Abstract
SHetA2 is a novel anticancer drug with poor aqueous solubility. In formal toxicological studies, Kolliphor HS 15 was used as a solubilizing agent to increase the oral bioavailability of SHetA2. The purpose of this study was to formulate SHetA2 and Kolliphor HS 15 as solid powders to facilitate their filling in hard gelatin capsules for clinical trials. Two manufacturing processes, ultra-rapid freeze-drying (URFD) and spray freeze drying (SFD), were employed to fabricate solid powders of SHetA2-Kolliphor HS 15 and trehalose. The morphology, size, flowability, and compressibility of URFD-SHetA2 and SFD-SHetA2 powders were characterized. The crystallinity and apparent maximum solubility of SHetA2 in both powders were also determined. SFD-SHetA2 powders were spherical in shape, small, and with a wide size distribution while the URFD-SHetA2 powders were irregularly shaped and big but with a narrower distribution. DSC and XRD analyses indicated that SHetA2 was mostly amorphous in both powders. The flow of both powders was categorized as “good” (angle of repose < 35°). The uniformity of drug content in URFD-SHetA2 powders was more variable than that in SFD-SHetA2 powders. The solubility profile of SHetA2 in both powders SGF exhibited a transient supersaturation “spring effect” due to the drug’s amorphousness followed by extended supersaturation “parachute effect” at approximately 6 μg/ml for both powders compared to 0.02 ± 0.01 μg/ml for unprocessed drug. In conclusion, both URFD and SFD formed solid SHetA2 Kolliphor powders that are possible formulation candidates to be filled in hard gelatin capsules for clinical trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In the last decade, anticancer compounds with chemo-preventive activity have been considered as a more attractive therapeutic option than conventional cytotoxic compounds (1). However, most of these compounds are highly hydrophobic and have less than desirable oral bioavailability because of their poor dissolution or variable absorption in the gastrointestinal tract (2). This has challenged the formulation scientists to find ways to improve the solubility and dissolution rate of these compounds by using formulation strategies including the addition of solubilizing agents, micronization, co-precipitation, spray drying, and hot melt extrusion (2,3).
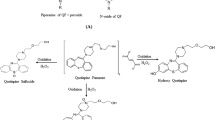
SHetA2 is a flexible heteroarotinoid that inhibits cancer cell growth and induces selective apoptosis, while retaining the differential resistance in normal cells (4,5,6,7), but has poor water solubility. The chemical structure of SHetA2 is shown in Fig. 1. SHetA2 inhibited the growth of all 60-cell lines in the National Cancer Institute (NCIs) human tumor panel at concentrations in the micromolar range (4). To overcome the limitation of drug solubility in the studies with cell lines, SHetA2 was dissolved in small amounts (less than or equal to 0.01%) of DMSO after establishing that this DMSO concentration was not toxic to the cells and it did not induce differentiation (8). In initial efficacy studies (4), SHetA2 was effective in inhibiting the growth of OVCAR-3 ovarian cancer xenografts when dissolved in super refined sesame oil and administered to NU/NU CD1 mice daily by gavage for 35 days. Subsequent studies performed in the renal cancer xenograft mouse model also demonstrated that SHetA2 was effective in inhibiting the growth of Caki-1 kidney cancer xenografts when dissolved in PEG 400 and administered daily to these mice by oral gavage for 30 days (6). SHetA2 was selected for preclinical development in the NCI Rapid Access to Intervention Development (RAID) and the rapid access to preventive intervention development (RAPID) programs. For the formal toxicological and pharmacokinetic studies performed through these programs, SHetA2 was administered in 1% methylcellulose/ 0.2% tween 80 by oral gavage to Cr1:CD (SD) rats and in 30% aqueous Kolliphor HS 15 (previously known as Solutol HS15) to dogs (9). These studies indicated that there were no adverse effects after consecutive administration of SHetA2 at concentrations that were 50 times greater than the effective dose, thus supporting the advancement of SHetA2 to clinical trials to prevent the progression of cervical dysplasia to cervical cancer. However, the pharmacokinetic studies revealed that the systemic bioavailability of SHetA2 in rats was less than 1% for all doses tested. In contrast, systemic bioavailability in dogs was significantly larger (> 10%) (9). The differences in SHetA2 bioavailability between rats and dogs may be due to differences in how these species metabolize the drug, but also largely attributed to the excipients in the solutions administered. This is no surprise as Kolliphor HS 15 is a non-ionic solubilizing and emulsifying agent developed to improve the oral absorption of highly lipophilic drugs (10).
Preventive therapies usually require the continuous long-term administration of the chemo-preventive agent and thus non-invasive routes of administration, such as the oral route are preferred. Even though simple formulations such as active pharmaceutical ingredient (API) in a capsule or powder in a bottle (PIB) are used for phase 0 or I clinical trials, the results of the formal pharmacokinetic/toxicological studies performed with SHetA2 (9) suggest that a more complex formulation should be developed for use in humans. Hard gelatin capsules for oral administration offer several advantages for use in clinical trials, such as the versatility of the formulation that can be encapsulated, the possibility of self-administration and of administering several capsules at a time as needed for dose escalation. Given that the formal toxicological studies were performed with a formulation using only SHetA2 and Kolliphor HS 15, it is likely that if other ingredients were added to the formulation for use in humans, the toxicological studies would have to be repeated with the new formulation.
Initially, we formulated SHetA2 as a semi-solid dispersion in Kolliphor HS 15, either by mixing SHetA2 with Kolliphor HS 15 using mortar and pestle, or by addition of SHetA2 to melted Kolliphor HS 15 then left to congeal at room temperature, to be manually filled in hard gelatin capsules. The integrity of the capsules did not change (melt or distort) upon filling with the semisolid dispersion and storing them at room temperature for 7 days. However, the in-batch drug content uniformity was highly variable, the dissolution times were excessively long (more than 45 min) and the capsule filling process was difficult. Therefore, we proposed to manufacture free flowing powder formulation of SHetA2/Kolliphor HS 15 to increase the uniformity of drug content, decrease the dissolution time and facilitate capsule filling. Due to the semi-solid paste-like nature of Kolliphor HS 15 (melting point 30°C), all granulation techniques requiring heat were disregarded. Thus, cryogenic granulation processes of ultra-rapid freeze drying (URFD) or spray freeze drying (SFD, also known as freeze granulation) were used to manufacture solid free flowing powders of SHetA2 and Kolliphor HS 15 using trehalose as the powder core.
URFD is a simple and robust FD technique which involves instantaneous freezing of a thin liquid film of a drug and excipient solution deposited on a pre-frozen substrate causing ultra-rapid freezing. SFD involves the atomization of a solution of drug and excipients into small droplets that partially freeze while flying into the vapor phase until they land into the liquid nitrogen in which they freeze completely (11). A faster cooling rate could be achieved in URFD compared to SFD by circumventing the Leidenfrost effect, in which an insulating vapor layer resulting from the boiling of the liquid nitrogen is formed resulting in a slower cooling rate of sprayed droplets (12,13).
The goal of this study was to develop an oral formulation of SHetA2 for use in clinical trials that will evaluate its efficacy in preventing the progress of cervical dysplasia into cervical cancer. We employed the same ingredients used in the toxicological studies and a generally regarded as safe (GRAS) ingredient to make it into a powder that facilitates its dissolution and absorption in the human GI tract.
MATERIALS AND METHODS
SHetA2 was synthesized by Cayman Chemical Company, Inc. under a contract from RAPID NCI program. Kolliphor HS 15 was a gift from BASF Corporation Pharma Ingredients & Services, (Lot # GMCP699Q, batch # 0419264). D-(+)-Trehalose dihydrate, sodium hydroxide, potassium dihydrogen phosphate were purchased from Sigma-Aldrich. All solvents were of HPLC grade and were supplied from Sigma-Aldrich.
Formulation of SHetA2 Powders
The processes to manufacture powders using SHetA2, Kolliphor HS 15, and trehalose by URFD and SFD illustrated in Fig. 2.
Preparation of SHetA2 Suspension
Finely triturated SHetA2 was added to Kolliphor HS 15 (1.5 g) that was previously melted at 60°C to form a Kolliphor/SHetA2 mixture. Trehalose was added to the mixture to form the powder core. Three concentrations of trehalose were evaluated in the formulation (F1, F2, and F3, Table I). An aqueous solution of trehalose was prepared in 5 ml of deionized water, then heated to 60°C. The trehalose solution was then added portion-wise to the Kolliphor HS 15/ SHetA2 mixture and homogenized using an OMNI mixer GLH homogenizer at speed of 13,500 rpm for 3 min.
Ultra-Rapid Freeze Drying (URFD) of SHetA2 Suspension
The homogenized mixture of SHetA2, Kolliphor HS 15 and trehalose was added dropwise to the walls of a jar pre-chilled at − 80°C for 2 h and the jar attached to the manifold of the freeze dryer via a vacuum valve. The mixture was then lyophilized for 48 h using a manifold temperature of − 55°C and a pressure of 25 mtorr (Kinetics Flexi-Dry, Kinetics Thermal Systems, Stone Ridge, NY). A blank powder containing only trehalose and Kolliphor HS 15 was prepared similarly. SHetA2 and the blank powder were stored in tightly closed, light protective containers and kept in a desiccator until further analysis.
Spray Freeze Drying (SFD) of SHetA2 Suspension
SHetA2 suspension was sprayed as fine droplets into a vessel containing liquid nitrogen (approximately 2 L) using a two-fluid pneumatic spray nozzle (7 mm diameter, Mini spray dryer, Buchi, Flawil, Switzerland) at a liquid feed rate of 10 ml/min, nitrogen gas at a flow rate of 742 L/h and atomization pressure of 5 bars. The SHetA2 suspension was constantly stirred while passing through the nozzle to ensure uniform drug content in the powders. After all the SHetA2 suspension was sprayed, the vessel was transferred to a − 80°C freezer for 2 h to evaporate the liquid nitrogen. The frozen droplets were transferred to a pre-chilled jar and lyophilized for 48 h at a manifold temperature of −55°C and a vacuum pressure of 25 mTorr (Flexi-Dry, Kinetics Thermal Systems, Stone Ridge, NY).
Physical Characterization of SFD-SHetA2 and URFD-SHetA2 Powders
Particle Morphology and Size Determination
The morphologies of crystalline SHetA2, SFD-SHetA2, and URFD-SHetA2 powders were examined using scanning electron microscopy (SEM) (HITACHI TM3000). Samples were prepared by deposition on a double-coated carbon conductive tape (Ted Pella Inc., Redding, CA), mounted on aluminum stubs and imaged with SEM at an acceleration voltage 5 Kv. The volume diameters (Dv) and the geometric standard deviation (GSD) of URFD-SHetA2 and SFD-SHetA2 powders were measured by laser diffraction using a HELOS system with RODOS dry dispersing unit (Sympatec Inc., Lawrenceville, NJ). Measurements were done in triplicate at a pressure of 0.5 bars.
Crystallinity
The crystallinity of SHetA2 in URFD-SHetA2 and SFD-SHetA2 powders was analyzed by powder X-ray diffraction (XRD) using a Rigaku Ultima IV X-ray diffractometer (Woodlands, TX). Cu-K-alpha radiation was used with a scintillation detector at a generation voltage of 40 kV and a current of 44 mA. Data were collected by the 2 θ method at a scan speed of 2°/min at the range of 5–50°.
Thermal Analysis
Thermal analyses of unprocessed SHetA2, SFD-SHetA2, and URFD-SHetA2 powders were performed using differential scanning calorimetry (DSC) (DSC-60, Shimadzu) (Kyoto, Japan). Approximately 10 mg of powder samples were loaded in aluminum pans, crimpled and heated from 25 to 250°C at the rate of 10°C/min. Differences in the heat flow rate were measured against an empty reference pan. Exothermic and endothermic peaks were analyzed using ta60, version 2.21, Shimadzu software.
Flowability and Compressibility of SHetA2 Powders
The flowability of URFD-SHetA2 and SFD-SHetA2 powders was determined to evaluate the reproducibility in which hard gelatin capsules may be filled with the powders. The static powder flow was characterized using the angle of repose, and Carr’s compressibility index (CCI) (14) as follows:
The angle of repose of URFD-SHetA2 and SFD-SHetA2 powders was determined as outlined in chapter <1174> of the USP. Briefly, powders were placed in a glass funnel and allowed to flow continuously onto a horizontal surface. The height of the formed powder cone and the angle of repose were measured using to the following equation (14):
The bulk and tapped densities were determined as described in chapter <616> of USP (14). For bulk density, a pre-weighed amount of URFD-SHetA2 or SFD-SHetA2 powders was introduced without compacting into a 10-ml graduate cylinder and the volume was measured with a 0.1-ml accuracy. The tapped density was calculated by measuring the volume resulting after mechanically tapping the cylinder onto a flat surface, until the powder volume did not decrease further with tapping.
Drug Content Uniformity
Three samples, 10 mg each, were collected from URFD-SHetA2 and SFD-SHetA2 powders and added to 5 ml of acetonitrile. After centrifugation, 850 μl of the supernatants were collected, diluted with 150 μl of deionized water and the SHetA2 content was determined using HPLC analysis.
HPLC Assay
HPLC analysis was performed using a Waters Alliance HPLC System with a Vydac 201 TP C18 5 μm (250 mm × 2.1 mm) column equipped with a guard column (Vydac 201TP, Grace) at 25°C, and a UV detector set at 341 nm. The mobile phase consisted of 80% methanol and 20% sodium acetate solution in water (pH = 3) at a flow rate of 0.3 ml/min. The retention time of the SHetA2 peak was 3.65 min and the total run time was 10 min. Peak areas were calculated using the Empower software.
Apparent Solubility Studies in Simulated Gastric Fluid (SGF) Under Non-Sink Conditions
Conventional dissolution studies under sink conditions were not feasible due to the poor aqueous solubility of SHetA2. Based on the aqueous solubility of SHetA2 (0.02 μg/ml), at least 50 L of dissolution medium would be needed to perform a dissolution study for 1 mg of SHetA2, which is not feasible with the USP dissolution apparatuses. The dissolution studies of SHetA2 in URFD-SHetA2 and SFD-SHetA2 formulations were performed in simulated gastric fluid (SGF) using unprocessed SHetA2 as a control as described by Nielsen et al. (15) SGF was prepared as 0.1 N HCl with a final pH = 1.2 and without enzymes. An excess amount of each SHetA2 formulation was added to screw cap vessels containing 5 ml of SGF and the vessels were shaken for 24 h at 37°C inside an incubator shaker (New Brunswick Scientific Co., Inc. Edison, NJ). At predetermined time points, 0.083, 0.5, 1, 1.5, 2, 4, 6, 8, and 24 h, three 500 μl samples were withdrawn and replaced with equal volumes of fresh SGF. The SHetA2 concentration at each time point was determined by HPLC as described above.
Statistical Analysis
Significant differences in the data obtained in results were determined using one-way ANOVA Tukey’s test and Student’s t test using at a level of significance of P < 0.05.
RESULTS
Both URFD and SFD methods were suitable to produce dry SHetA2 powders. Regardless of the manufacturing method, the critical factor that influenced the dryness of the powders was the amount of trehalose added to the formulation. The higher the trehalose amount, the better powder formation (Table I). Among the three formulations evaluated for both URFD-SHetA2 and SFD-SHetA2, only the one corresponding to the 1:1 ratio of Kolliphor HS 15: trehalose (F3), resulted in solid and dry powders (Fig. 3). Therefore, the best formulation composition was determined to have a 1:1.5:1.5 proportion of SHetA2: Kolliphor HS 15: trehalose.
Powders Size, Shape, and Morphology
The shape and morphology of crystalline SHetA2, blank URFD, URFD-SHetA2, blank SFD, and SFD-SHetA2 powders are shown in Fig. 4. The SEM micrograph of crystalline unprocessed SHetA2 (Fig. 4a) revealed big and irregularly shaped powders covered with finer crushed powders. Blank URFD powders were big and non-uniform aggregates of thin and scale-like powders (Fig. 4b). Similarly, URFD-SHetA2 powders were big, dense, and irregularly shaped with smooth surface with few porous structures (Fig. 4c). Blank SFD were irregularly shaped and had a melted-like appearance (Fig. 4d), clearly showing precipitated trehalose crystals, while SFD-SHetA2 powders were smaller fragile aggregates, mostly in the form of hollow spheres with porous surface (Fig. 4e). URFD-SHetA2 powders were much bigger in size with volume diameter, Dv = 157.37 ± 0.127 μm compared to SFD-SHetA2 powders (Dv = 23.54 ± 0.68 μm). These volume diameters were in agreement with the geometric size observed from SEM images. In contrast, the GSD of URFD-SHetA2 powders (1.45 ± 0.07) was significantly smaller than the GSD of SFD-SHetA2 powders (2.33 ± 0.10) indicating a narrower size distribution for URFD than SFD powders.
Crystallinity
The XRD diffractograms of unprocessed SHetA2, URFD-SHetA2, and SFD-SHetA2 powders and their corresponding blanks are shown in Fig. 5. Unprocessed SHetA2 exhibited larger characteristic peaks at 5.00°, 17.50°, 19.83°, and 24.75° indicating its crystalline form (Fig. 5a). The XRD diffractogram of unprocessed SHetA2 also had other peaks at 16.5° and 24° that although being smaller than the main peaks, were present in a zone voided of peaks in the diffractograms of SFD and URFD blanks (Fig. 5b, c, respectively). These diffractograms were mostly identical to each other and included sharp peaks for trehalose with a prominent peak at 23.8°. The XRD diffractograms of SFD-SHetA2 and URFD-SHetA2 powders (Fig. 5d, e, respectively) shared most of the peaks, which were dominated by the trehalose peaks corresponding to those in the SFD and URFD blank diffractograms. Notably, the SHetA2 peak at 5.00° was absent in the SFD-SHetA2 and URFD-SHetA2 diffractograms. Tracking the remaining characteristic SHetA2 peaks in SFD-SHetA2 and URFD-SHetA2 diffractograms was not feasible due to the overlap with the trehalose peaks.
Thermal Analysis
Figure 6 compares the thermograms of URFD-SHetA2 and SFD-SHetA2 with those of unprocessed SHetA2, trehalose dihydrate alone, and their physical blend. Unprocessed SHetA2 had one endothermic peak at 168°C corresponding to its melting temperature (Tm). Trehalose dihydrate had two characteristic endothermic peaks at 100°C and 210°C corresponding to the evaporation of bound water and the melting point of trehalose, respectively. A few uncharacteristic peaks appeared at the temperature range of 110–130°C in the trehalose dihydrate thermogram due to the evaporation of unbound water. The thermogram of the physical mixture exhibited the characteristics of endothermic peaks of trehalose dihydrate for bound and unbound water evaporation and its melting peak. The thermograms of URFD-SHetA2 and SFD-SHetA2 powders were identical, showing only one well-defined peak corresponding to the melting point of trehalose.
Flow Properties of SHetA2 Powders
The flowability of URFD-SHetA2 and SFD-SHetA2 powders, as determined by their angle of repose and CCI, is presented in Table II. There was no significant difference in the angle of repose of URFD-SHetA2 and SFD-SHetA2 powders. According to USP <1174>, both powders have ‘good flow’ properties having their angles of repose in the range between 31 and 35°. In contrast, The CCI of URFD-SHetA2 and SFD-SHetA2 powders were significantly different (P < 0.05). URFD-SHetA2 powders had significantly higher compressibility index than SFD-SHetA2 powders. The USP scale (14) for the CCI categorizes the compressibility of URFD-SHetA2 powders as ‘very poor’ and compressibility of SFD-SHetA2 powders as ‘passable’.
Drug Content Uniformity
SHetA2 content in URFD-SHetA2 and SFD-SHetA2 powders was 104.57% ± 7.78 and 104.30% ± 2.12 respectively. There was no significant difference in SHetA2 content between the two powders with both having drug content within usual USP specifications for most dosage forms (90–110%). The drug content in SFD-SHetA2 powders was more uniform than of the URFD- SHetA2 powders as indicated by the variability (standard deviation) in the content.
Apparent Maximum Solubility of SHetA2 Formulations
The concentrations of SHetA2 as a function of time profiles of unprocessed, URFD-SHetA2, and SFD-SHetA2 in SGF are shown in Fig. 7. The initial solubility of unprocessed SHetA2 in SGF was very low (0.02 ± 0.01 μg/ml) and remained at this low level throughout the study period (Fig. 7 inset). In contrast, SHetA2 formulated as both URFD and SFD powders showed a prompt increase in concentration in SGF within 5 min reaching to a maximum solubility of 10.26 ± 0.24 μg/ml and 8.14 ± 2.2 μg/ml for URFD-SHetA2 and SFD-SHetA2 powders, respectively (Fig. 7). After this, the concentration of SHetA2 decreased gradually to a minimum of 6.47 ± 0.54 μg/ml and 5.84 ± 0.48 μg/ml for both URFD-SHetA2 and SFD-SHetA2 and remained relatively constant for 24 h. The highest and the lowest concentrations of SHetA2 observed in the solubility profiles of both powders were significantly higher (P < 0.05) than the solubility of unprocessed SHetA2 throughout the study. Furthermore, the maximum concentration of SHetA2 in URFD-SHetA2 powder (10.26 ± 0.24 μg/ml) was significantly higher than that of SFD-SHetA2 powder (8.14 ± 2.2 μg/ml). In contrast, no significant difference was observed between the lowest SHetA2 concentrations (approximately 6 μg/ml) observed for both powders.
DISCUSSION
SHetA2 is a novel chemopreventive drug with demonstrated efficacy in a number of cancer cell lines and xenograft animal models, but with poor aqueous solubility (0.02 ± 0.01 μg/ml). The addition of Kolliphor HS 15 significantly improved the solubility and oral bioavailability of SHetA2 after administration to dogs (9), as previously shown with the Akt Inhibitor SRI3668 in phase 0 clinical trials (16). Based on its efficacy and lack of toxicity, SHetA2 was advanced to phase 0 clinical trials funded by the NCI to determine its pharmacokinetic profile using dose escalation studies at very low exposure levels (e.g., 1/50 of rat’s No Observed Adverse Effect Level (NOAEL)).
The present work aimed to develop an oral dosage form of SHetA2, using the same ingredients as in the formal toxicological studies, for use in patients with cervical dysplasia. Our goal was to develop a formulation so that the SHetA2 dose could be adjusted according to the weight of patients in the trial. The allometric scaling studies performed by Sharma et al. predicted the “first in human dose” of SHetA2 to be 100 mg/Kg (17). For simplicity, our initial formulation consisted of a semisolid dispersion of SHetA2 in Kolliphor HS 15 but posed a challenge in the content uniformity of the dose. Thus, we formulated SHetA2 into a dry solid powder that could be easily metered.
We selected cryogenic technologies (URFD and SFD) to fabricate powders containing SHetA2 and Kolliphor HS (Fig. 2) to avoid difficulties that may arise due to the low melting point of Kolliphor HS 15, if techniques that require heat were used. To the best of our knowledge, this is the first study to report the lyophilization of Kolliphor HS 15 for the fabrication of solid powders by adding trehalose dihydrate as inert excipient widely used in the stabilization of lyophilized formulations, to the SFD and URFD formulations. The Kolliphor HS 15: trehalose ratio was optimized by visual inspection of the formed powders as described in Table I. A 1:1 ratio of Kolliphor HS 15 to trehalose was needed to form solid and dry powders with good flow properties (Fig. 3). Thus, F3 formulation (SHetA2: Kolliphor HS 15: trehalose 1:1.5:1.5) was used for SFD-SHetA2 and URFD-SHetA2.
The SEM micrographs (Fig. 4) showed the influence of both, the formulation composition and methodology, on the shape and morphology of the formed powders. The URFD-SHetA2 and SFD-SHetA2 powders (Fig. 4c, e) had more rugged morphologies compared to their corresponding blank powders (Fig. 4b, d). In the absence of the drug, trehalose in the blank URFD formed layered powders. In contrast, the high viscosity of the Kolliphor HS 15-trehalose that was SFD resulted in soft and molten like structures. The additional step of spraying SHetA2 suspension in SFD-SHetA2 powders resulted in small, hollow and spherically shaped powders compared to the irregularly shaped URFD-SHetA2 powders. SFD is well known to produce porous powders which can lead to high fragility. The SFD process parameters such as atomization pressure, feed flow rate as well as the feed composition can significantly contribute to the porosity and the fragility of powders formed by SFD. Zijstra et al. reported high fragmentation of their SFD cetrorelix acetate during mixing with lactose carrier due to the excessive fragility of the SFD powders (18). Thus, it is plausible that the wide size distribution of the SFD-SHetA2 powders compared to the URFD-SHetA2 powders may be due to the fragmentation of the powders caused by the shear force of the powder dispersion during particle size measurement by laser diffraction as reported by Rahmati et al. when they measured the size of SFD powders containing salmeterol xinafoate and lactose or mannitol (19). The URFD-SHetA2 powders were big and irregularly shaped, which correlates with the freezing of the suspension forming a film rather than droplets. Overhoff et al. reported the formation of non-uniform and plate-like URFD powders of 1:2 danazol and polyvinyl pyrolidone (PVP 15) in tert-butanol. Overhoff attributed the shape of the powders to the slow cooling rate of the solvent, which results in particle growth (12).
Flowability and compressibility are key characteristics for powders intended for oral dosage forms (20). Particle size, size distribution, and shape can significantly affect powder flowability (21). The angle of repose could not elucidate differences between the flowabilities of URFD-SHetA2 and SFD-SHetA2 powders despite their differences in size, shape, and porosity as shown by the equal values of the angle of repose (approximately 31°) that categorizes them as having “good” flowability. In contrast, the CCI indicated that the flowability of SFD-SHetA2 powders was better than that of URFD-SHetA2 powders (Table II). A few studies have reported contradicting flowability properties when using these two parameters of angle of repose versus CCI. Garmise et al. reported contradiction between the flow of SFD trehalose powders, which was “fair-no aid needed” according to the static angle of repose (36.1 ± 2.1) versus “very poor” according to the CCI (34.8 ± 0.90) (22). Likewise, El-Gendy et al. reported that freeze-dried fluticasone-albuterol nanoaggregates had “excellent flow” properties according to the angle of repose scale, yet their CCI showed “fair flow” (23). The discrepancy between the results of the angle of repose and CCI could be due to the difference in the measurement method as well as the rating scale for flow properties (20).
The crystallinity of SHetA2 in URFD-SHetA2 and SFD-SHetA2 could not be assessed using DSC analysis as the melting point of SHetA2 was not observed in the thermogram of the physical mixture (Fig. 5c). However, both the DSC analysis and the XRD diffractogram of unprocessed SHetA2 indicated its crystalline form prior to processing. According to the manufacturer (BASF), Kolliphor should be heated and melted to solubilize a drug. Thus, a physical mixture of SHetA2 and Kolliphor without increasing temperature does not solubilize SHetA2; it only does it when Kolliphor is melted. Therefore, a simple physical mixture of unprocessed SHetA2, Kolliphor, and trehalose before temperature ramping should not modify the crystalline form of the drug, yet the peak of the melting point of SHetA2 is absent in the thermogram of the physical mixture (Fig. 5c). A comparative analysis of the XRD diffractograms of all powders (Fig. 6) revealed the existence of two peaks (at 16.5° and 24°) in the X-ray diffractogram of the unprocessed drug and in the SHetA2 URFD and SFD powders that are not present in the diffractograms of the URFD and SFD blank powders. Thus, it is plausible that the drug may be in a partial amorphous state or in a molecular dispersion. Further evaluations of these powders are required to support either of these assumptions.
Our findings agree with those of Seo et al. (24) who found that after a drug was dissolved in Kolliphor, it becomes partially amorphous based on the disappearance of some peaks in the X-ray diffractograms and the remaining peaks becoming smaller as the proportion of Kolliphor in the mixture increases. In Seo’s study like in ours, the melting peak of the drug also disappeared in the DSC thermograms when the drug was in a physical blend with Kolliphor, or dissolved in it. Likewise, in a study using PEG 4000 to enhance the solubility of alpha-asarone (25), DSC analysis indicated that the crystallinity of the drug was completely lost in the physical mixture and the solid dispersion with PEG. In contrast, XRD analysis revealed that alpha-asarone was in a microcrystalline form in the solid dispersion with PEG 4000 (25). Similar results of DSC and XRD analyses were reported by Zahedi, et al. using diclofenac sodium and poly hydroxyethyl methacrylate (26) and by Hu et al. using curcumin, Kolliphor RH40, poloxamer 188, and PEG 4000 (27).
Evaluation of the dissolution rate of drugs that are poorly soluble in water under sink conditions is challenging without a flow through cell apparatus (USP apparatus 4). Thus, apparent maximum solubility studies in non-sink conditions are used for the determination of the level and duration of supersaturation of the drug in a relevant fluid. Childs et al. showed a strong correlation between the level and duration of supersaturation of danazol: vanillin cocrystal achieved by 1% vitamin E TPGS as a solubilizer and 2% hydroxypropyl cellulose as precipitation inhibitor (28). Therefore, this approach was used in the present study. In the apparent solubility profiles of URFD-SHetA2 and SFD-SHetA2 powders (Fig. 7), the high transit supersaturation phase (approximately 0–2 h) followed by an extended supersaturation phase (2–24 h) may be attributed to the “spring” and “parachute” effects, respectively. The “spring” effect refers to transient, and elevated apparent solubility (in this case, about 300–500 fold increase compared to the unprocessed drug) for high energy and unstable forms of the drug leading to a transient supersaturation in the dissolution medium. The subsequent parachute effect refers to the extended enhancement of the drug’s solubility (about 300–400 fold increase in solubility compared to unprocessed drug) by preventing its recrystallization and precipitation using a “parachute” or precipitation inhibitor (29).
Previously, we reported an enhancement in the solubility of SHetA2 caused by the transformation into the amorphous form while being spray dried into microparticles for inhalation (30). This solubility enhancement was observed for microparticles formed of drug alone, and in a 50:50 proportion with either mannitol or PLGA. However, this enhancement was transient (5–45 min, spring effect) and was comparable to that of the unprocessed drug within 4 h because none of these formulations included excipients to stabilize the molecular mobility of the drug in the microparticles. Therefore, in the present study, it is likely that the presence of Kolliphor in the powder formulation acted as the “parachute” that prevented the precipitation of SHetA2, as reported by the use of surface active agents or self-emulsifiers used in third generation solid dispersions (31). Kalivoda et al. hot-melt extruded a blend of Soluplus®, a solubilizing agent similar to Kolliphor, copovidone, and hypromellose to improve the solubility of oxeglitazar, a novel compound to treat type II diabetes (32). The solubility of the drug in the resulting extrudate was three-fold higher than that of the unprocessed drug, and at least 20% higher than when extruded without the Soluplus®.
Future studies will explore the use of other excipients, such as low molecular weight polymers to reduce the conversion of SHetA2 from the amorphous to the crystalline form as well as other methods of manufacture. Alternatively, nanocrystal formulations of the drug will also be considered.
CONCLUSION
Dry powders of SHetA2 and Kolliphor HS 15 mixture were fabricated in a small scale using SFD and URFD methods. SFD-SHetA2 powder had better flow properties that URFD-SHetA2 powders, which would facilitate manual capsule filling. The apparent maximum solubility was enhanced in SHetA2 powders fabricated with both methods. Thus, it is expected that the SHetA2 powder formulation would result in an oral bioavailability of SHetA2 in humans similar to that determined in the formal toxicological studies performed in dogs with the suspension.
References
Benbrook DM, Guruswamy S, Wang Y, Sun Z, Mohammed A, Zhang Y, et al. Chemoprevention of colon and small intestinal tumorigenesis in APC(min/+) mice by SHetA2 (NSC721689) without toxicity. Cancer Prev Res (Phila). 2013;6(9):908–16.
Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:195727.
Benbrook DM, Kamelle SA, Guruswamy SB, Lightfoot SA, Rutledge TL, Gould NS, et al. Flexible heteroarotinoids (Flex-Hets) exhibit improved therapeutic ratios as anti-cancer agents over retinoic acid receptor agonists. Investig New Drugs. 2005;23(5):417–28.
Liu S, Brown CW, Berlin KD, Dhar A, Guruswamy S, Brown D, et al. Synthesis of flexible sulfur-containing heteroarotinoids that induce apoptosis and reactive oxygen species with discrimination between malignant and benign cells. J Med Chem. 2004;47(4):999–1007.
Liu T, Masamha CP, Chengedza S, Berlin KD, Lightfoot S, He F, et al. Development of flexible-heteroarotinoids for kidney cancer. Mol Cancer Ther. 2009;8(5):1227–38.
Liu T-Z, Hannafon B, Gill L, Kelly B, Benbrook DM. Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria. Mol Cancer Ther. 2007;6:1814–22.
Guruswamy S, Lightfoot S, Gold MA, Hassan R, Berlin KD, Ivey RT, et al. Effects of retinoids on cancerous phenotype and apoptosis in organotypic cultures of ovarian carcinoma. J Natl Cancer Inst. 2001;93(7):516–25.
Kabirov KK, Kapetanovic IM, Benbrook DM, Dinger N, Mankovskaya I, Zakharov A, et al. Oral toxicity and pharmacokinetic studies of SHetA2, a new chemopreventive agent, in rats and dogs. Drug Chem Toxicol. 2013;36(3):284–95.
PHARMA B. Excipients for Drug Formulation. BASF; 2018 [cited 2018 03/01/2018]; Available from: https://pharmaceutical.basf.com/en/Drug-Formulation/Kolliphor-HS-15.html.
Cheow WS, Ng ML, Kho K, Hadinoto K. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: effect of freeze-drying adjuvants. Int J Pharm. 2011;404(1–2):289–300.
Overhoff KA, Engstrom JD, Chen B, Scherzer BD, Milner TE, Johnston KP, et al. Novel ultra-rapid freezing particle engineering process for enhancement of dissolution rates of poorly water-soluble drugs. Eur J Pharm Biopharm. 2007;65(1):57–67.
Wanning S, Suverkrup R, Lamprecht A. Pharmaceutical spray freeze drying. Int J Pharm. 2015;488(1–2):136–53.
USP USP. <1174> Powder Flow. USP29-NF24.28(2):618.
Nielsen LH, Gordon S, Holm R, Selen A, Rades T, Mullertz A. Preparation of an amorphous sodium furosemide salt improves solubility and dissolution rate and leads to a faster Tmax after oral dosing to rats. Eur J Pharm Biopharm. 2013;85(3 Pt B):942–51.
Reid JM, Walden CA, Qin R, Ziegler KL, Haslam JL, Rajewski RA, et al. Phase 0 clinical chemoprevention trial of the Akt inhibitor SR13668. Cancer Prev Res. 2011;4(3):347–53.
Sharma A, Benbrook DM, Woo S. First-in-human dose determination for a phase 0 study of SHetA2, a novel anticancer agent using the interspecies scaling. American Association of Pharmaceutical Scientists (AAPS): San Antonio; 2013.
Zijlstra GS, Hinrichs WL, de Boer AH, Frijlink HW. The role of particle engineering in relation to formulation and de-agglomeration principle in the development of a dry powder formulation for inhalation of cetrorelix. Eur J Pharm Sci. 2004;23(2):139–49.
Rahmati MR, Vatanara A, Parsian AR, Gilani K, Khosravi KM, Darabi M, et al. Effect of formulation ingredients on the physical characteristics of salmeterol xinafoate microparticles tailored by spray freeze drying. Adv Powder Technol. 2013;24(1):36–42.
Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008;9(1):250–8.
Liu LX, Marziano I, Bentham AC, Litster JD, White ET, Howes T. Effect of particle properties on the flowability of ibuprofen powders. Int J Pharm. 2008;362(1–2):109–17.
Garmise RJ, Staats HF, Hickey AJ. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech. 2007;8(4):E81.
El-Gendy N, Pornputtapitak W, Berkland C. Nanoparticle agglomerates of fluticasone propionate in combination with albuterol sulfate as dry powder aerosols. Eur J Pharm Sci. 2011;44(4):522–33.
Seo SW, Han HK, Chun MK, Choi HK. Preparation and pharmacokinetic evaluation of curcumin solid dispersion using Solutol(R) HS15 as a carrier. Int J Pharm. 2012;424(1–2):18–25.
Deng L, Wang Y, Gong T, Sun X, Zhang ZR. Dissolution and bioavailability enhancement of alpha-asarone by solid dispersions via oral administration. Drug Dev Ind Pharm. 2017;43(11):1817–26.
Zahedi P, Lee PI. Solid molecular dispersions of poorly water-soluble drugs in poly(2-hydroxyethyl methacrylate) hydrogels. Eur J Pharm Biopharm. 2007;65(3):320–8.
Hu L, Shi Y, Li JH, Gao N, Ji J, Niu F, et al. Enhancement of oral bioavailability of curcumin by a novel solid dispersion system. AAPS PharmSciTech. 2015;16(6):1327–34.
Childs SL, Kandi P, Lingireddy SR. Formulation of a danazol cocrystal with controlled supersaturation plays an essential role in improving bioavailability. Mol Pharm. 2013;10(8):3112–27.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72.
Ibrahim M, Hatipoglu MK, Garcia-Contreras L. SHetA2 dry powder aerosols for tuberculosis: formulation, design, and optimization using quality by design. Mol Pharm. 2018;15(1):300–13.
Vo CL, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3 Pt B):799–813.
Kalivoda A, Fischbach M, Kleinebudde P. Application of mixtures of polymeric carriers for dissolution enhancement of oxeglitazar using hot-melt extrusion. Int J Pharm. 2012;439(1–2):145–56.
Acknowledgments
We appreciate the generous gift of SHetA2 by Dr. Doris Benbrook at the Stephenson Cancer Center and of Kolliphor HS 15 from the BASF Company. We are thankful for Dr. Andrew Madden and Brittany Pritchett for performing the XRD analysis. This work was supported by start-up funds for Dr. Garcia-Contreras provided by the University of Oklahoma, College of Pharmacy. Ms. Ibrahim was partially supported by a Fulbright scholarship from Egypt.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, M., Hatipoglu, M.K. & Garcia-Contreras, L. Cryogenic Fabrication of Dry Powders to Enhance the Solubility of a Promising Anticancer Drug, SHetA2, for Oral Administration. AAPS PharmSciTech 20, 20 (2019). https://doi.org/10.1208/s12249-018-1204-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1204-z