ABSTRACT
Purpose
The aim of the present study is to evaluate the formulation effect on the oral absorption of poorly water-soluble drugs using a dissolution/permeation system (D/P system).
Methods
This D/P system, consisting of apical and basal chambers and a Caco-2 cell monolayer mounted between chambers, can be used to perform simultaneous analysis of drug dissolution and permeation process of drugs applied as various dosage forms. Oral administration study with rats was also performed for both drugs as the same dosage forms.
Results
When danazol, a low-soluble and high-permeable drug, was applied to the D/P system as various formulations, dissolved and permeated amounts were significantly high compared with those from a suspension form. On the other hand, whereas the dissolved amount of pranlukast, a low-soluble and low-permeable drug, was significantly increased by formulations, there were no significant changes observed in the permeated amount between suspension and formulation. The oral availability of danazol was significantly increased by formulations but not pranlukast, which corresponded well to in vitro evaluations.
Conclusion
These results indicated that the D/P system might be applicable for selection of formulation on the basis of physicochemical drug properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The number of poorly water-soluble drug candidates in drug discovery has recently increased (1). Their limited water solubility often causes low and variable oral absorption because poor dissolution of drugs in the gastrointestinal (GI) fluid is insufficient for drug absorption to show a pharmacological effect. Except for first-pass metabolism, the rate and amount of drug absorption are expressed by the following equations:
where Peff is the effective permeability of drugs, S is the surface area of the intestinal membrane, and CGI is the luminal concentration of drugs. AUCGI expresses the area under the drug concentration-time curve in the GI tract during the intestinal transit time, t. In order to estimate the fraction of absorbed drugs (Fa) after oral administration, these parameters in the equations should be assessed. In particular, in the case of poorly water-soluble drugs, solubility and dissolution rate of a drug to the gastrointestinal fluid are key factors to consider the extent of oral absorption. Maintenance of a high concentration of dissolved drugs in the gastrointestinal fluid results in high Fa. Hence, various techniques to enhance drug dissolution have been used to improve the oral absorption of poorly water-soluble drugs (2,3).
Recently, many researchers have focused on supersaturation-generating formulations to improve the oral absorption of poorly water-soluble drugs. Drugs in a state of supersaturation are kinetically soluble in solution at a concentration above their thermodynamic equilibrium solubility. If a supersaturated drug solution exists in the intestinal fluid for a sufficient length of time to be absorbed, it may result in an enhanced flux across the intestinal wall and thus improve the absorption. Several formulations that induce supersaturation in vitro and enhance oral absorption in vivo include amorphous ones, such as solid dispersion (4–6), crystalline salts (7), cocrystals (8,9), higher-energy polymorphic forms (10), and self-emulsified drug delivery system (SEDDS) (11). Moreover, the existence of several polymers in SEDDS (supersaturable SEDDS, S-SEDDS) such as hydroxypropyl methylcellulose (HPMC) at a supersaturable state of drugs can enhance the oral absorption of poorly water-soluble drugs (12,13).
In order to achieve successful drug development for oral use, the effect of such techniques on the oral absorption of poorly water-soluble drug candidates in humans should be assessed in advance of pre-clinical and clinical studies. In particular, drug concentration at supersaturable state and its sustained period in the intestinal fluid are important factors to determine the oral absorption of drugs from a supersaturation-generating formulation. However, it is still difficult to evaluate quantitatively what effect supersaturation has on the enhanced absorption because these formulations often increase not only the dissolved concentration but also the dissolution rate of drugs. In addition, the in vitro/in vivo correlation of supersaturable formulation has not yet been understood clearly.
We have developed an in vitro system for simultaneous evaluation of drug dissolution and permeation in oral drug absorption (14). This system (dissolution/permeation system, D/P system) consists of apical and basal chambers with a Caco-2 cell monolayer mounted between the two chambers as a model membrane of human intestine. Previous studies with the D/P system indicate that it enables evaluation of the effect of various factors such as food intake (15,16), solid dosage forms (17), and dose strength (16) in terms of their influence on the oral absorption of poorly water-soluble drugs.
In this study, we focused on evaluation of the effect of SEDDS and co-solvent formulations with or without water-soluble polymers on the oral absorption of poorly water-soluble drugs using the D/P system. In addition, animal study with rats was also performed to compare the results with in vitro estimation.
MATERIALS AND METHODS
Materials
The human colorectal adenocarcinoma cell line Caco-2 (passage: 17 times) was purchased from American Type Culture Collection (Rockville, MD). Dulbecco’s Modified Eagle’s Medium (DMEM) was obtained from Sigma-Aldrich (St. Louis, MO). Non-essential amino acids (10 mM), fetal bovine serum (FBS), L-glutamine (200 mM), trypsin-EDTA (trypsin: 0.25%, EDTA: 1 mM), and antibiotic-antimycotic (penicillin: 10,000 U/mL, streptomycin: 10 mg/mL, amphotericin B: 25 μg/mL; dissolved in 0.85% (w/v) sodium chloride aqueous solution) were purchased from Gibco Laboratories (Lenexa, KS). Cell culture inserts with polyethylene terephthalate filters (pore size: 3.0 μm, growth area: 4.20 cm2) were obtained from Becton Dickinson Bioscience (Bedford, MA).
Bovine serum albumin (BSA), egg-phosphatidylcholine (lecithin), ethanol, polyethyleneglycol 400 (PEG400), pranlukast, sodium taurocholate (NaTC), and Tween 80 were obtained from Wako Pure Chemical Industries Co., Ltd. (Osaka, Japan). Polyoxyl 35 hydrogenerated castor oil (Cremophor® EL), danazol, and HPMC were purchased from Sigma-Aldrich (St. Louis, MO). Mono/diglyceride of capric acid (Capmul® MCM) was obtained from ABITEC Corp. (Janesville, WI). Polyoxyl 40 hydrogenerated castor oil (Cremophor® RH40) was purchased from BASF SE (Ludwigshafen, Germany). Hydroxypropylcellulose (HPC) and methylcellulose were obtained from Acros Organics (Morris Plains, NJ). Glyceryl monolinoleate (Maisine 35–1) was purchased from GATTEFOSSÉ SAS (Saint Priest, France).
Preparation of Various Formulations
The standard suspension of danazol and pranlukast was prepared as follows. Danazol (20 mg/mL) and pranlukast (10 mg/mL) were suspended into purified water containing 0.5% (w/v) methylcellulose and 0.1% (w/v) Tween 80. Various formulations of danazol and pranlukast were prepared according to the composition of each formulation shown in Tables I and II, respectively. Danazol and pranlukast were completely dissolved into vehicle of all formulations.
In Vitro Experiments Using the D/P System
Preparation of the Caco-2 Cell Monolayer
Caco-2 cells were grown in D-MEM supplemented with 10% FBS, 1% L-glutamate, 1% NEAA, and 5% antibiotic-antimycotic solution as culture medium at 37°C in culture flasks (Nippon Becton Dickinson Co., Ltd., Tokyo, Japan) in humidified air with a 5% CO2 atmosphere. Cells were harvested with trypsin-EDTA and seeded onto polycarbonate filters (3.0 μm pores, 4.20 cm2 growth area) inside a cell culture insert (Nippon Becton Dickinson Co., Ltd., Tokyo, Japan) at a density of 3 × 105 cells/filter. The culture medium (1.5 mL in the insert and 2.6 mL in the well) was replaced every 48 h for the first 6 days and every 24 h thereafter. After 18–21 days in culture, the Caco-2 cell monolayer was utilized for the following experiments.
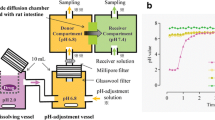
Chambers for the D/P System
In the D/P system, the Caco-2 cell monolayers are mounted in side-by-side chambers. The effective surface area of the Caco-2 cell monolayer in the D/P system is 1.77 cm2. Both sides of the Caco-2 cell monolayer are consistently stirred at 200 rpm with magnetic stirrers. The volumes of apical and basal sides are set to 8 mL and 5.5 mL, respectively. As a buffer solution in this study, Hank’s balanced salt solution (HBSS), containing 5.36 mM KCl, 136.89 mM NaCl, 0.34 mM Na2HPO4, 0.44 mM KH2PO4, 4.17 mM NaHCO3, 1.26 mM CaCl2, 0.49 mM MgCl2, 0.41 mM MgSO4, and 25 mM glucose, was used (transport medium, TM). Simulated intestinal medium as the apical side of the D/P system was prepared, which was based on TM with the addition of NaTC (3 mM) and lecithin (0.75 mM) for FaSSIFmod. The pH of FaSSIFmod was adjusted to 6.5 with HEPES. As a basal medium, TM containing BSA (4.5% w/v) with a pH adjusted to 7.4 with HEPES was used in the D/P system.
Dissolution and Permeation Study with the D/P System
A solution (TM, pH adjusted to 6.5) and the basal medium were introduced to the apical and basal sides of the Caco-2 monolayer in the well, respectively. After preincubation for 20 min, the Caco-2 monolayer with support filter was mounted between the chambers of the D/P system. Then, the apical side of the Caco-2 monolayer was filled with the apical medium, FaSSIFmod. The basal side was filled with the basal medium in all experiments. Drugs were applied to the apical side as a standard suspension or formulation. The applied volume of all forms was set to 50 μL for danazol and 225 μL for pranlukast which contains 1 mg and 2.25 mg (1% of the clinical dose), respectively. Then, aliquots of samples were routinely taken from the basal solution for 2 h. The volume of the basal solution was maintained by adding fresh medium. After completion of the experiment, the apical solution was immediately collected and filtered through a cellulose acetate filter. All apical samples (0.2 mL) were mixed with 0.2 mL of the solution consisting of 50 mM phosphate buffer (pH 2.5) and acetonitrile (50/50) to prevent the dissolved drug from precipitating in the apical solution. The transepithelial electric resistance (TEER) of the Caco-2 cell monolayer was checked before and after the experiment. All experiments were performed at 37°C.
Prediction of the Oral Absorption of Drugs from In Vitro Data
In order to predict the fraction of dose absorbed (Fa values) of each drug in fasted human from in vitro data, the following equation was used:
where Absmax is the maximum absorption (defined as 100%), PA is the in vitro permeated amount in the D/P system (% of dose/2 h), PA50 is the permeated amount, which corresponds to 50% of the absorption in vivo, and γ is Hill’s coefficient. In a previous study, PA50 and γ were obtained by fitting the permeated amount (PA) of non-P-gp substrate drugs in the D/P system and their oral absorption in humans; there was a good correlation between oral absorption in humans and% of dose/2 h with these substitutions (Kataoka et al., 2006). PA50 and γ of 0.334 and 0.883, respectively, that were obtained in a previous study were used for prediction of the oral absorption in fasted humans.
Animal Study
All animal experiments were approved by the Ethical Review Committee of Setsunan University and were performed in accordance with the Principles of Laboratory Animal Care (NIH publication No. 85–23, revised 1985). Wistar male rats weighing 250–300 g were deprived of food but given free access to water for 18 h before the experiments. The doses of danazol and pranlukast were set to 1.5 mg/kg and 4.0 mg/kg, respectively, on the basis of their clinical doses. The volume of each formulation was set to 0.075 mL/kg for danazol and 0.4 mL/kg for pranlukast. Standard suspensions and supersaturation-generating formulations of danazol and pranlukast were immediately administered to rats after suspensions and formulations were mixed with appropriate volumes of purified water. The total volume of administered solution with drugs was set to 2 mL/kg. At pre-determined time points, blood samples were collected from the jugular vein. Blood samples were centrifuged and plasma was kept at −30°C before quantification of drugs.
Assay
The sample solutions from in vitro study were pre-treated as follows. All basal samples (0.1 mL) were mixed with 0.9 mL of acetonitrile. The mixture was shaken and the supernatant was used for the assay after centrifugation at 15,000 rpm for 20 min. The plasma samples from in vivo study were pre-treated as follows. All plasma samples (0.1 mL) were mixed with 0.9 mL of acetonitrile. The mixture was shaken and the supernatant (0.7 mL) was collected after centrifugation at 15,000 rpm for 20 min. After removal of the solvent under a vacuum at 40°C, the residue was dissolved in 0.1 mL of the solution consisting of 50 mM phosphate buffer (pH 2.5) and acetonitrile (50/50). These solutions were used for the assay.
The amounts of danazol and pranlukast in the solutions were determined using a UPLC system (ACQUITY® UPLC, Waters, Milford, MA) equipped with a tandem mass spectrometer (ACQUITY® TQD, Waters, Milford, MA). A reversed-phase Waters ACQUITY® UPLC BEH C18 analytical column of 50 × 2.1 mm and 1.7 μm particle size (Waters, Milford, MA) was used with a mobile phase consisting of 0.1% (v/v) formic acid in water (solvent A) and acetonitrile containing 0.1% (v/v) formic acid (solvent B) with gradient time period. The initial mobile phase was 98% solvent A and 2% solvent B pumped at a flow rate of 0.3 mL/min. Between 0 and 1.0 min, the percentage of solvent B was increased linearly to 95%, where it was held for 1.0 min. Between 2.01 and 2.5 min, the percentage of solvent B was decreased linearly to 2%. This condition was maintained until 3 min, at which time the next sample was injected into the UPLC system. All treated samples were injected at 5 μL into the UPLC system. Ionization conditions for analysis of danazol were as follows: electrospray ionization, positive mode; source temperature, 150°C; desolvation temperature, 400°C; cone voltage, 50 V; and collision energy, 50 eV. Precursor and production ions (m/z) of danazol were 338.19 and 91.02, respectively. Ionization conditions for analysis of pranlukast were as follows: electrospray ionization, positive mode; source temperature, 150°C; desolvation temperature, 400°C; cone voltage, 40 V; and collision energy, 30 eV. Precursor and production ions (m/z) of pranlukast were 482.52 and 121.13, respectively.
Data Analysis
In vitro data obtained from experiments using the D/P system are expressed as a fraction of the dose (% of dose); the dose is the drug amount applied to the apical side of the Caco-2 cell monolayer attached to the D/P system. In in vivo study, the area under the plasma concentration-time curve (AUC) from 0 to the time of final sampling point was calculated using a linear trapezoidal rule. Data are presented as means and standard deviations (s.d.) for individual groups. Statistical significance was assessed with the unpaired Student’s t-test and p values of 0.05 or less were considered significant.
RESULTS
In Vitro Estimation of the Effect of Various Formulations on the Dissolution and Permeation of Poorly Water-Soluble Drugs Using the D/P System
Figure 1 shows the effect of various supersaturation-generating formulations on the danazol dissolution (Fig. 1a) and permeation (Fig. 1b) in the D/P system. The dissolved and permeated amounts of danazol for 2 h are summarized in Table III. The PEG400-based formulation (Co-solvent) enhanced danazol dissolution in the apical medium and then the amount that permeated to the basal solution was increased compared with that from standard suspension. Moreover, when HPC or HPMC was added to PEG400-based formulation (S-Co-solvent/HPC, S-Co-solvent/HPMC), both dissolution and permeation of danazol were significantly increased, indicating that these polymers could maintain a supersaturated state in the apical solution. This effect by HPMC was stronger than that by HPC. As lipid-based formulation, SEDDS was used and significantly enhanced danazol dissolution and permeation. In comparison with Co-solvent, there was no observed prolonged effect by HPC or HPMC on danazol dissolution. In addition, no significant difference in the permeated amount of danazol was observed between SEDDS and SEDDS containing HPC or HPMC (S-SEDDS/HPC, S-SEDDS/HPMC). Significant change in the TEER value of the monolayers was not observed.
Effect of various dosage forms on the dissolution (a) and permeation (b) of danazol in the D/P system. The dissolved amount of danazol at 2 h and the time-courses of danazol permeation were observed for 2 h in the D/P system. As formulations of danazol, standard suspension (◊), SEDDS (○), S-SEDDS/HPC (∆), S-SEDDS/HPMC (□), Co-solvent (●), S-Co-solvent/HPC (▲), and S-Co-solvent/HPMC (■) were applied to the D/P system. The data are expressed as the mean ± s.d. of three independent experiments.
Dissolution and permeation study with the D/P system was performed for pranlukast (Table IV). When SEDDS and S-SEDDS formulations were applied to the apical side of the D/P system, pranlukast was completely dissolved for 2 h but decrease in the TEER value (30% of initial value at 2 h) across the Caco-2 cell monolayer during the experiment was observed. In case of other dosing conditions, no significant change in the TEER value of the monolayers was observed. However, the permeated amount of pranlukast was significantly lower in spite of complete dissolution. In the case of co-solvent formulations, whereas the dissolved amount was significantly increased compared with that from standard suspension and an inhibitory effect on the precipitation by HPMC was observed, permeation to the basal compartment was not enhanced.
Prediction of Oral Absorption from In Vitro Data
The in vitro data (% of dose/2 h) were substituted for PA in Eq. 3 in order to predict the in vivo oral absorption (Fa%) of each drug from various dosage forms. As shown in Table III, the oral absorption of danazol was estimated to be 32% when 100 mg of danazol was administered as standard suspension to fasted human. Although the oral absorption of danazol from Co-solvent (49%) could be enhanced by addition of polymer, the oral absorption was predicted not to be affected by the difference in the addition of HPC (72%) and HPMC (78%). In the case of lipid-based formulations, the oral absorption of danazol was predicted to be high compared with that from standard suspension and the predicted absorption of danazol, ranging from 58% to 65%, was not affected by the presence of polymer in SEDDS.
The prediction of the oral absorption of pranlukast from various dosage forms was performed in the same manner as for danazol and is shown in Table IV. The oral absorption of pranlukast from standard suspension and PEG400-based formulation (Co-solvent and S-Co-solvent/HPMC) was predicted to be lower than that from lipid-based formulation (SEDDS and S-SEDDS/HPMC). There were no differences in the predicted oral absorption of pranlukast between SEDDS and S-SEDDS/HPMC. The rank order of formulation efficacy on the oral absorption of pranlukast was SEDDS = S-SEDDS/HPMC > Co-solvent/HPMC > standard suspension > Co-solvent.
In Vivo Evaluation of the Effect of Various Formulations on the Oral Absorption of Poorly Water-Soluble Drugs by Rats
The plasma concentration versus time profiles of danazol after oral administration as various dosage forms are shown in Fig. 2. The pharmacokinetic parameters are listed in Table V. The oral absorption of danazol (AUC0-8h) from standard suspension was the lowest of all values. The rank order of the PEG400-based formulation on the AUC0-8h values was S-Co-solvent/HPMC > S-Co-solvent/HPC > S-Co-solvent, corresponding to the present in vitro results. In the case of lipid-based formulations, although the rank order of the AUC0-8h values did not agree with that of in vitro results, the oral absorption of danazol was significantly increased by SEDDSs.
The time-profile of plasma concentration of danazol after oral administration as various dosage forms. As formulations of danazol, standard suspension (◊), SEDDS (○), S-SEDDS/HPC (∆), S-SEDDS/HPMC (□), Co-solvent (●), S-Co-solvent/HPC (▲), and S-Co-solvent/HPMC (■) were administered to rats (1.5 mg/kg). The data are expressed as the mean ± s.d. of three or four independent experiments.
Figure 3 shows the plasma concentration-time profile of pranlukast after oral administration as various dosage forms; pharmacokinetic parameters are summarized in Table VI. Although significant change in the oral availability (AUC0-24h) of pranlukast could not be observed between all formulations used in this study, the oral availability from co-solvent formulations seemed to be lower than that from other formulations. Maximum drug concentration times (Tmax) when pranlukast was administered as SEDDS formulations delayed compared to that after administration as other formulations.
The time-profile of plasma concentration of pranlukast after oral administration as various dosage forms. As formulations of pranlukast, standard suspension (◊), SEDDS (○), S-SEDDS/HPMC (□), Co-solvent (●), and S-Co-solvent/HPMC (■) were administered to rats (4.0 mg/kg). The data are expressed as the mean ± s.d. of three or four independent experiments.
DISCUSSION
Self-emulsifying drug delivery systems (SEDDS) to improve the oral bioavailability of poorly water-soluble drugs are well known and have been demonstrated with various drugs (18–20). SEDDS formulation is composed of drug, oil, surfactant, and co-surfactant, which are emulsified in aqueous media under conditions of gentle agitation (21). SEDDS formulation increases dissolution rate and solubility of poorly water-soluble drugs. Gao et al. have revealed that supersaturable SEDDS (S-SEDDS) that was developed employing HPMC significantly improved oral availability of poorly water-soluble drugs (12,13). For example, paclitaxel rapidly precipitated after dispersal into buffer solution as SEDDS formulation. On the other hand, precipitation of paclitaxel was significantly inhibited by the presence of HPMC. In vivo oral availability of paclitaxel from S-SEDDS was 10-fold higher than that from SEDDS, corresponding to the findings of in vitro study (12). However, although similar correspondences were observed between in vitro and in vivo studies, absolute values of inhibition effect on precipitation observed by in vitro study did not necessarily correspond to those calculated from in vivo results.
Miller et al. have reported the impact of micellar solubilization on the intestinal membrane permeability (22). They demonstrated that a trade-off exists between micellar apparent solubility increase and permeability decrease that must take into account to consider the oral absorption of drugs. Moreover, some surfactants affect the membrane permeability of drug due to inhibition of transporters such as P-gp (23). In case of co-solvent formulations, these effects, effect on solubility and permeability, should be considered. It was reported that co-solvent agents such as PEG400 had no impact on the membrane permeation of drugs (24,25); however, several observations that co-solvent agents decrease membrane permeability of drugs were shown (26). In these cases, drug concentration related to permeation across a membrane could be important to determine the absorption. The permeated amount of drug in the D/P system could reflect the various phenomena which affected in vivo drug absorption because the system could evaluate drug dissolution and permeation, simultaneously.
In order to assess the food effect by micelles, the limiting process of oral drug absorption (Fa < 95%) can be considered as three categories, permeability limited (PL), dissolution rate limited (DRL), and solubility limited (SL) oral absorption (27). The permeability limited case can be further categorized as epithelial membrane permeation limited (PL-E) and unstirred water layer limited (PL-U). In addition, the solubility limited case can be further categorized as epithelial membrane permeation limited (SL-E) and unstirred water layer (UWL) limited (SL-U). In drugs categorized into SL-E, the increase of solubility by bile micelles would be cancelled out by the decrease of effective permeability due to decreasing free drug as a fraction of the total dissolved amount. This would result in slightly positive food effect on the oral absorption. On the other hand, in drugs categorized into DRL and SL-U, a positive food effect by bile micelles was anticipated. The extent of positive food effect would depend on the balance of change of effective diffusion coefficient in the UWL and an increase of solubility. This categorizing could be applicable to consider not only the food effect on the oral absorption of poorly water-soluble drugs by bile micelles but also the formulation effect on it.
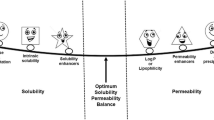
Figure 4 shows a scheme of the relationship between the limiting steps of oral absorption of poorly water-soluble drugs and the formulation effect based on the report by Sugano et al. (27). In the case of liquid formulation, UWL limited can be translated into partition rate limited (SL-P) from micelles and emulsions to water phase. In low-soluble compounds with high permeability (DRL and/or SL-P), significant increase in the oral absorption by formulation techniques was anticipated. The extent of the formulation effect would depend on the balance of free fraction and bound fraction with micelles or emulsions and an increase of solubility. In low-soluble compounds with low permeability (SL-E), slight or no formulation effect on the oral absorption was anticipated. Danazol (28) and pranlukast (29) used in this study are high-permeability and low-permeability compounds with low solubility, respectively. Therefore, danazol could be classified into the DRL and/or SL-P category and pranlukast could be classified into the SL-E category. In addition, it can be mainly considered that drug permeation from a solution with the micelle and emulsion depends on free drug concentration (unbound and unenclosed into micelle and emulsion). The free drug concentration is decreased by permeation across the membrane. In case of DRL and/or SL-P categorized drugs, the free concentration of drug in a solution significantly decreases and then drug is released from the micelle and emulsion on the basis of the partition ratio which results in the maintaining high drug permeation. On the other hand, SL-E categorized drugs do not lead to such permeation cycle due to low membrane permeation. According to this categorizing, a significant effect of formulation on the oral absorption of danazol was anticipated but not of pranlukast.
Two types of formulation, SEDDS and simple co-solvent formulations, were used in this study. Moreover, a water-soluble cellulosic polymer, HPC or HPMC, was added to each formulation to generate a supersaturated state in a solution. In the apical side of the D/P system, formulation effect on the dissolution and inhibition effect on precipitation by polymers could be clearly detected (Tables III and IV). In danazol, the high dissolution from various formulations led to high permeation to the basal solution compared with that for a standard suspension (Table III). Whereas supersaturated state could be generated in the apical solution of the D/P system because significant increase in the dissolved amount after applying pranlukast as co-solvent formulation with HPMC was observed compared to that as co-solvent formulation, permeated amount of pranlukast was slightly increased (Table IV). It was considered that the flux of drug across the membrane depended upon the product of the drug concentration related to permeation, the surface area and permeation rate. If the detectability of the D/P system was increased (e.g. by extension of the surface area of the monolayer), the effect of supersaturation from co-solvent with HPMC on the permeated amount of pranlukast could be detected, clearly. When SEDDS and S-SEDDS formulations were applied to the apical side of the D/P system, pranlukast was completely dissolved for 2 h but a decrease in the TEER value across the Caco-2 cell monolayer during the experiment was observed. The decrease in the TEER value of the monolayer indicated disruption of the monolayer stricture which led to enhancing drug permeation through paracellular route. Accordingly, the observed permeated amount and estimated oral absorption of pranlukast from SEDDS formulations should be used as references. These results supported the scheme of the relationship between the limiting steps of oral absorption of poorly water-soluble drugs and the formulation effect shown in Fig. 4.
In animal study with rats, all formulations improved the oral availability of danazol (Fig. 2, Table V). In SEDDS formulations, the oral availabilities in rats were similar between three formulations. In co-solvent formulations, in vivo rank order of the formulation effect corresponded well to that from oral absorption estimated using the D/P system, indicating that a similar inhibition effect by polymers observed in vitro occurred in the in vivo gastrointestinal absorption process. Significant difference in the oral availability of danazol between SEDDS and S-SEDDS forms was not observed. In contrast, each formulation performance could be evaluated from in vitro experiment with the D/P system. This discrepancy in the results could be caused by the difference in the sensitivity between the in vitro and in vivo studies. In vitro study provides repeatable data with small deviation. In contrast, in vivo study provides data with large deviation due to intra-species difference. From Fig. 5 a, significant correlation (r2 = 0.83) between in vivo oral availability (AUC0−8h) and estimated absorption (Fa%) from the permeated amount in the D/P system was obtained for danazol. In the case of pranlukast, because significant formulation effect on the oral availability was not observed, irrespective of formulation type (Table VI), we did not obtain a correlation coefficient (Fig. 5 b). However, when pranlukast was administered as SEDDS formulations, the maximum drug concentration time (Tmax) was seemed to be delayed compared to that after administration as standard suspension and co-solvent formulations (Table VI). As the reason for the change in Tmax, several possibilities were considerable as follows: due to decrease in the free fraction of drugs in the luminal solution and due to change in the gastrointestinal movement of drug solution. Although the reason for the change in Tmax was not clarified, the oral availability of pranlukast was not affected by any formulations used in this study. The estimated absorption from the permeated amount in the D/P system indicated that the oral absorption of pranlukast was not increased by co-solvent formulations, irrespective of the presence of HPMC, corresponding well to the in vivo observations. The increase of solubility by SEDDS formulations would be cancelled out by the decrease of effective permeability, because free fraction of drugs in the luminal solution might be the same between drug only and lipid-based formulation. In the case of co-solvent formulation, PEG400 in a solution enhances drug solubility but reduces effective permeability with an increase in the concentration of PEG400 (26). PEG400 is regarded as a safe and non-toxic agent and there is no positive evidence for direct interaction of PEG400 with passive permeation process. Thus, the indirect effects of PEG400, such as the increased viscosity of the apical solution and interaction between PEG400 and pranlukast, are considered to influence effective permeability of pranlukast. In order to clarify the effect, more detailed studies are necessary. However, these effects of PEG400 could be observed both in vitro and in vivo studies, suggesting that the D/P system detects the similar effect observed in vivo.
These in vitro/in vivo correlations in the oral absorption indicated that limiting steps in the in vivo gastrointestinal absorption of poorly water-soluble drugs followed the scheme shown in Fig. 4. Therefore, the D/P system may be a useful tool not only for the estimation of drug absorption from various formulations but also for understanding of the formulation effect on oral absorption of poorly water-soluble drugs. In addition, the D/P system may provide valuable information to select the oral dosage form in drug development.
In this paper, the effect of lipolysis on the oral absorption of poorly water-soluble drugs after administration as lipid-based formulation was not discussed since the formulation effect on the drug dissolution and permeation was focused on. Whereas lipolysis is also the key factor to determine the oral absorption of drugs from lipid-based formulation, how lipolysis impacts on the absorption has not been reported. By using the D/P system, the effect of lipolysis on the drug absorption from lipid-based formulation may be estimated and is a subject for future study.
CONCLUSION
In conclusion, we have successfully demonstrated that the D/P system enables evaluation of the formulation effect on oral drug absorption and have indicated that the use of supersaturation-generated formulation could be a favorable strategy to improve the oral absorption of poorly water-soluble drugs with high permeability. The D/P system may be a useful tool for formulation selection in the early stage of drug development.
REFERENCES
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int J Pharm. 2006;317(1):61–8.
Hamaguchi T, Shinkuma D, Irie T, Yamanaka Y, Morita Y, Iwamoto B, et al. Effect of a high-fat meal on the bioavailability of phenytoin in a commercial powder with a large particle size. Int J Clin Pharmacol Ther Toxicol. 1993;31(7):326–30.
Kennedy M, Hu J, Gao P, Li L, Ali-Reynolds A, Chal B, et al. Enhanced bioavailability of a poorly soluble VR1 antagonist using an amorphous solid dispersion approach: a case study. Mol Pharm. 2008;5(6):981–93.
Gao P. Amorphous pharmaceutical solids: characterization, stabilization, and development of marketable formulations of poorly soluble drugs with improved oral absorption. Mol Pharm. 2008;5(6):903–4.
Chokshi RJ, Zia H, Sandhu HK, Shah NH, Malick WA. Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution: pros and cons. Drug Deliv. 2007;14(1):33–45.
Guzmán HR, Tawa M, Zhang Z, Ratanabanangkoon P, Shaw P, Gardner CR, et al. Combined use of crystalline salt forms and precipitation inhibitors to improve oral absorption of celecoxib from solid oral formulations. J Pharm Sci. 2007;96(10):2686–702.
Bak A, Gore A, Yanez E, Stanton M, Tufekcic S, Syed R, et al. The co-crystal approach to improve the exposure of a water-insoluble compound: AMG 517 sorbic acid co-crystal characterization and pharmacokinetics. J Pharm Sci. 2008;97(9):3942–56.
Shiraki K, Takata N, Takano R, Hayashi Y, Terada K. Dissolution improvement and the mechanism of the improvement from cocrystallization of poorly water-soluble compounds. Pharm Res. 2008;25(11):2581–92.
Lu GW, Hawley M, Smith M, Geiger BM, Pfund W. Characterization of a novel polymorphic form of celecoxib. J Pharm Sci. 2006;95(2):305–17.
Kommuru TR, Gurley B, Khan MA, Reddy IK. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212(2):233–46.
Gao P, Rush BD, Pfund WP, Huang T, Bauer JM, Morozowich W, et al. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92(12):2386–98.
Gao P, Akrami A, Alvarez F, Hu J, Li L, Ma C, et al. Characterization and optimization of AMG 517 supersaturatable self-emulsifying drug delivery system (S-SEDDS) for improved oral absorption. J Pharm Sci. 2009;98(2):516–28.
Kataoka M, Masaoka Y, Yamazaki Y, Sakane T, Sezaki H, Yamashita S. In vitro system to evaluate oral absorption of poorly water-soluble drugs: simultaneous analysis on dissolution and permeation of drugs. Pharm Res. 2003;20(10):1674–80.
Kataoka M, Masaoka Y, Sakuma S, Yamashita S. Effect of food intake on the oral absorption of poorly water-soluble drugs: in vitro assessment of drug dissolution and permeation assay system. J Pharm Sci. 2006;95(9):2051–61.
Kataoka M, Itsubata S, Masaoka Y, Sakuma S, Yamashita S. In vitro dissolution/permeation system to predict the oral absorption of poorly water-soluble drugs: effect of food and dose strength on it. Biol Pharm Bull. 2011;34(3):401–7.
Buch P, Langguth P, Kataoka M, Yamashita S. IVIVC in oral absorption for fenofibrate immediate release tablets using a dissolution/permeation system. J Pharm Sci. 2009;98(6):2001–9.
Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50(1):179–88.
Khoo SM, Porter CJ, Charman WN. The formulation of Halofantrine as either non-solubilizing PEG 6000 or solubilizing lipid based solid dispersions: physical stability and absolute bioavailability assessment. Int J Pharm. 2000;205(1–2):65–78.
Kim JY, Ku YS. Enhanced absorption of indomethacin after oral or rectal administration of a self-emulsifying system containing indomethacin to rats. Int J Pharm. 2000;194(1):81–9.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, et al. The Solubility-Permeability Interplay: Mechanistic Modeling and Predictive Application of the Impact of Micellar Solubilization on Intestinal Permeation. Mol Pharm. 2011; in press.
Katneni K, Charman SA, Porter CJ. Impact of cremophor-EL and polysorbate-80 on digoxin permeability across rat jejunum: delineation of thermodynamic and transporter related events using the reciprocal permeability approach. J Pharm Sci. 2007;96(2):280–93.
Takahashi Y, Kondo H, Yasuda T, Watanabe T, Kobayashi S, Yokohama S. Common solubilizers to estimate the Caco-2 transport of poorly water-soluble drugs. Int J Pharm. 2002;246(1–2):85–94.
Hugger ED, Audus KL, Borchardt RT. Effects of poly(ethylene glycol) on efflux transporter activity in Caco-2 cell monolayers. J Pharm Sci. 2002;91(9):1980–90.
Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci. 2000;10(3):195–204.
Sugano K, Kataoka M, Mathews Cda C, Yamashita S. Prediction of food effect by bile micelles on oral drug absorption considering free fraction in intestinal fluid. Eur J Pharm Sci. 2010;40(2):118–24.
Sunesen VH, Vedelsdal R, Kristensen HG, Christrup L, Müllertz A. Effect of liquid volume and food intake on the absolute bioavailability of danazol, a poorly soluble drug. Eur J Pharm Sci. 2005;24(4):297–303.
Nakajima M, Kanamaru M, Umematsu T, Tsubokura S. A phase I clinical study of a leukotriene C4, D4 and E4 receptor antagonist; ONO-1078 in healthy volunteers. Rynsho iyaku 1993;Suppl. 1:9.
ACKNOWLEDGMENTS & DISCLOSURES
In vitro experiments with the D/P system were financially supported by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataoka, M., Sugano, K., da Costa Mathews, C. et al. Application of Dissolution/Permeation System for Evaluation of Formulation Effect on Oral Absorption of Poorly Water-Soluble Drugs in Drug Development. Pharm Res 29, 1485–1494 (2012). https://doi.org/10.1007/s11095-011-0623-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0623-2