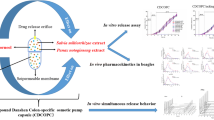
Abstract
In the current study, a novel colon-specific osmotic pump capsule of Panax notoginseng saponins was developed to achieve colon-specific release, a zero-order, thus to promote the efficacy of Panax notoginseng saponins. The capsule was assembled using a semi-permeable capsule shell with contents including Panax notoginseng saponins, sodium chloride (NaCl), and Ludipress. The semipermeable membrane was made of cellulose acetate (CA), along with polyethylene glycol (PEG) 6000 for flexibility and strength, and Eudragit® S100 for colon-specific targeting. The in vitro dissolution test showed an approximately zero-order release of Panax notoginseng saponins over 12 h at pH 7.8 through the pores on the membrane. Meanwhile, the drug release from the optimal formulation was found to be independent of equipment type or agitation speed. Rather, it depended on mainly the osmotic pressure of the dissolution media. The in vivo test in beagle dogs demonstrated that the relative bioavailability of the current system was 487.42% in comparison to that of the marketed product, yet with a prolonged retention time. The novel controlled delivery system for Panax notoginseng saponins in the current study utilizing colon-specific and osmotic pump system therefore offered the advantages of avoiding stomach and enteric irritation, reducing dosage frequency, minimizing the drug fluctuation in plasma, and improving its oral bioavailability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Panax notoginseng saponins, extracted from the traditional Chinese herbal medicine Panax notoginseng (1), and it have some unique physiological effects including the promotion of arterial blood flow, the dilation of blood vessels, the reduction of arterial blood pressure, the reduction of myocardial oxygen consumption, the enhancement the body’s immune function, and the improvement of tolerance to hypoxia (2,3). Panax notoginseng saponins are used to treat the cardiovascular disease in elderly patients by oral administration (4,5). However, Panax notoginseng saponins can be degraded into aglycone and carbohydrate in gastric fluid, which results in a reduced oral bioavailability (6). To compensate the poor bioavailability, frequent dosing is usually required which causes fluctuation in plasma concentration and poor patient compliance. At present, the commercial preparations mainly include injections and oral preparations, the injection of Panax notoginseng saponins, such as Xuesaitong injection, Xuesaitong freeze-dried powder needle, Xueshuantong injection, and Xueshuantong freeze-dried powder, have been widely used in clinical practice. But the impurities and purity of traditional Chinese medicine injections are not easy to control, and the incidence of adverse reactions is high (7). On the other hand, common oral preparation, such as tablets, granules, and capsules, is easily destroyed in acidic digestive juices of the gastrointestinal tract, with low the bioavailability, and large dose and many times of daily dose, which is inconvenient for the patients. Therefore, the current study aims to develop a delivery system to control the release and improve the oral bioavailability of Panax notoginseng saponins.
The colon is located in the right iliac fossa to the cecum and is connected to the rectum in the third sacral vertebra (8). The close to neutral environmental pH and lower biological enzymatic activities in colon make it an ideal site for the delivery of drugs which suffer from gastric degradation (9,10,11,12). The oral colonization drug delivery system was first developed at the late 1980s (13). After oral administration, the system started to release drug when it was transported to the appendix part of the colon; thus, this drug release way avoided the drug exposure in the upper gastrointestinal tract environment, greatly enhancing the oral bioavailability of multiple drugs (14,15,16,17). On the other hand, the osmotic drug delivery systems (OPS) which utilize osmotic pressure as energy source and driving force for sustained drug release can improve patient compliance by reducing the dosing frequency (18,19,20,21,22,23). Moreover, the drug release generally follows zero-order kinetics which is not affected by various factors such as gastrointestinal motility, media pH, and presence of food (24,25,26,27,28). Therefore, the colon-specific OPS may solve both the problems of Panax notoginseng saponins: gastric degradation and frequent dosing.
In this study, a new Panax notoginseng saponins colon-specific osmotic pump capsule has been developed, combine the oral colon-targeting with osmotic pump techniques. It can achieve colon positioning and zero-order release thus improves the bioavailability of Panax notoginseng saponins. And, this new delivery system also features simple formulation and preparation process, with a potential for delivering a variety of active pharmaceutical ingredient.
MATERIALS AND METHODS
Materials
Panax notoginseng saponins was obtained from Kunming Jintaide Pharmaceutical Company (Yunnan, China). Eudragit® S100 was gifted by Evonik Company (Essen, Germany). NaCl, Cellulose acetate (CA) was gotten from Sinopharm Chemical Reagent Company (Shanghai, China). Polyethylene glycol (PEG) with average molecular weight (MW) of 400 (PEG 400), 4000 (PEG 4000), and 6000 (PEG 6000) was obtained from Anhui Shanhe Pharmaceutical Excipient Company (Anhui, China). Ludipress was received from BASF SE (Ludwigshafen, Germany). Acetone was obtained from Shanghai Pilot Chemical Company (Shanghai, China). Astragaloside was purchased from Chinese Food and Drug Inspection Institute (Beijing, China). Stainless steel capsule molds (size 1) and colon-specific capsule shells were obtained from Anhui Huangshan Capsule Company (Anhui, China). Xuesaitong capsule (regular Panax notoginseng saponins capsules, 100 mg/capsule) was manufactured by Yunnan Weihe Pharmaceutical Company (Yunnan, China). All other chemicals were of analytical grade, and deionized double-distilled water was used throughout the study.
Methods
Preparation of the Colon-Specific Osmotic Pump Capsule Shells
The shells (body and cap) of the osmotic pump capsules composed of semipermeable membrane were prepared by the dip plastic method (29,30,31). Briefly, a coating solution was formed by dissolving CA, PEG (PEG 400, PEG 4000 or PEG 6000), and Eudragit® S100 in the mixed solvent of acetone and water (9.5 mL/0.5 mL). The stainless steel mold was firstly dipped into the coating solution. Then, the mold was slowly withdrawn at a constant speed before it was rotated horizontally. The above operations were repeated for 3 times. The mold was dried in a temperature and humidity-controlled chamber (25°C, 40–50% RH) for 2 h. The capsule shells were removed from the molds by a capsule plier and then cut into the desired size. Finally, the capsule caps were drilled at the tip by a Lumonics Phase I laser (Tianjin Institute of Industrial Automation Instrument, Tianjin, China) and then the 0.8 mm holes were sealed with 15% (w/v) Eudragit® S100 solution. The compositions of the capsule shells are given in Table I.
Preparation of the Colon-Specific Osmotic Pump Capsules
Panax notoginseng saponins (150 mg), NaCl (different dosage) and Ludipress (different dosage) powders were passed through a 100-mesh screen separately and then mixed. The mixture was placed into the capsule body. The capsule body and cap were sealed with the coating solution to ensure that no drug would leak out.
In Vitro Release Test
Release studies were carried out by the dissolution and release of the first law (basket method) in the 15th edition of the Chinese Pharmacopoeia in a RCZ-8M dissolution device (Tianda Tianfa Technology Co., Ltd., Tianjin, China). The rotation speed was kept at 100 rpm, the temperature was maintained at 37.5 ± 0.5°C, and the dissolution media volume is 900 mL. The capsules were merged first in the simulated gastric fluid (SGF, pH 1.2) for 2 h, and then in the phosphate buffer saline (PBS, pH 6.8) for 3 h, and at last in another PBS (pH 7.8) for 12 h. Samples of 5 mL were withdrawn and replaced by the fresh medium of the same volume at the predetermined time intervals (2, 3, 5, 7, 9, 11, 13, 15, and 17 h). All samples were treated with 5% vanillin-acetic acid (0.2 mL) and perchloric acid (0.8 mL) based on colorimetric method prior to passing through a 0.45-μm membrane filter. Panax notoginseng saponins were measured at 560 nm by an UV-Vis spectrophotometer, and the cumulative release was calculated at each time point. The similarity factor (ƒ2) suggested by the Chinese FDA (CFDA) was employed to evaluate the similarity between the release profiles. It was calculated as follows: (32,33).
where Rt and Tt represent the drug release percentage at time t of the reference formulation and test formulation, respectively; n is the number of the time points. The two dissolution profiles were considered similar when the ƒ2 value was more than 50.
Evaluation of Drug Release Kinetics
Release data obtained from the optimized formulation in PBS (pH 7.8) were fitted to zero-order, first-order and Higuchi models. The coefficient of correlation was utilized to assess the fitting goodness of the drug release profiles.
Pharmacokinetics in Beagle Dogs
The pharmacokinetic study in beagle dogs was carried out by a randomized and single-dose design. The healthy male beagle dogs with a mean age of 3 ± 1 years, mean weight of 15 ± 2.5 kg were purchased from the Animal Experimental Center of Anhui Medical University (Hefei, China). The animal experiments were approved and supervised by the Animal Experimental Ethical Committee of Anhui Medical University (Hefei, China). Nine beagle dogs were fasted for 12 h and randomly divided into three groups prior to the drug administration. Group A were given three commercial Xuesaitong capsules each containing Panax notoginseng saponins 100 mg, while Group B two colon-specific osmotic pump capsules each containing Panax notoginseng saponins 150 mg. All the capsules were administered orally with 200 mL water via a feeding tube. About 3 mL of venous blood samples was collected in heparinized tubes before drug administration and at the predetermined time intervals. The sampling time points were 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 24, and 36 h for Group A and 2, 3, 5, 6, 7, 9, 11, 13, 15, 17, 24, 30, and 36 h for Group B, respectively. Plasma samples were obtained by centrifugation of the blood samples (3000 rpm, 10 min) and stored at − 20°C until analysis. Twenty-microliter internal standard solution (methanol solution of astragaloside, 150 ng/mL) and 450 μL acetonitrile were added in each 150 μL plasma. The mixture was vortexed for 2 min and centrifuged at 12,000 rpm for 10 min. The supernatant was separated and dried at 40°C in the water bath. The residue was reconstituted with 150 μL methanol and vortexed for 1 min, followed by centrifugation for 10 min at 12,000 rpm. Four microliters of the supernatant was directly injected for the UPLC-MS/MS analysis (34).
UPLC-MS/MS Assay Method for Panax notoginseng Saponins
The UPLC-MS/MS analyses were carried out with an Agilent 1290 UPLC system and a Triple QuadTM 4500 Mass Spectrometers (AB SCIEX Company, USA), equipped with an electrospray ionization source and an auto-sampler. The drug was separated at 40°C by a Waters ACQUITY UPLC® BEH C18 column (100 mm × 2.1 mm, 1.7 μm) at a flow rate of 0.4 mL/min. Gradient elution with water (A) and acetonitrile (B) was used according to the following profile: 0–1 min, 80%A, 1–2.5 min, 60% A, 2.5–3.0 min, 42% A, 3.0–4.0 min, 30%A, 4.0–4.5 min, 30%A, 4.5–4.6 min, 80%A, 4.6–5.0 min, 80%A (35). The ESI source, positive ion mode, and the optimized mass spectrometry conditions were as follows: IV 5500 V, TEM 500°C, CUR 20 psi, Gas 1 50 psi, and Gas 2 50 psi. The optimized parameters of the five kinds of saponins (notoginsenoside R1, ginsenoside Rg1, Rb1, Re, Rd) and IS mass spectrometry multi-reaction monitoring (MRM) were presented in Table II.
Statistical Analysis
The DAS 2.0 pharmacokinetics software was used to calculate pharmacokinetics parameters. All data in this study were expressed as mean ± standard deviation. Data were analyzed by one-way analysis of variance (ANOVA).
RESULTS AND DISCUSSIONS
Influence of the Concentration of CA on Shell Forming
The osmotic pump capsule shells were prepared by dip plastic method with CA concentration of 6, 8, and 10%, respectively. During the preparation, the shell was hard to be pulled off with a poor toughness when the CA concentration was 6%. On the other hand, when the CA concentration was 10%, different degrees of bubbles occurred and toughness was poor also. A CA concentration at 8% resulted in the best shell forming.
Influence of Plasticizer Type and Concentration on Drug Release
PEG was used as the plasticizer to improve the flexibility and strength of the capsule shell in the current study. The type and level of PEG were evaluated by the cumulative release of drug and the similarity factor. The release profiles of capsules with different types of the plasticizers (PEG 400, PEG 4000, PEG 6000) were shown in Fig. 1. Compared with the other two plasticizers, PEG 6000 resulted in more drug release and the release profile closer to zero-order kinetics. The optimal concentration of PEG 6000 in the capsule shell was further evaluated (Fig. 2). A 20% PEG 6000 yielded less than 60% total cumulative drug release. When the concentration of PEG 6000 was increased to 25%, the drug release curve fitted better to zero-order kinetics and more than 90% drug release was achieved. Although a further increase to 30% of PEG 6000 demonstrated a similar total release as 25% PEG 6000, more drug release were also observed during the first 3–5 h, i.e., in pH 6.8 PBS, which was undesired. The increased drug release rate at higher PEG 6000 concentration could be due to its porogenic property. More micropores would be formed on the capsule shell to facilitate the drug diffusion. Therefore, PEG 6000 (25% of CA in dry weight, w/w) was selected as the optimized plastisizer.
Influence of Colon-Dissolving Agent Concentration on Drug Release
Colon-dissolving materials (soluble at pH 7.8) can be used as a pore former to adjust the permeability of the capsule shells to achieve the desired drug releasing rate. In the present study, pH-sensitive material Eudragit® S100 was used as the colon-dissolving agent. The effects of different concentrations of Eudragit®S100 (30% 35, and 40%, of CA dry weight, w/w) were investigated on the release of the drugs (Fig. 3). The different concentration of Eudragit® S100 had a significant impact on the drug release. The release of the drug was accelerated as the concentration of Eudragit® S100 increased. Taken into consideration that a better fitting to zero-order kinetics and a complete release were both achieved by 35% of Eudragit® S100, this concentration was used in the optimized formulation.
Influence of Osmotic Agent Concentration on Drug Release
Drug release from the osmotic pump capsules requires certain osmotic pressure. In general, the drug itself does not produce enough osmotic pressure. Therefore, osmotic active substances are usually incorporated into the capsule content in order to boost the osmotic pressure gradient between the inside and outside of the capsule shell (36). NaCl is an osmotic active substance commonly used in oral osmotic pump preparations, providing a higher osmotic pressure at the equivalent amount when compared with other osmotic agents. In the current study, NaCl was tested at 35, 40, and 45% (w/w) of the total content. The corresponding drug release curves were illustrated in Fig. 4.
The results indicate a faster and more complete release as the NaCl concentration increased. When the concentration of NaCl was 45%, the release of the drug was too rapid and a complete release was achieved within 12 h. Contrarily, 35% NaCl was not able to maintain sufficient pumping force in the later stage and resulted incomplete release. When 40% NaCl was used, the release rate was consistent and close to zero-order kinetics. Therefore, 40% NaCl was selected as the optimum concentration.
In Vitro Release Test
In Vitro Release Test Influence of Rotation Speed on Drug Release
Factors such as age, gender, and physical condition have impacts on gastrointestinal motility. Therefore, the rotation speed of dissolution apparatus (50, 75, and 100 rpm) was investigated. The results demonstrate identical drug release manner despite of the rotation speed of the equipment, indicating that the release of drug was free from the impact of the gastrointestinal motility in the body.
Influence of Dissolution Apparatus on Drug Release
The effects of dissolution apparatus on drug release were investigated by basket method and paddle method. The results demonstrate no impact of the types of the device on drug release kinetics. But in the process of the paddle method, the capsule floats on the water and is easily broken by the blades, causing the drug to leak. Therefore, we finally chose the basket method.
Evaluation of Drug Release Kinetics
Drug Release Pathway
To investigate whether Panax notoginseng saponins were released from the micropores on the surface of the capsule or through the punched pore on the cap, capsules without laser-drilled holes on the cap were compared to the capsules with the holes. The release curve of the holed and non-holed capsule was presented in Fig. 5. More than 90% Panax notoginseng saponins was released from the holed-capsules within 12 h, while less than 20% of the drug was released from its counterpart. Therefore, drug was primarily released through the hole on the capsule cap, driven by the osmotic pressure. Yet diffusion through micropores on the surface had minor impact on drug release.
Influence of Dissolution Medium on Drug Release
The osmotic pressure of 0.45, 0.9, and 1.8% sodium chloride solution was determined as 149, 285, and 561 mOsm/kg by AUTO.F.P. Osmometer (Shanghai Medical University Instrument, Shanghai, China), respectively. As illustrated in Fig. 6, slower and incomplete drug releases were observed when the osmotic pressure of the medium increased.
Drug Release Kinetics
The results of study on drug release kinetics revealed that after a certain lag time (5 h), as summarized in Table III, zero-order kinetics best describes the release, with a regression coefficient higher than 0.99.
Pharmacokinetic Studies
The plasma concentration versus time profiles of the five active ingredients in Panax notoginseng saponins after oral administration of the commercially available capsules and the colon-specific osmotic pump capsules in beagle dogs were illustrated in Fig. 7. It could be seen that, the commercially available capsules reached significantly higher peak in a shorter time period but dropped abruptly as compared with the other. Contrarily, the colon-specific osmotic pump capsules showed a delayed peak after 5 h, yet declined slowly. In addition, the peak concentrations were observed during 7 to 15 h in the case of the colon-specific osmotic pump capsules, suggesting delayed drug release. And, the peaks by the colon-specific osmotic pump capsules were wider than those by the commercial capsules, indicating prolonged absorption. The main pharmacokinetics parameters of five active ingredients of Panax notoginseng saponins were summarized in Table IV. Compared with the commercial capsule, colon-specific osmotic pump capsules exhibited a prolonged MRT (14 versus 7 h of commercial capsule), demonstrating that a more long-acting effect and more stable drug plasma concentration. Furthermore, the integrated AUC0-t of the five active ingredients increased from 227.04 ng h mL−1 for the commercial capsules to 1106.64 ng h mL−1 for the colon-specific osmotic pump capsules. In other words, a 487.42% relative bioavailability was achieved by the colon-specific osmotic pump capsules as compared with the commercial capsules.
CONCLUSIONS
In the present study, a novel colon-specific osmotic pump capsule for controlled delivery of Panax notoginseng saponins was successfully designed and investigated. The capsule shell prepared by dipping method consisted of CA, PEG 6000 and Eudragit® S100 at optimized concentrations to achieve zero-order drug release. In addition, drug release was not affected by the rotation speed or the type of the dissolution apparatus. Panax notoginseng saponins was released mainly through the orifice on the capsule cap by osmotic pump effect and a small portion diffusing through the micropores on the capsule body. The in vivo study in beagle dogs after oral administration of commercial capsule and colon-specific OPS of Panax notoginseng saponins indicated a delayed and controlled release of Panax notoginseng saponins from the novel colon-specific OPS, resulting in 487.42% relative bioavailability compare with the commercial capsules. In summary, the oral colonization drug delivery system combined with osmotic drug delivery system opened a feasible and promising way to improve the oral bioavailability, reduce dosing frequency and minimize the plasma fluctuation for drugs which are labile to acidic pH in the stomach.
References
Wang D, Jie Q, Liu B, Li Y, Dai L, Luo J, et al. Saponin extract from panax notoginseng promotesangiogenesis through ampk and enosdependent pathways in huvecs. Mol Med Rep. 2017;16(4):5211–8.
Li Y, Zhang Y, Zhu CY. Pharmacokinetics and correlation between in vitro release and in vivo absorption of bio-adhesive pellets of panax notoginseng saponins. Chin J Nat Med. 2017;15(2):142–51.
Song H, Wang P, Liu J, Wang C. Panax notoginseng preparations for unstable angina pectoris: a systematic review and meta-analysis. Phytother Res. 2017;31(8):1162–72.
Pang HH, Li MY, Wang Y, Tang MK, Ma CH, Huang JM. Effect of compatible herbs on the pharmacokinetics of effective components of panax notoginseng in fufang xueshuantong capsule. J Zhejiang Univ Sci B. 2017;18(4):343–52.
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603.
Duan L, Xiong X, Hu J, Liu Y, Li J, Wang J. Panax notoginseng saponins for treating coronary artery disease: a functional and mechanistic overview. Front Pharmacol. 2017;8:1–18.
Chen YM, Li YW, Zhu GH. Panax notoginseng saponins injection induced 103 cases of adverse reactions analysis. Chin J Chin Mater Med. 2010;35(2):237–9.
Banerjee A, Pathak S, Subramanium VD, G D, Murugesan R, Verma RS. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discov Today. 2017;22(8):1224–32.
Vong LB, Mo J, Abrahamsson B, Nagasaki Y. Specific accumulation of orally administered redox nanotherapeutics in the inflamed colon reducing inflammation with dose-response efficacy. J Control Release. 2015;210:19–25.
Chaudhary A, Tiwari N, Jain V, Singh R. Microporous bilayer osmotic tablet for colon-specific delivery. Eur J Pharm Biopharm. 2011;78(1):134–40.
SSDaG V. Rational design and development of colon-specific prodrugs. Curr Top Med Chem. 2011;11:2318–45.
Jin L, Ding YC, Zhang Y, Xu XQ, Cao Q. A novel ph-enzyme-dependent mesalamine colon-specific delivery system. Drug Des Devel Ther. 2016;10:2021–8.
Sanjay K, Jain AJ. Target specific drug release to the colon. Expert Opin Drug Deliv. 2008;5(5):483–98.
Ruiz JF, Kedziora K, Windle H, Kelleher DP, Gilmer JF. Investigation into drug release from colon-specific azoreductase-activated steroid prodrugs using in-vitro models. J Pharm Pharmacol. 2011;63(6):806–16.
Wen P, Feng K, Yang H, Huang X, Zong MH, Lou WY, et al. Electrospun core-shell structured nanofilm as a novel colon-specific delivery system for protein. Carbohydr Polym. 2017;169:157–66.
Patel MM. Colon targeting: an emerging frontier for oral insulin delivery. Expert Opin Drug Deliv. 2013;10(6):731–9.
Fang WY. Studies on oral colon-specific capsules of panax notoginsens saponins liposomes [master]: Anhui University of Chinese Medicine. 2015.
Xie Y, Lu Y, Qi J, Li X, Zhang X, Han J, et al. Synchronized and controlled release of multiple components in silymarin achieved by the osmotic release strategy. Int J Pharm. 2013;441(1–2):111–20.
Liu X, Wang S, Chai L, Zhang D, Sun Y, Xu L, et al. A two-step strategy to design high bioavailable controlled-release nimodipine tablets: the push-pull osmotic pump in combination with the micronization/solid dispersion techniques. Int J Pharm. 2014;461(1–2):529–39.
Pan H, Jing H, Yang X, Pan W, Chen T. Synchronized and controlled release of metformin hydrochloride/glipizide from elementary osmotic delivery. Drug Dev Ind Pharm. 2017;43(5):780–8.
Wu L, LI HY, YIN XZ, LI Y, CHEN JX, HU RF, et al. Applicability of a natural swelling matrix as the propellant of osmotic pump tablets. Acta Pharm Sin. 2013;48(08):1319–24.
Liu D, Yu S, Zhu Z, Lyu C, Bai C, Ge H, et al. Controlled delivery of carvedilol nanosuspension from osmotic pump capsule: in vitro and in vivo evaluation. Int J Pharm. 2014;475(1–2):496–503.
Banerjee A, Verma PR, Gore S. Controlled porosity solubility modulated osmotic pump tablets of gliclazide. AAPS PharmSciTech. 2015;16(3):554–68.
Zeng QP, Liu ZH, Huang AW, Zhang J, Song HT. Preparation and characterization of silymarin synchronized-release microporous osmotic pump tablets. Drug Des Devel Ther. 2016;10:519–31.
Waterman KC, Goeken GS, Konagurthu S, Likar MD, MacDonald BC, Mahajan N, et al. Osmotic capsules: a universal oral, controlled-release drug delivery dosage form. J Control Release. 2011;152(2):264–9.
Li S, Wang X, Wang Y, Zhao Q, Zhang L, Yang X, et al. A novel osmotic pump-based controlled delivery system consisting of ph-modulated solid dispersion for poorly soluble drug flurbiprofen: in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2015;41(12):2089–99.
Guan J, Zhou L, Pan Y, Han H, Xu H, Pan W. A novel gastro-retentive osmotic pump capsule using asymmetric membrane technology: in vitro and in vivo evaluation. Pharm Res. 2010;27(1):105–14.
Hill A, Geissler S, Weigandt M, Mader K. Controlled delivery of nanosuspensions from osmotic pumps: zero order and non-zero order kinetics. J Control Release. 2012;158(3):403–12.
Bhatt B, Kumar V. Regenerated cellulose capsules for controlled drug delivery: part iii. Developing a fabrication method and evaluating extemporaneous utility for controlled-release. Eur J Pharm Sci. 2016;91:40–9.
Bhatt B, Kumar V. Regenerated cellulose capsules for controlled drug delivery: part iv. In-vitro evaluation of novel self-pore forming regenerated cellulose capsules. Eur J Pharm Sci. 2017;97:227–36.
Bhatt B, Kumar V. Regenerated cellulose capsules for controlled drug delivery, part 2: modulating membrane permeability by incorporation of depolymerized cellulose and altering membrane thickness. J Pharm Sci. 2015;104(12):4266–75.
Viness Pillay RF. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J Control Release. 1998;55:45–55.
Shah VP, Tsong Y, Sathe P, Liu JP. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15(6):889–96.
Guo Q, Li P, Wang Z, Cheng Y, Wu H, Yang B, et al. Brain distribution pharmacokinetics and integrated pharmacokinetics of panax notoginsenoside r1, ginsenosides rg1, rb1, re and rd in rats after intranasal administration of panax notoginseng saponins assessed by uplc/ms/ms. J Chromatogr B. 2014;969:264–71.
Wu H, Liu H, Bai J, Lu Y, Du S. Simultaneous determination of notoginsenoside r1, ginsenoside rg1, ginsenoside re and 20(s) protopanaxatriol in beagle dog plasma by ultra high performance liquid mass spectrometry after oral administration of a panax notoginseng saponin preparation. J Chromatogr B. 2015;974:42–7.
Khan ZA, Tripathi R, Mishra B. Floating elementary osmotic pump tablet (feopt) for controlled delivery of diethylcarbamazine citrate: a water-soluble drug. AAPS PharmSciTech. 2011;12(4):1312–23.
Acknowledgements
We thank Dr. Jun Shao of St. John’s University for his support in the design and writing of the thesis.
Funding
This project was supported by the National Natural Science Foundation of China (No.81573615, No.81274100) and Project of Scientific Research Fund of Anhui University of Chinese medicine (No. 2016zr009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jin, D., Wang, B., Hu, R. et al. A Novel Colon-Specific Osmotic Pump Capsule of Panax notoginseng Saponins (PNS): Formulation, Optimization, and In Vitro-In Vivo Evaluation. AAPS PharmSciTech 19, 2322–2329 (2018). https://doi.org/10.1208/s12249-018-1068-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1068-2