in vitro dissolution, and therefore the decision on the dose setting is important for in vitro in vivo correlation (IVIVC). The DS can also influence LLPS during ASD dissolution. A high DS can dramatically influence crystallization without LLPS. Limited DS can lead to LLPS. LLPS has been observed in dissolution experiments with a permeability compartment. The presence of an absorption sink influences the DS due to mass transport across the membrane that reduces the thermodynamic drive for precipitation, thereby maintaining SS (92). An absorptive compartment is decisive for predictive dissolution. However, the surface area to volume is an important issue as the area/volume ratio in the intestinal tract of humans is 1.9 cm−1 to 2.3 cm−1 which is difficult to replicate in an in vitro setup which is typically < 0.5 cm−1 in a conventional side-by-side diffusion cell (93).
Excipients can have contradictory effects on the performance outcome. On the one hand, polymers/surfactants can stabilize SS and LLPS; on the other hand, they can reduce the thermodynamic activity of the drug which can influence its absorptive flux. In a recent report, it was observed that a combination of a polymer and surfactant was able to reduce the size of the nanodroplets formed during LLPS by its incorporation into the droplet phase (62). Incorporation of these excipients however reduces the thermodynamic activity with a resultant lowering of the DS and absorptive flux. Methods to screen excipients that maximize this thermodynamic drive are therefore required. It has been reported that the phase distribution of excipients determines the thermodynamic drive for mass transport across an absorptive membrane (62).
ROLE OF NON-SINK CONDITION IN DISSOLUTION OF ASDS
The ability of an ASD formulation to generate and sustain SS is studied under non-sink conditions (4). All the competing kinetic processes which occur during the dissolution of ASDs are influenced by the deviation from the sink condition. The degree of deviation from sink condition is contingent on dissolution conditions like the media volume, its composition, and dose of the drug. Therefore, a careful study of the conditions under which the dissolution is carried out is critical while designing a dissolution study (94). The onset of nucleation and crystallization and the rate and extent of drug SS depends on the non-sink condition of the study. Therefore, characterizing the level of sink condition is a critical aspect for the design of a dissolution study. The effect of SS on phase behavior can be studied under non-sink conditions which mimic the in vivo behavior (95) The USP also provides the flexibility to use non-sink dissolution, provided the scientific rationality of the study is justified. However, due to regulatory concerns, industries try to rely on compendial methodologies which are based on dissolution under sink condition. The problem of setting up specifications and regulations for carrying out non-sink dissolution has been a subject of discussion (87, 89). To account for the deviation from traditionally used sink condition, a dimensionless parameter called the sink index (SI) has been proposed (96).
SI is the ratio of Cs (solubility of the crystalline drug) to the drug concentration upon dissolution of the complete dose in the dissolution media with a volume V. SI is the reciprocal of the DS mentioned in Eq. 3. A perfect sink condition as defined by the USP corresponds to a SI > 3. A SI value of around 0.1 corresponds to a non-sink condition which accentuates the DS and corresponding increase in the rate of nucleation and crystallization demonstrating the spring and parachute effect. The shape of the dissolution curve depends on the SI (95). It is therefore critical to state the SI values as it is a key determinant of the kinetic processes occurring during dissolution.
Although non-sink dissolution can capture the kinetics of dissolution, nucleation, and crystallization, the lack of a permeability compartment can have a substantial effect on these kinetic processes which governs the evolution of a dynamic SS state (Fig. 3). Non-sink dissolution with an absorption sink has been used as a discriminatory tool to study ASD formulation which shows complex phase changes during dissolution (24). Dissolution under absorptive conditions has also been used to study the impact of the presence of seed crystals in an ASD formulation (97). A high SS shows a significant impact on drug crystallization despite the presence of a precipitation inhibitor in an absorption sink. At low SS, the impact is however reduced.
ROLE OF IN SILICO MODELLING AND SIMULATION APPROACHES
In silico modelling and simulation approaches are valuable tools to understand the effect of the drug, formulation, and physiological consideration on in vivo performance. Modelling using in silico methods can complement in vitro methods to reduce the complexity in media selection which can then be integrated with physiologically based pharmacokinetic (PBPK) models for in vivo prediction. Drug-related factors like pKa, partition coefficient, and permeability coefficient can be integrated to dissolution models to predict the rate of dissolution as a function of physiological and drug-related factors (67). Accurate estimation of dissolution and permeation is critical for the quantitative prediction of oral drug absorption. In silico absorption modelling is a powerful tool used to determine the rate-limiting step in oral absorption and to define the critical performance attribute of ASDs. Mechanistic aspects of in vitro dissolution and how it translates into in vivo performance are the most important concern and a challenge for the pharmaceutical industry. Application of biorelevant techniques and modern analytical tools for real-time analysis of kinetic and dynamics processes happening during dissolution studies can improve the predictive capacity of in silico methods (32). The nano colloidal and micellar form of the drug produced during the dissolution of ASDs when incorporated into in silico permeability simulation was found to agree with in vivo data than unmodified effective permeability. This represents the contribution of the nanoaggregates and colloids formed during dissolution on the performance of ASDs especially when absorption is UWL-limited (34). The process of intestinal drug precipitation during supersaturation is highly complex due to several factors which result in variability of in vitro dissolution. In silico test can be used to decipher the subtler aspects of intestinal dynamics critical for absorption (96, 98, 99). Complex molecular approaches are being used to simulate molecular interaction with biological membrane to gain insight into the transport mechanism to understand permeability at the molecular level (100, 101). Complex in silico models are used to predict overall drug absorption and plasma drug concentration taking into account all the physicochemical and physiological parameters.
Molecular dynamic simulation has been used to understand the dissolution and performance of ASDs. Simulation of interaction between a drug and bile salts and partitioning of the drug in the phospholipid bilayer has been simulated, and it is observed that hydrogen bond between drug and taurocholate determines the relative solubilization of the drug (102). It has been observed that during the dissolution of ASDs, the supersaturation stability of a drug in water is related to the relative H-bond between drug-drug, drug-polymer, and drug-solvent molecule (103).
PREDICTIVE ROLE OF DISSOLUTION METHODS IN AN ABSORPTIVE ENVIRONMENT
A dissolution method should be able to predict the in vivo absorption of the drug from a formulation (104). Forecasting the in vivo absorption of a drug from in vitro dissolution is a challenging task as it is confounded by several gastrointestinal and formulation variables which results in poor predictability (105). Regiospecific changes in the hydrodynamics and media composition in GIT like the UWL, media pH, and the presence of bile salts can influence product performance (106, 107). Physiologically relevant in vitro dissolution methods which can capture the biophysical and formulation aspects reproducibly require a permeability component in the dissolution setup. A one-compartment setup, conventionally used for dissolution testing, was found to overestimate drug precipitation than a two-compartment setup with a permeability component (108). Precipitation can be overestimated under non-absorptive conditions, both in the presence and absence of polymers (109). At high DS, precipitation event dominates which may compromise mass transport across the membrane. Therefore, controlling the DS during dissolution is associated with SS stabilization (104).
Drug concentration in the stomach while emptying into the intestine is a critical factor for in vivo performance of ASDs (33). The solubility, dissolution, and residence time of a drug and polymer in the stomach can affect in vivo exposure to the drug. For example, in the case of enteric polymers, pH has a significant impact on polymer dissolution and consequently on the drug release and absorption from the ASD (109). pH shift is also an important aspect of the predictive dissolution of weakly basic and acidic drugs. Non-linearity of drug absorption is often observed with supersaturating systems at different doses (110, 111). This has been attributed to dose-dependent increase in DS which accelerates the rate of precipitation and destabilizes SS, thereby reducing absorption and systemic availability of a drug (112,113,114).
CONCLUSIONS
Understanding the interplay between the competitive processes that determine the SS and phase behavior requires the development of a biopredictive dissolution method. Dissolution and absorption happen simultaneously in the intestinal lumen, and therefore, permeability is a critical component that governs all kinetic processes during dissolution of ASDs. The evolution of SS and LLPS is critical determinants of membrane flux and performance of ASDs. Apart from this, the role of surfactant as excipient in the formulation or as part of the physiological media can have a profound influence on the DS, LLPS, absorptive flux, nucleation, and crystallization of a drug. Absorptive dissolution has been shown to have a remarkable correlation with in vivo data in comparison to conventional non-sink closed dissolution system (115).
In silico methods are getting more and more acceptance from pharmaceutical industry and regulatory agencies for decision-making in the drug development process. Integrating in silico PBPK modelling with in vitro biorelevant data is gaining prominence among regulators and the pharmaceutical industry (116, 117). With the improvement of computational power and the understanding of complex dissolution of ASDs, the in silico methods will gain more and more regulatory acceptance.
Crystallization inhibition, maintaining physical and chemical stability, achieving the target in vivo drug concentration along with low cost and manufacturability are key attributes of an excipient that should be tailored according to the property of the selected drug. Recent literature shows that thermodynamic activity of the supersaturated solution during dissolution is a key determinant of the behavior of ASDs (50, 118). The thermodynamic activity of a drug is substantially influenced by solubilizing and polymeric excipients. In vitro dissolution methods are tools to determine the critical performance criteria which influence the choice of excipients for an ASD. Complex dissolution systems can however increase the operational variables, and therefore, dissolution tests should be simple to improve reproducibility, but it should capture the rate-limiting events in the absorption of a drug. The complex nature of the interaction between drug, polymer, and surfactant during dissolution impacts product performance of ASDs. Permeability is a critical component for translating in vitro methods to the in vivo performance by developing IVIVC models that can reduce product failures and help in the risk assessment of ASDs.
References
Arce FA, Setiawan N, Campbell HR, Lu X, Nethercott MJ, Bummer P, et al. Toward developing discriminating dissolution methods for formulations containing nanoparticulates in solution: the impact of particle drift and drug activity in solution. Mol Pharm. 2020;17(11):4125–40. https://doi.org/10.1021/acs.molpharmaceut.0c00599.
Butler JM, Dressman JB. The developability classification system: application of biopharmaceutics concepts to formulation development. J Pharm Sci. 2010;99:4940–54. https://doi.org/10.1002/jps.22217.
Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–6. https://doi.org/10.1038/nrd1470.
Ashwathy P, Anto AT, Sudheesh MS. A mechanistic review on the dissolution phase behavior and supersaturation stabilization of amorphous solid dispersions. Drug Dev Ind Pharm. 2021;47:1–11. https://doi.org/10.1080/03639045.2021.1879843.
Vidhya KM, Saranya TR, Sreelakshmy KR, Nair AS, Nair SC. Pharmaceutical solid dispersion technology: a promising tool to enhance oral bioavailability. Int Res J Pharm ApplSci. 2013;3:214–8.
Shammika P, Aneesh TP, Viswanad V. Solubility enhancement of synthesized quinazolinone derivative by solid dispersion technique. Int J Pharm Sci Rev Res. 2016;41:197–206.
Buckley ST, Frank KJ, Fricker G, Brandl M. Biopharmaceutical classification of poorly soluble drugs with respect to “enabling formulations.” Eur J Pharm Sci. 2013;50:8–16.
Frank KJ, Westedt U, Rosenblatt KM, Hölig P, Rosenberg J, Mägerlein M, et al. What Is the mechanism behind increased permeation rate of a poorly soluble drug from aqueous dispersions of an amorphous solid dispersion? J Pharm Sci. 2014;103:1779–86.
Fong SYK, Bauer-Brandl A, Brandl M. Oral bioavailability enhancement through supersaturation: an update and meta-analysis. Expert Opin Drug Deliv. 2017;14:403–26.
Guzman H, Tawa M, Zhang Z, Ratanabanangkoon P, Shaw P, Mustonen P, Gardner C, Chen H, Moreau JP, Almarsson O, Remenar J. Spring and parachute approach to designing solid celecoxib formulations having enhanced oral absorption. AAPS J. 2004;6:T2189.
Yamashita T, Ozaki S, Kushida I. Solvent shift method for anti-precipitant screening of poorly soluble drugs using biorelevant medium and dimethyl sulfoxide. Int J Pharm. 2011;419(1):170–4. https://doi.org/10.1016/j.ijpharm.2011.07.045.
Palmelund H, Madsen CM, Plum J, Mullertz A, Rades T. Studying the propensity of compounds to supersaturate: a practical and broadly applicable approach. J Pharm Sci. 2016;105:3021–9. https://doi.org/10.1016/j.xphs.2016.06.016.
Plum J, Bavnhoj CG, Eliasen JN, Rades T, Müllertz A. Comparison of induction methods for supersaturation: amorphous dissolution versus solvent shift. Eur J Pharm Biopharm. 2020;152:35–43. https://doi.org/10.1016/j.ejpb.2020.04.017.
He X, Kadomura S, Takekuma Y, Sugawara M, Miyazaki K. A new system for the prediction of drug absorption using a pH controlled Caco-2 model: evaluation of pH-dependent soluble drug absorption and pH-related changes in absorption. J PharmSci. 2004;93:71–7.
He X, Sugawara M, Kobayashi M, Takekuma Y, Miyazaki K. An in vitro system for prediction of oral absorption of relatively water-soluble drugs and ester prodrugs. Int J Pharm. 2003;263:35–44.
Bevernage J, Brouwers J, Annaert P, Augustijns P. Drug precipitation-permeation interplay: supersaturation in an absorptive environment. Eur J Pharm Biopharm. 2012;82(2):424–8. https://doi.org/10.1016/j.ejpb.2012.07.009.
Sironi D, Christensen M, Rosenberg J, Bauer-Brandl A, Brandl M. Evaluation of a dynamic dissolution/permeation model: mutual influence of dissolution and barrier-flux under non-steady state conditions. Int J Pharm. 2017;522:50–7. https://doi.org/10.1038/nrd1470.
Kataoka M, Masaoka Y, Yamazaki Y, Sakane T, Sezaki H, Yamashita S. In vitro system to evaluate oral absorption of poorly, water-soluble drugs: simultaneous analysis on dissolution and permeation of drugs. Pharm Res. 2003;20:1674–80.
Kataoka M, Sugano K, Mathews C, Wong JW, Jones KL, et al. Application of dissolution/permeation system for evaluation of formulation effect on oral absorption of poorly water-soluble drugs in drug development. Pharm Res. 2012;29:1485–94.
Tajiri T, Morita S, Sakamoto R, Mimura H, Ozaki Y, et al. Developing dissolution testing methodologies for extended-release oral dosage forms with supersaturating properties. Case example: solid dispersion matrix of indomethacin. Int J Pharm. 2015;490:368–74.
Shi Y, Gao P, Gong Y, Ping H. Application of a biphasic test for characterization of in vitro drug release of immediate release formulations of celecoxib and its relevance to in vivo absorption. Mol Pharm. 2010;7:1458–65.
Jacobsen AC, Krupa A, Brandl M, Bauer-Brandl A. High-throughput dissolution/permeation screening a 96-well two-compartment microplate approach. Pharmaceutics. 2019;11(5):227. https://doi.org/10.3390/pharmaceutics11050227.
Puppolo MM, Hughey JR, Dillon T, Storey D, Jansen-Varnum S. Biomimetic dissolution: a tool to predict amorphous solid dispersion performance. AAPS PharmSciTech. 2017;18(8):2841–53. https://doi.org/10.1208/s12249-017-0783-4.
Hate SS, Reutzel-Edens SM, Taylor LS. Insight into amorphous solid dispersion performance by coupled dissolution and membrane mass transfer measurements. Mol Pharm. 2019;16:448–61. https://doi.org/10.1021/acs.molpharmaceut.8b01117.
Indulkar AS, Gao Y, Raina SA, Zhang GGZ, Taylor LS. Exploiting the phenomenon of liquid-liquid phase separation for enhanced and sustained membrane transport of a poorly water-soluble drug. Mol Pharm. 2016;13:2059–69. https://doi.org/10.1021/acs.molpharmaceut.6b00202.
Raina SA, Zhang GG, Alonzo DE, Wu J, Zhu D, Catron ND, Gao Y, Taylor LS. Enhancements and limits in drug membrane transport using supersaturated solutions of poorly water-soluble drugs. J Pharm Sci. 2014;103(9):2736–48. https://doi.org/10.1002/jps.23826.
Li J, Li LB, Nessah N, Huang Y, Hidalgo C, et al. Simultaneous analysis of dissolution and permeation profiles of nanosized and microsized formulations of indomethacin using the in vitro dissolution absorption system. J Pharm Sci. 2019;108(7):2334–40.
Li ZQ, Tian S, Gu H, Wu ZG, Nyagblordzro M, et al. In vitro-in vivo predictive dissolution-permeation-absorption dynamics of highly permeable drug extended-release tablets via drug dissolution/absorption simulating system and pH alteration. AAPS Pharm Sci Tech. 2018;19(4):1882–93. https://doi.org/10.1208/s12249-018-0996-1.
Porat D, Dahan A. Active intestinal drug absorption and the solubility-permeability interplay. Int J Pharm. 2018;537(1–2):84–93. https://doi.org/10.1016/j.ijpharm.2017.10.058.
Korjamo T, Heikkinen AT, Mönkkönen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharm Sci. 2009;98(12):4469–79. https://doi.org/10.1002/jps.21762.
Stewart AM, Grass EM, Mudie DM, Morgen MM, Friesen DT, Vodak DT. Development of a biorelevant, material-sparing membrane flux test for rapid screening of bioavailability-enhancing drug product formulations. Mol Pharm. 2017;14(6):2032–46. https://doi.org/10.1021/acs.molpharmaceut.7b00121.
Stewart AM, Grass ME, Brodeur TJ, Goodwin AK, Morgen MM, Friesen DT, Vodak DT. Impact of drug-rich colloids of itraconazole and HPMCAS on membrane flux in vitro and oral bioavailability in rats. Mol Pharm. 2017;14(7):2437–49. https://doi.org/10.1021/acs.molpharmaceut.7b00338.
Stewart AM, Yates I, Mudie D, Pivette P, Goodwin A, Sarmiento A, et al. Mechanistic study of belinostat oral absorption from spray-dried dispersions. J Pharm Sci. 2019;108:326–36. https://doi.org/10.1016/j.xphs.2018.09.031.
Stewart AM, Grass ME. Practical approach to modelling the impact of amorphous drug nanoparticles on the oral absorption of poorly soluble drugs. Mol Pharm. 2020;17(1):180–9. https://doi.org/10.1021/acs.molpharmaceut.9b00889.
Sugano K. Estimation of effective intestinal membrane permeability considering bile micelle solubilisation. Int J Pharm. 2008;368:116–22. https://doi.org/10.1016/j.ijpharm.2008.10.001.
Fagerberg JH, Bergstrom CA. Intestinal solubility and absorption of poorly water soluble compounds: predictions, challenges and solutions. Ther Deliv. 2015;6:935–59. https://doi.org/10.4155/tde.15.45.
Harmon P, Galipeau K, Xu W, Brown C, Wuelfing WP. Mechanism of dissolution-induced nanoparticle formation from a copovidone based amorphous solid dispersion. Mol Pharm. 2015;13(5):1467–81. https://doi.org/10.1021/acs.molpharmaceut.5b00863.
Dahan A, Miller JM. The solubility-permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012;14(2):244–51. https://doi.org/10.1208/s12248-012-9337-6.
Dahan A, Beig A, Lindley D, Miller JM. The solubility–permeability interplay and oral drug formulation design: two heads are better than one. Adv Drug Deliv Rev. 2016;101:99–107. https://doi.org/10.1016/j.addr.2016.04.018.
Beig N, Lindley D, Miller JM, Agbaria R, Dahan A. Hydrotropic solubilization of lipophilic drugs for oral delivery: the effects of urea and nicotinamide on carbamazepine solubility-permeability interplay. Front Pharmacol. 2016;7:379. https://doi.org/10.3389/fphar.2016.00379.
Beig A, Fine-Shamir N, Lindley D, Miller JM, Dahan A. Advantageous solubility-permeability interplay when using amorphous solid dispersion formulation for the BCS class IV p-gp substrate rifaximin: simultaneous increase of both the solubility and the permeability. AAPS J. 2017;19(3):806–13. https://doi.org/10.1208/s12248-017-0052-1.
Dahan A, Beig A, Ioffe-Dahan V, Agbaria R, Miller JM. The twofold advantage of the amorphous form as an oral drug delivery practice for lipophilic compounds: Increased apparent solubility and drug flux through the intestinal membrane. AAPS J. 2013;15(2):347–53. https://doi.org/10.1208/s12248-012-9445-3.
Miller JM, Beig A, Carr RA, Spence JK, Dahan A. A win-win solution in oral delivery of lipophilic drugs: Supersaturation via amorphous solid dispersions increases apparent solubility without sacrifice of intestinal membrane permeability. Mol Pharm. 2012;9(7):2009–16. https://doi.org/10.1021/mp300104s.
Beig A, Fine-Shamir N, Porat D, Lindley D, Miller JM, Dahan A. Concomitant solubility-permeability increase: vitamin E TPGS vs. amorphous solid dispersion as oral delivery systems for etoposide. Eur J Pharm Biopharm. 2017;121:97–103. https://doi.org/10.1016/j.ejpb.2017.09.012.
Frank KJ, Westedt U, Rosenblatt KM, Hölig P, Rosenberg J, Mägerlein M, Fricker G, Brandl M. The amorphous solid dispersion of the poorly soluble ABT-102forms nano/microparticulate structures in aqueous medium: impact on solubility. Int J Nanomedicine. 2012;7:5757–68. https://doi.org/10.2147/IJN.S36571.
Nawroth T, Buch P, Buch K, Langguth P, Schweins R. Liposome formation from bile salt-lipid micelles in the digestion and drug delivery model FaSSIF(mod) estimated by combined time-resolved neutron and dynamic light scattering. Mol Pharm. 2011;8(6):2162–72. https://doi.org/10.1021/mp100296w.
Khoshakhlagh P, Johnson R, Langguth P, Nawroth T, Schmueser L, Hellmann N, Decker H, Szekely NK. Fasted-state simulated intestinal fluid “FaSSIF-C”, a cholesterol containing intestinal model medium for in vitro drug delivery development. J Pharm Sci. 2015;104(7):2213–24. https://doi.org/10.1002/jps.24470.
Li Z, Johnson LM, Ricarte RG, Yao LJ, Hillmyer MA, Bates FS, Lodge TP. Enhanced performance of blended polymer excipients in delivering a hydrophobic drug through the synergistic action of micelles and HPMCAS. Langmuir. 2017;33:2837–48. https://doi.org/10.1021/acs.langmuir.7b00325.
Li Z, Lenk TI, Yao LJ, Bates FS, Lodge TP. Maintaining hydrophobic drug supersaturation in a micelle corona reservoir. Macromolecules. 2018;51:540–51. https://doi.org/10.1021/acs.macromol.7b02297.
Wilson V, Lou X, Osterling DJ, Stolarik DF, Jenkins G, Gao W, Zhang GGZ, Taylor LS. Relationship between amorphous solid dispersion in vivo absorption and in vitro dissolution: phase behaviour during dissolution, speciation, and membrane mass transport. J Control Rel. 2018;292:172–82. https://doi.org/10.1016/j.jconrel.2018.11.003.
Higuchi T. Physical chemical analysis of percutaneous absorption process from creams and ointments. J Soc Cosmet Chem. 1960;11:85–97.
Myerson AS. Handbook of industrial crystallization. 2nd ed. Woburn: Butterworth-Heinemann, New Delhi; 2000.
Raina SA, Zhang GGZ, Alonzo DE, Wu J, Zhu D, Catron ND, Gao Y, Taylor LS. Impact of solubilizing additives on supersaturation and membrane transport of drugs. Pharm Res. 2015;32(10):3350–64. https://doi.org/10.1007/s11095-015-1712-4.
Elkhabaz A, Moseson DE, Brouwers J, Augustijns P, Taylor LS. Interplay of supersaturation and solubilization: lack of correlation between concentration-based supersaturation measurements and membrane transport rates in simulated and aspirated human fluids. Mol Pharm. 2019;16(12):5042–53. https://doi.org/10.1021/acs.molpharmaceut.9b00956.
Ozaki S, Minamisono T, Yamashita T, Kato T, Kushida I. Supersaturation-nucleation behaviour of poorly soluble drugs and its impact on the oral absorption of drugs in thermodynamically high-energy forms. J Pharm Sci. 2012;101(1):214–22. https://doi.org/10.1002/jps.22760.
LakshmanJP Cao Y, Kowalski J, Serajuddin ATM. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol Pharm. 2008;5:994–1002. https://doi.org/10.1021/mp8001073.
Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int J Pharm. 2007;328:119–29. https://doi.org/10.1016/j.ijpharm.2006.08.010.
Solanki NG, Lam K, Tahsin M, Gumaste SG, Shah AV, Serajuddin ATM. Effects of surfactants on itraconazole-HPMCAS solid dispersion prepared by hot melt extrusion: miscibility and drug release. J Pharm Sci. 2019;108:1453–65. https://doi.org/10.1016/j.xphs.2018.10.058.
Indulkar AS, Box KJ, Taylor R, Ruiz R, Taylor LS. pH-dependent liquid-liquid phase separation of highly supersaturated solutions of weakly basic drugs. Mol Pharm. 2015;12:2365–77. https://doi.org/10.1021/acs.molpharmaceut.5b00056.
Mosquera-Giraldo LI, Trasi NS, Taylor LS. Impact of surfactants on the crystal growth of amorphous celecoxib. Int J Pharm. 2014;461:251–7. https://doi.org/10.1016/j.ijpharm.2013.11.057.
Chen Y, Liu C, Chen Z, Su C, Hageman M, Hussain M, et al. Drug–polymer–water interaction and its implication for the dissolution performance of amorphous solid dispersions. Mol Pharm. 2015;12:576–89. https://doi.org/10.1021/mp500660m.
Ueda K, Taylor LS. Partitioning of surfactant into drug-rich nanodroplets and its impact on drug thermodynamic activity and droplet size. J Control Rel. 2021;330:229–43. https://doi.org/10.1016/j.jconrel.2020.12.018.
Sugano K. Theoretical comparison of hydrodynamic diffusion layer models used for dissolution simulation in drug discovery and development. Int J Pharm. 2008;363:73–7. https://doi.org/10.1016/j.ijpharm.2008.07.002.
Di Cagno MP, Stein PC. Studying the effect of solubilizing agents on drug diffusion through the unstirred water layer (UWL) by localized spectroscopy. Eur J Pharm Biopharm. 2019;139:205–12. https://doi.org/10.1016/j.ejpb.2019.04.005.
Ueda K, Higashi K, Limwikrant W, Sekine S, Horie T, et al. Mechanistic differences in permeation behavior of supersaturated and solubilized solutions of carbamazepine revealed by nuclear magnetic resonance measurements. Mol Pharm. 2012;9(11):3023–33. https://doi.org/10.1021/mp300083e.
Batrakova EV, Han HY, Alakhov VY, Miller DW, Kabanov AV. Effects of pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm Res. 1998;15(6):850–5. https://doi.org/10.1023/a:1011964213024.
Boyd BJ, Bergström CAS, Vinarov Z, Kuentz M, Brouwers J, Augustijns P, Brandl M, Bernkop-Schnürch A, Shrestha N, Préat V, Müllertz A, Bauer-Brandl A, Jannin V. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur J Pharm Sci. 2019;1(137):104967. https://doi.org/10.1016/j.ejps.2019.104967.
Vinarov Z, Katev V, Burdzhiev N, Tcholakova S, Denkov N. Effect of surfactant-bile interactions on the solubility of hydrophobic drugs in biorelevant dissolution media. Mol Pharm. 2018;15(12):5741–53. https://doi.org/10.1021/acs.molpharmaceut.8b00884.
Rosen MJ. Surfactants and interfacial phenomena. 3rd ed. New Jersey: Wiley; 2004.
Feng D, Peng T, Huang Z. Polymer-surfactant system based amorphous solid dispersion: precipitation inhibition and bioavailability enhancement of itraconazole. Pharmaceutics. 2018;10(2):53. https://doi.org/10.3390/pharmaceutics10020053.
Dintaman JM, Silverman JA. Inhibition of p-glycoprotein by d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm Res. 1999;16(10):1550–6. https://doi.org/10.1023/a:1015000503629.
Prasad YR, Puthli S, Eaimtrakarn S, Ishida M, Yoshikawa Y, Shibata N, Takada K. Enhanced intestinal absorption of vancomycin with labrasol and D-α-tocopheryl PEG 1000 succinate in rats. Int J Pharm. 2003;250(1):181–90. https://doi.org/10.1016/s0378-5173(02)00544-6.
Sugano K. Possible reduction of effective thickness of intestinal unstirred water layer by particle drifting effect. Int J Pharm. 2010;387:103–9. https://doi.org/10.1016/j.ijpharm.2009.12.014.
Roos C, Westergren J, Dahlgren D, Lennernas H, Sjogren E. Mechanistic modelling of intestinal drug absorption - the in vivo effects of nanoparticles, hydrodynamics, and colloidal structures. Eur J Pharm Biopharm. 2018;133:70–6. https://doi.org/10.2147/ijn.s596.
Kesisoglou F, Wang M, Galipeau K, Harmon P, Okoh G, Xu W. Effect of amorphous nanoparticle size on bioavailability of anacetrapib in dogs. J Pharm Sci. 2019;108(9):2917–25. https://doi.org/10.1016/j.xphs.2019.04.006.
Borbás E, Kádár S, Tsinman K, Tsinman O, Csicsák D, Takács-Novák K, et al. Prediction of bioequivalence and food effect using flux- and solubility-based methods. Mol Pharm. 2019;16(10):4121–30. https://doi.org/10.1021/acs.molpharmaceut.9b00406.
Wilson VR, Mugheirbi NA, Mosquera-Giraldo LI, Deac A, Moseson DE, Smith DT, et al. Interaction of polymers with enzalutamide nanodroplets-impact on droplet properties and induction times. Mol Pharm. 2021;18(3):836–49. https://doi.org/10.1021/acs.molpharmaceut.0c00833.
Saboo S, Mugheirbi NA, Zemlyanov DY, Kestur US, Taylor LS. Congruent release of drug and polymer: a “sweet spot” in the dissolution of amorphous solid dispersions. J Control Rel. 2019;298:68–82. https://doi.org/10.1016/j.jconrel.2019.01.039.
Morrison JS, Nophsker MJ, Haskell RJ. A combination turbidity and supernatant microplate assay to rank-order the supersaturation limits of early drug candidates. J Pharm Sci. 2014;103(10):3022–32. https://doi.org/10.1002/jps.24090.
Mosquera-Giraldo LI, Taylor LS. Glass-liquid phase separation in highly supersaturated aqueous solutions of telaprevir. Mol Pharm. 2015;12:496–503. https://doi.org/10.1021/mp500573z.
Tres F, Hall SD, Mohutsky MA, Taylor LS. Monitoring the phase behaviour of supersaturated solutions of poorly water-soluble drugs using fluorescence techniques. J Pharm Sci. 2018;107:94–102. https://doi.org/10.1016/j.xphs.2017.10.002.
Ilevbare GA, Liu H, Pereira J, Edgar KJ, Taylor LS. Influence of additives on the properties of nano droplets formed in highly supersaturated aqueous solutions of ritonavir. Mol Pharm. 2013;10:3392–403. https://doi.org/10.1021/mp400228x.
Sun DD, Lee PI. Evolution of supersaturation of amorphous pharmaceuticals: the effect of rate of supersaturation generation. Mol Pharm. 2013;10:4330–46. https://doi.org/10.1021/mp400439q.
Sun DD, Ju TR, Lee PI. Enhanced kinetic solubility profiles of indomethacin amorphous solid dispersions in poly(2-hydroxyethyl methacrylate) hydrogels. Eur J Pharm Biopharm. 2012;81(1):149–58. https://doi.org/10.1016/j.ejpb.2011.12.016.
Han YR, Lee PI. Effect of extent of supersaturation on the evolution of kinetic solubility profiles. Mol Pharm. 2017;14:206–20. https://doi.org/10.1021/acs.molpharmaceut.6b00788.
Li Z, Van Zee NJ, Bates FS, et al. Polymer nanogels as reservoirs to inhibit hydrophobic drug crystallization. ACS Nano. 2019;13:1232–43. https://doi.org/10.1021/acsnano.8b06393.
Sun DD, Lee PI. Crosslinked hydrogels – a promising class of insoluble solid molecular dispersion carriers for enhancing the delivery of poorly soluble drugs. Acta Pharm Sin B. 2014;4:26–36. https://doi.org/10.1016/j.apsb.2013.12.002.
Sun DD, Lee PI. Probing the mechanisms of drug release from amorphous solid dispersions in medium-soluble and medium-insoluble carriers. J Control Release. 2015;211:85–93. https://doi.org/10.1016/j.jconrel.2015.06.004.
Ilevbare GA, Taylor LS. Liquid–liquid phase separation in highly supersaturated aqueous solutions of poorly water-soluble drugs: implications for solubility enhancing formulations. Cryst Growth Des. 2013;13(4):1497–509. https://doi.org/10.1021/cg301679h.
Ueda K, Higashi K, Moribe K. Mechanistic elucidation of formation of drug-rich amorphous nanodroplets by dissolution of the solid dispersion formulation. Int J Pharm. 2019;561:82–92. https://doi.org/10.1016/j.ijpharm.2019.02.034.
Ueda K, Higashi K, Moribe K. Direct NMR monitoring of phase separation behavior of highly supersaturated nifedipine solution stabilized with hypromellose derivatives. Mol Pharm. 2017;14:2314–22. https://doi.org/10.1021/acs.molpharmaceut.7b00178.
Tsume Y, Matsui K, Searls AL, Takeuchi S, Amidon GE, Sun D, Amidon GL. The impact of supersaturation level for oral absorption of BCS class IIb drugs, dipyridamole and ketoconazole, using in vivo predictive dissolution system: Gastrointestinal Simulator (GIS). Eur J Pharm Sci. 2017;102:126–39. https://doi.org/10.1016/j.ejps.2017.02.042.
Mudie DM, Shi Y, Ping H, Gao P, Amidon GL, Amidon GE. Mechanistic analysis of solute transport in an in vitro physiological two-phase dissolution apparatus. Biopharm Drug Dispos. 2012;33:378–402. https://doi.org/10.1002/bdd.1803.
Sun DD, Lee PI. Haste makes waste: the interplay between dissolution and precipitation of supersaturating formulations. AAPS Journal. 2015;17(6):1317–26. https://doi.org/10.1208/s12248-015-9825-6.
Speybroeck VM, Mellaerts R, Mols R, Thi TD, Martens JA, Humbeeck JV, Annaert P, Mooter GVD, Augustijns P. Enhanced absorption of the poorly soluble drug fenofibrate by tuning its release rate from ordered mesoporous silica. Eur J Pharm Sci. 2010;41(5):623–30. https://doi.org/10.1016/j.ejps.2010.09.002.
Sun DD, Wen H, Taylor LS. Non-sink dissolution conditions for predicting product quality and in vivo performance of supersaturating drug delivery systems. J Pharm Sci. 2016;105:1–12. https://doi.org/10.1016/j.xphs.2016.03.024.
Hate SS, Reutzel-Edens SM, Taylor LS. Absorptive dissolution testing: an improved approach to study the impact of residual crystallinity on the performance of amorphous formulations. J Pharm Sci. 2020;109(3):1312–23. https://doi.org/10.1016/j.xphs.2019.11.016.
Takano R, Takata N, Saito R, Furumoto K, Higo S, Hayashi Y, Machida M, Aso Y, Yamashita S. Quantitative analysis of the effect of supersaturation on in vivo drug absorption. Mol Pharmaceutics. 2010;7:1431–40. https://doi.org/10.1021/mp100109a.
Sugano K. Computational oral absorption simulation of free base drugs. Int J Pharm. 2010;398:73–82. https://doi.org/10.1016/j.ijpharm.2010.07.027.
Awoonor-Williams E, Rowley CN. Molecular simulation of nonfacilitated membrane permeation. Biochim Biophys Acta (BBA) Biomembr. 2016;1858:1672–87. https://doi.org/10.1016/j.bbamem.2015.12.014.
Lee CT, Comer J, Herndon C, Leung N, Pavlova A, Swift RV, Tung C, Rowley CN, Amaro RE, Chipot C. Simulation-based approaches for determining membrane permeability of small compounds. J Chem Inf Model. 2016;56:721–33. https://doi.org/10.1021/acs.jcim.6b00022.
Holmboe M, Larsson P, Anwar J, Bergstrom CAS. Partitioning into colloidal structures of fasted state intestinal fluid studied by molecular dynamics simulations. Langmuir. 2016;32(48):12732–40. https://doi.org/10.1021/acs.langmuir.6b03008.
Edueng K, Mahlin D, Larsson P, Bergstrom CAS. Mechanism-based selection of stabilization strategy for amorphous formulations: insights into crystallization pathways. J Control Release. 2017;256:193–202. https://doi.org/10.1016/j.jconrel.2017.04.015.
Hens B, Kataoka M, Ueda K, Ping Gao Y, Tsume P, Augustijns K, Kawakami S, Yamashita S. Biopredictive in vitro testing methods to assess intestinal drug absorption from supersaturating dosage forms. J Drug Deliv Sci Technol. 2020;56:101275. https://doi.org/10.1016/j.jddst.2019.101275.
Carlert S, Pålsson A, Hanisch G, von Corswant C, Nilsson C, Lindfors L, et al. Predicting intestinal precipitation-a case example for a basic BCS class II drug. Pharm Res. 2010;27:2119–30. https://doi.org/10.1007/s11095-010-0213-8.
Chen J, Mosquera-Giraldo LI, Ormes JD, Higgins JD, Taylor LS. Bile salts as crystallization inhibitors of supersaturated solutions of poorly water-soluble compounds. Cryst Growth Des. 2017;15:2593–7. https://doi.org/10.1021/acs.cgd.5b00392.
Li N, Giraldo LIM, Borca CH, Ormes JD, Lowinger M, Higgins JD, Slipchenko LV, Taylor LS. A comparison of the crystallization inhibition properties of bile salts. Cryst Growth Des. 2016;16:7286–300. https://doi.org/10.1021/acs.cgd.6b01470.
Tanaka Y, Kawakami A, Nanimatsu A, Horio M, Matsuoka J, Wada T, Kasaoka S, Yoshikawa H. In vivo evaluation of supersaturation/precipitation/re-dissolution behaviour of cinnarizine, a lipophilic weak base, in the gastrointestinal tract: the key process of oral absorption. Eur J Pharm Sci. 2017;96:464–71. https://doi.org/10.1016/j.ejps.2016.10.023.
Alhayali A, Selo MA, Ehrhardt C, Velaga S. Investigation of supersaturation and in vitro permeation of the poorly water-soluble drug ezetimibe. Eur J Pharm Sci. 2018;117:147–53. https://doi.org/10.1016/j.ejps.2018.01.047.
Psachoulias D, Vertzoni M, Butler J, Busby D, Symillides M, Dressman J, Reppas C. An in vitro methodology for forecasting luminal concentrations and precipitation of highly permeable lipophilic weak bases in the fasted upper small intestine. Pharm Res. 2012;29:3486–98. https://doi.org/10.1007/s11095-012-0844-z.
Knopp MM, Chourak N, Khan F, Wendelboe J, Langguth P, Rades T, Holm R. Effect of polymer type and drug dose on the in vitro and in vivo behavior of amorphous solid dispersions. Eur J Pharm Biopharm. 2016;105:106–14. https://doi.org/10.1016/j.ejpb.2016.05.017.
Bevernage J, Brouwers J, Clarysse S, Vertzoni M, Tack J, Annaert P, Augustijns P. Drug supersaturation in simulated and human intestinal fluids representing different nutritional states. J Pharm Sci. 2010;99:4525–34. https://doi.org/10.1002/jps.22154.
Ueda K, Higashi K, Kataoka M, Yamashita S, Yamamoto K, Moribe K. Inhibition mechanism of hydroxypropyl methylcellulose acetate succinate on drug crystallization in gastrointestinal fluid and drug permeability from a supersaturated solution. Eur J Pharm Sci. 2014;62:293–300. https://doi.org/10.1016/j.ejps.2014.06.007.
Kawakami K, Sato K, Fukushima M, Miyazaki A, Yamamura Y, Sakuma S. Phase separation of supersaturated solution created from amorphous solid dispersions: Relevance to oral absorption. Eur J Pharm Biopharm. 2018;132:146–56. https://doi.org/10.1016/j.ejpb.2018.09.014.
Jackson MJ, Kestur US, Hussain MA, Taylor LS. Dissolution of danazol amorphous solid dispersions: supersaturation and phase behaviour as a function of drug loading and polymer type. Mol Pharm. 2016;13(1):223–31. https://doi.org/10.1021/acs.molpharmaceut.5b00652.
Wagner C, Zhao P, Pan Y, Hsu V, Grillo J, Huang S, Sinha V. Application of physiologically based pharmacokinetic (PBPK) modeling to support dose selection: Report of an FDA public workshop on PBPK. CPT Pharmacomet Syst Pharmacol. 2015;4:226–30. https://doi.org/10.1002/psp4.33.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, Jamei M, Lloyd R, Pepin X, Rostami-Hodjegan A, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2013;57:300–21. https://doi.org/10.1016/j.ejps.2013.09.008.
Berben P, Brouwers J, Augustijns P. The artificial membrane insert system as predictive tool for formulation performance evaluation. Int J Pharm. 2018;537:22–9. https://doi.org/10.1016/j.ijpharm.2017.12.025.
Funding
MSS is thankful to the management of Amrita Vishwa Vidyapeetham for the financial seed grant support [AVV-ASP-SG/31/10/19].
Author information
Authors and Affiliations
Contributions
GR: literature survey and writing the original draft. MSS: conceptualization, reviewing, and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramachandran, G., Sudheesh, M.S. Role of Permeability on the Biopredictive Dissolution of Amorphous Solid Dispersions. AAPS PharmSciTech 22, 243 (2021). https://doi.org/10.1208/s12249-021-02125-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02125-4