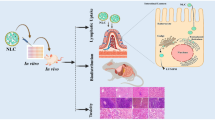
ABSTRACT
Darunavir has a low oral bioavailability (37%) due to its lipophilic nature, metabolism by cytochrome P450 enzymes and P-gp efflux. Lipid nanoparticles were prepared in order to overcome its low bioavailability and to increase the binding efficacy of delivery system to the lymphoid system. Darunavir-loaded lipid nanoparticles were prepared using high-pressure homogenization technique. Hydrogenated castor oil was used as lipid. Peptide, having affinity for CD4 receptors, was grafted onto the surface of nanoparticles. The nanoparticles were evaluated for various parameters. The nanoparticles showed size of less than 200 nm, zeta potential of − 35.45 mV, and a high drug entrapment efficiency (90%). 73.12% peptide was found conjugated to nanoparticles as studied using standard BSA calibration plot. Permeability of nanoparticles in Caco-2 cells was increased by 4-fold in comparison to plain drug suspension. Confocal microscopic study revealed that the nanoparticles showed higher uptake in HIV host cells (Molt-4 cells were taken as model containing CD4 receptors) as compared to non-CD4 receptor bearing Caco-2 cells. In vivo pharmacokinetic in rats showed 569% relative increase in bioavailability of darunavir as compared to plain drug suspension. The biodistribution study revealed that peptide-grafted nanoparticles showed higher uptake in various organs (also in HIV reservoir organs namely the spleen and brain) except the liver compared to non-peptide-grafted nanoparticles. The prepared nanoparticles resulted in increased binding with the HIV host cells and thus could be promising carrier in active targeting of the drugs to the HIV reservoir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Darunavir is a second generation potent protease inhibitor used in treatment of HIV-1 infection. It has low oral bioavailability (37%) due to various factors such as its lipophilic nature (log P of 3.9), low water solubility (0.15 mg/mL as ethanolate salt at 20°C), first pass metabolism by intestinal as well as hepatic cytochrome P450 enzymes, and is a substrate of ABC transporters like P-glycoprotein (P-gp) leading to P-gp efflux [1, 2]. Moreover, the bioavailability of darunavir is highly food dependent and is increased in presence of high-fat meals [1]. High dose of darunavir is associated with various complications including liver disease and skin rashes [2, 3]. It is always co-administered with ritonavir at an adult dose of 600 mg with 100 mg ritonavir twice a day. Studies have shown that combination of darunavir and ritonavir was associated with lower incidence of treatment failure and lower emergence of mutations than the widely used anti-HIV combination therapy of lopinavir/ritonavir [4]. Co-administration of ritonavir leads to increase in bioavailability of darunavir from 37 to 82%. However, long-term use of ritonavir causes its resistance and further cross resistance for other protease inhibitors [5, 6]. It can also cause other side effects like perioral and peripheral paresthesiae, liver problems, pancreatitis, heart problems, and severe allergic reactions [7]. Few approaches have been reported to increase bioavailability of darunavir including pellet formation of darunavir using K-carrageenan [8], solid self-microemulsifying drug delivery system [9], and tablet prepared by melt extrusion containing poloxamer 188 as solubilizer [10].
One of the approaches for enhancement of bioavailability of drugs undergoing hepatic metabolism is the use of lipid-based carriers such as solid lipid nanoparticles. Apart from its increased lymphatic uptake, lipids can also decrease the effect of cytochrome P450 enzymes on substrate drugs and reduce efflux transport activity at intestinal wall thereby increasing permeability of substrate drugs [11,12,13]. Among various lipid systems, solid lipid nanoparticles (SLNs) are widely accepted due to non-toxicity, biocompatibility, scalability facilities, and particulate uptake by Peyer’s patch that increases the lymphatic transport [14].
One of the drawbacks of anti-retroviral therapy is the inability of drug to reach the cellular and anatomical reservoirs of HIV where the HIV resides and replicates and thus the therapy cannot eliminate HIV completely from the body [15]. Lymphoid organs are the major reservoirs of HIV. Ninety-nine percent of the HIV replication occurs in CD4+ receptors containing T cells of the blood and lymphoid tissues such as peripheral secondary lymphoid organs, lymph nodes, GALT, and spleen [16, 17]. Lymphoid tissues have a greater level of infection compared to peripheral blood since only 98% of lymphocytes are distributed in the lymphoid tissues and the rest 2% in the general blood circulation [17]. Targeting of the anti-HIV drug to lymphoid tissues can greatly reduce the viral load and decrease the replication rate of the HIV. The solid lipid nanoparticles (SLNs) can thus enhance the bioavailability as well as target the drug to the lymphoid tissues. Thus, in the present study, SLNs of darunavir were developed for increasing its oral bioavailability. In order to increase the binding of formulated SLNs to the T cells, a peptide having known specificity for CD4+ molecules (present on T cells) was grafted on the surface of cells.
MATERIALS AND METHODS
Materials
Darunavir was obtained as a gift sample from Matrix Laboratories (Hyderabad, India). Hydrogenated castor oil (HCO) was kindly gifted by Meril Life Sciences Pvt. Ltd. DSPE-Mal-m PEG 2000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (poly ethylene glycol) 2000]) was purchased from Avanti Polar Lipids (Alabaster, AL). Peptide sequence CARRPKFYRAPYVKN HPNVWGPWVAYGP was obtained from Genscript (Piscataway, NJ). Human epithelial colorectal adenocarcinoma cell line (Caco-2) and human T cell leukemia cell line (Molt-4) were procured from National Centre for Cell Science (NCCS), Pune. Dithiothreitol (DTT), dimethyl sulphoxide (DMSO), and glycerol were purchased from S. D. Fine Chem Ltd. (Mumbai, India). Trehalose dihydrate and cycloheximide were procured from Himedia Lab. (Mumbai, India). Acrylamide, N,N,N′,N′-tetramethylethylenediamine (TEMED), bis-acrylamide and trishydroxymethyl aminomethane (trisbase) were obtained from Himedia Lab. Ammonium persulfate was purchased from Sigma-Aldrich. Dulbecco’s MEM medium (DMEM), RPMI 1640, trypsin-EDTA solution, fetal bovine serum (FBS), Hank’s balanced salt solution (HBSS), penicillin-streptomycin solution, and trypan blue were purchased from Himedia, Mumbai. 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI), Lucifer yellow, coumarin, chlorpromazine, nystatin, and amiloride were purchased from Sigma-Aldrich, Germany. Double-distilled water was prepared in the laboratory. All other chemicals and reagents were of analytical grade and were used without further purification.
Preparation of Darunavir-Loaded SLNs (D-SLNs)
Darunavir-loaded SLNs were prepared using high-pressure homogenization technique with few modifications [18, 19]. Briefly, 80 mg of darunavir and 480 mg of hydrogenated castor oil (HCO) were accurately weighed and dissolved in 2 mL of dichloromethane. Twenty milliliters of aqueous phase was prepared by adding sodium oleate (0.7 mL) in distilled water to obtain a concentration of 3.5% v/v. The organic phase was dispersed into the aqueous phase using an Ultra-Turrax (IKA T25, IKA, India) at 12000 rpm for 20 min. The obtained pre-emulsion was then passed through high-pressure homogenizer (Emulsiflex C5, Avestin, Canada). Three homogenization cycles (first cycle at 10000 kpa, second and third cycle at 15000 kpa) were applied along with intermittent cooling using ice bath followed by stirring of magnetic stirrer to evaporate organic solvent at room temperature until complete evaporation. The unentrapped drug was removed by centrifugation at 10, 000 rpm for 10 min at 4°C (Sigma centrifuge) and supernatant-containing nanoparticles were collected and lyophilized using Vertis lyophilizer (Vertis Advantage-plus, USA). Trehalose solution (30 mg/mL in distilled water) was added as a cryoprotectant.
Preparation of Peptide Grafted-Darunavir Loaded SLNs (Pept-D-SLNs)
The peptide sequence used in the present study (CARRPKFYRAPYVKNHPNVWG) has been previously reported to increase binding and cellular internalization of nanoparticles to CD4+ cells [20]. Peptide-grafted SLNs of darunavir were prepared by high-pressure homogenization technique as discussed earlier with few modifications. DSPE-Mal-m PEG 2000 was added in organic phase during preparation. For peptide coupling to this preformed nanoparticles, 100 μg of peptide and SLNs equivalent to 1 μM of lipid (molecular weight of lipid 939.50) were combined in 100 μL of buffer (0.9% NaCl, 10 mM NaHCO3) at pH 6.5 at room temperature and were agitated for 8 h in an incubator (Orbitek, Scigenics, India) at 50 rpm. To remove any unbound peptide, nanoparticles were dialyzed against 1 L of buffer and lyophilized using trehalose as per earlier procedure [20].
Particle Size and Zeta Potential
The particle size and zeta potential of darunavir-loaded formulations (D-SLNs and Pept-D-SLNs) were measured using Malvern Zetasizer (Nano ZS, Malvern instrument, UK). The samples were diluted 10 times with double-distilled water to avoid multi-scattering phenomena and placed in disposable sizing cuvette. Zeta potential calculation was performed by Zetasizer software using Smoluchowski equation from the electrophoretic mobility. Each measurement for size and zeta potential was performed in triplicate, and the results were expressed as mean value ± SD (standard deviation).
Entrapment Efficiency and Drug Loading
The nanoparticle dispersions were centrifuged at 25, 000 rpm, 4°C for 120 min (3K30, Sigma laboratory centrifuge, Osterode, Germany) to separate the free drug in solubilized form. Methanol: dichloromethane (7:3) solvent mixture was added to the sediment to dissolve nanoparticles, diluted suitably, and analyzed using UV spectrophotometer at 267 nm to estimate the amount of entrapped drug. Percent EE and drug loading were calculated using the following formulae:
Morphology Study
The prepared nanoparticle dispersion (Pept-D-SLNs) was observed under transmission electron microscope (Hitachi H-7500, 120 kV) by negative staining, and the images were captured. Nanoparticles were also examined for surface morphology using field emission scanning electron microscopy. The nanoparticles (after lyophilization) were coated with platinum (3 nm thick) using an ion sputter for 5 min at 20 mA. Observation was performed at an accelerating voltage for 60 kV and a working distance of 7.7 mm. The images were recorded using field emission scanning electron microscope (JEOL, JSM7600F).
In Vitro Drug Release
The prepared nanoparticles (D-SLNs and Pept-D-SLNs) were studied for in vitro release using procedure described by Neves et al. (Neves et al., 2013) with few modifications. Briefly, nanoparticle dispersion (containing 20 mg drug) was taken in dialysis membrane (MWCO-12000, Hi media, India) closed at one end. The other end was closed and suspended in a beaker containing 50 mL simulated gastric fluid (SGF) for 2 h. After 2 h, the media was replaced by 50 mL simulated intestinal fluid (SIF) for 10 h. The contents were maintained at constant stirring of 100 rpm using magnetic stirrer at 37 ± 0.5°C throughout the experiment. At predetermined time intervals, 2-mL samples were withdrawn from the receptor compartment (media) and replaced with equal volume of fresh media. The withdrawn samples were diluted appropriately and analyzed by UV spectrophotometer at the absorbance maxima of 267 nm. The cumulative percentage of drug release was determined using the average of triplicate samples. The release from darunavir suspension (prepared using 0.4% w/v methyl cellulose) as control was also studied similarly.
Qualitative Determination of Surface-Grafted Peptide
The peptide grafted onto the surface of nanoparticles was checked qualitatively by SDS-PAGE study using reported method [21, 22]. Briefly, the resolving gel solution (consisting of 2.3 mL distilled water, 5 mL of 30% acrylamide, 2.5 mL of 1 M Tris-Cl, 0.1 mL of 10% SDS, 0.1 mL of 10% ammonium persulfate, and 4 μL of TEMED) was poured into the assembly up to the marked level, and 1 mL isobutanol was added. The gel was allowed to polymerize for 1 ;, isobutanol was poured off; and stacking gel was added. The wells were prepared using comb. The stacking gel (consisting of 6.8 mL distilled water, 1.7 mL of 30% acrylamide, 1.25 mL of 1 M Tris-Cl, 0.1 mL of 10% SDS, 0.1 mL of 10% ammonium persulfate, and 0.1 mL of TEMED) was allowed to polymerize for 15 min, and then the comb was removed. Gel was placed in the buffer chamber, and running gel buffer was added into the chamber. The samples (Table I) were carefully loaded in the wells. The gel was run at a constant voltage of 100 V up to a fixed height and then removed and stained using staining reagent (consisting of 40% methanol, 10% acetic acid, and 0.1% Coomassie Blue R-250 in distilled water) for 2 h. After 2 h, the gel was destained using destaining solution (consisting 20% methanol and 10% glacial acetic acid in distilled water) until protein bands were visualized. The bands were observed using Gel-Doc instrument (Genaxy, India).
Quantitative Determination of Surface-Grafted Peptide
The quantitative determination of bound peptide was done by Lowry’s method [23]. The following solutions were prepared:
0.5 mL of the sample was taken in the 2 mL Eppendorf tube (for formulation sample, 0.25 mL of Pept-Dar-SLN was diluted to 0.5 mL with buffer (0.9% NaCl, 10 mM NaHCO3) and taken for study). 0.7 mL of Lowry solution was added, vortexed, and incubated in dark at room temperature for 15 min. After incubation, 0.1 mL of diluted Folin reagent was added, vortexed, and again incubated in dark for 30 min. After 30 min, the samples were analyzed colorimetrically at 660-nm wavelength using UV-Vis spectrophotometer. The calibration plot was prepared using BSA as standard, and concentration of peptide in sample was calculated from its absorbance and calibration equation. Percent of peptide conjugated to nanoparticles was calculated from the following equation:
Cell Line Studies
Identification of Internalization Pathway [24]
Subconfluent monolayer culture in one T-25 flask of Caco-2 cells was trypsinized and 5 mL of complete growth medium (DMEM supplemented with 10% FBS, 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate, and 1% penicillin-streptomycin solution) was added. Trypsin was removed by removing the medium, and 5 mL of complete growth medium-containing serum was replenished. Cells were aspirated gently with pipette. Tips of pipette were discarded after single use. Cells were counted and diluted to 5 × 103 cells/mL. Then, cell suspension (400 μl) was transferred to 24-well plate with a pipette to produce cell concentration of 2 × 103 cells per well. Cells were incubated in the incubator at 37°C and 5% CO2 exposure for 24 h to allow the cells to attach to the surface. Medium from the wells was removed and discarded. The following samples were added: (a) Control group wells (n = 6)—400 μl complete media was added. (b) Group A wells (n = 6)—400 μl complete media containing chlorpromazine (10 μg/mL) was added. (c) Group B wells (n = 6)—400 μl complete media containing nystatin (25 μg/mL) was added. (d) Group C wells (n = 6)—400 μl complete media containing amiloride (550 μM) was added. The wells were incubated for 1 h at 37°C. After 1 h, 100 μl of Pept-D-SLNs was added to the culture medium such that the drug concentration in final volume (500 μl) becomes 10 μg/mL. The plate was kept in an incubator for 1 h at 37°C. After 1 h, the culture medium was removed, the cell monolayer was rinsed thrice with ice cold PBS (pH 7.4). The cells were lysed using 200 μl NaOH solution (0.5 M) for 30 min at room temperature. The lysate was centrifuged at 3000 rpm, 10 min to settle the cell components. The supernatant was taken and drug amounts were estimated by HPLC. The concentration of drug obtained in control group (A) was taken as 100%, and the relative uptake efficiency was calculated as per following equation:
Intestinal Permeability Study [25]
Caco-2 cells were grown on Transwell® inserts (Nunc, Denmark) with (0.4-μ pore diameter, 1.13-cm2 area). The inserts were washed twice using transport buffer (Hank’s balanced salt solution (HBSS) containing 25 mM of HEPES, pH 7.4) and equilibrated for 30 min. The integrity of the monolayers was checked by monitoring the permeability of the paracellular leakage marker, Lucifer yellow across the monolayers. The cell monolayers were considered tight enough for the transport experiments when the apparent permeability coefficient (Papp) for Lucifer yellow was less than 0.5 × 10−6 cm/s. All transport studies were conducted at 37°C. 1.5 mL of transport buffer was added to basolateral side. 0.5 mL of transport buffer-containing sample (150 μl sample containing 0.1 mg drug diluted to 0.5 mL with transport buffer) was added to apical side. The study was performed for darunavir solution (1% DMSO in transport buffer) and Pept-D-SLNs. After the incubation of 0.5, 1, 2, 3, and 4 h, aliquot of 100 μL was withdrawn from the receiver chambers and was immediately replenished with an equal volume of pre-warmed HBSS. The concentrations of the drug in the transport medium were analyzed by developed HPLC methods. The apical-to-basolateral permeability coefficient (Papp in cm/s) was calculated according to following equation:
Where
- dQ/dt:
-
the amount of drug in basolateral compartment per min (mg/min)
- A:
-
the monolayer area (cm2) (=1.13 cm2)
- C:
-
the concentration of drug added in apical compartment initially (=0.2 mg/mL)
Confocal Microscopy
Cellular binding of peptide-grafted nanoparticles was monitored by confocal microscopy. The study was conducted in two different cell lines—Molt-4 cell line expressing high CD4+ and Caco-2 cell line as negative control (no CD4+ expression). The cells, at density of 5 × 105 cells/ well, were seeded onto six well plates with a glass cover slip at the bottom of each well. The cells were incubated in incubator for 24 h at 37°C and 5% CO2 exposure. After 24 h, medium from the wells was removed and discarded. Peptide-grafted solid lipid nanoparticles (Pept-D-SLNs) and non-peptide-grafted SLNs (D-SLNs) were diluted to lipid concentration of 100 nM in complete media, added to wells, and incubated. After 4 h of incubation, cells were washed with cold PBS immediately and fixed using ice cooled 4% paraformaldehyde solution for 10 min. Cells were stained by cell nuclei stain, 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI), for next 10 min. Cover slips were mounted on slides after washing with PBS three times and proceeded for confocal microscopy using confocal laser scanning microscope (LSM 710, Carl-Zeiss Inc., USA).
In Vivo Studies
All experiments and protocols described in this study were approved by the Institutional Animal Ethics Committee of The M.S. University of Baroda and were in accordance with guidelines of the committee for purpose of control and supervision of experiments on animals, ministry of social justice and empowerment, Government of India (protocol no: MSU/PHARM/IAEC/2011/29).
Pharmacokinetic Studies
Pharmacokinetic behavior of Pept-D-SLNs and D-SLNs was studied in male Wistar rats (weighing 250 ± 20 g). Rats were fasted overnight with free access to water and divided into five groups (n = 3). The first group was given D-SLNs, the second group was given Pept-D-SLNs, the third group was given darunavir suspension, and the fourth group was given saline (negative control). The samples were administered orally with the aid of syringe and infant feeding tube at the dose of 40 mg/kg. 0.4-mL blood samples were drawn by retro-orbital venous plexus puncture with the aid of capillary tubes at predose, 0.5, 1, 2, 4, 8, and 24 h post oral dose. The samples were collected in heparinized eppendorf tubes and centrifuged at 5000 rpm for 15 min and filtered. The plasma was collected and stored at − 20°C until analysis. Acetonitrile was added to plasma sample at a ratio of 4:1 (acetonitrile:plasma) and centrifuged to remove plasma proteins. The supernatant was taken and analyzed using LCMS. The chromatographic system consisted of an Ekspert ultraLC 100 HPLC system (Eksigent-AB Sciex, USA). The separation of darunavir was achieved at 40°C on a SUPELCO C18 column (5 μm particle size, 4.6 × 250-mm internal diameter; Sigma-Aldrich) with a C18 guard column under the following conditions: mobile phase—acetonitrile: water (60:40); flow rate 1 mL/min. The LC/MS measurements were achieved using 3200 QTRAP mass spectrometer (AB Sciex, USA) equipped with electrospray ionization (ESI) source. Data were acquired using Analyst 1.6.2 software (Applied Biosystems). The energy of darunavir was optimized at m/z 548.1 → m/z 156.20 Da. The plasma concentration-time profiles were subjected to non-compartmental model using Kinetica software (version 5.0.11, Thermofischer scientific), and various pharmacokinetic parameters were calculated. The relative bioavailability was defined as the ratio between AUC0-t values of sample to that of plain drug suspension.
In Situ Absorption from Stomach and Small Intestine
The absorption of darunavir suspension and optimized darunavir-loaded SLNs (D-SLNs) in the gastrointestinal tract (stomach and intestine) was investigated in rats by in situ method [26]. The animals were fasted overnight and anesthetized by intraperitoneal injection of ketamine (50 mg/kg). A small incision was made in the abdomen, and the pylorus of the stomach was ligated. SLNs were given to rats (40 mg/kg) by intragastric administration to evaluate its absorption in the stomach. Likewise, to measure the absorption of SLNs in the intestine, SLNs were intraduodenally administered to rats at the same dose (n = 3). The incisions were stitched and rats were kept under infrared lamps to maintain body temperature. 0.4 mL blood sample was collected at 15, 30, 45, 60, and 90 min, and plasma was separated by centrifugation at 3000 rpm for 15 min. 0.2 mL of supernatant was diluted to 1 mL using chilled acetonitrile to precipitate plasma proteins, centrifuged at 5000 rpm, 4°C for 10 min, and the amount of darunavir in supernatant was measured using developed LCMS method.
Organ Biodistribution Study
Male Wistar rats (weighing 250 ± 20 g) were divided into two groups with 12 animals in each group. They were fasted overnight before administration of dose. The first group was given darunavir suspension and the second group was given Pept-D-SLNs both at the dose of 40 mg/kg. Three animals of each group were sacrificed at each time points 1, 4, 8, and 24 h. The organs (spleen, liver, kidney, stomach, intestine, brain, heart, and lung) were excised, isolated, and washed with Ringer’s solution and dried using tissue paper. Organs were stored at − 80°C until assay. For estimation of drug in the different organs, the following procedure was followed: Various organs were weighed and cut into small pieces. One-gram organ was homogenized individually using tissue homogenizer in double-distilled water (10% homogenate concentration). In case of organs weighing less than 1 g, the whole organ was used. The homogenate was centrifuged at 5000 rpm for 15 min, acetonitrile was added to tissue homogenate (4:1—acetonitrile:homogenate) and centrifuged to remove plasma proteins. The supernatant was taken and analyzed using LCMS.
Biodistribution in Peyer’s patch (PP) and non-Peyer’s patch (non-PP): The distribution of SLNs in PP and non-PP was studied as per method described by desai et al. [27]. Pept-D-SLNs were administered at the dose of 40 mg/kg to eight male Wistar rats. Two animals were sacrificed at each time intervals (1, 4, 8, and 24 h) and the intestine was isolated, washed with Ringer’s solution, and blotted using tissue paper. The PP and non-PP regions were differentiated visually and separated. The regions were weighed, separately homogenized, and centrifuged at 5000 rpm for 15 min. Acetonitrile was added to tissue homogenate (4:1—acetonitrile:homogenate) and centrifuges to remove plasma proteins. The supernatant was taken and analyzed using LCMS method.
RESULTS AND DISCUSSION
Preparation of Darunavir-Loaded Solid Lipid Nanoparticles and Peptide Grafted-Darunavir Loaded SLNs
D-SLNs and Pept-D-SLNs were prepared by emulsification-solvent evaporation technique using HCO as solid lipid and dichloromethane as organic solvent. High-pressure homogenization was used followed by evaporation of organic solvent. The nanoparticles prepared using this technique showed high entrapment efficiency and particle size less than 200 nm (Table II). The high entrapment efficiency obtained is attributed to the lipophilic nature of the drug having high affinity for the lipid. The negative zeta potential might be due to hydrogenated castor oil. A good colloidal stability can be expected due to the high values of zeta potential [28]. There was an insignificant increase in particle size and entrapment efficiency of nanoparticles and a significant increase in zeta potential (from − 50.1 to − 35.45 mV) after attachment of peptide which might be due to ligand attachment on SLNs surface. The particle size of Pept-D-SLNs after lyophilization was found to be 198 ± 2.11 nm.
Morphology Study
The TEM and FE-SEM images of Pept-D-SLNs and the TEM image of non-grafted D-SLNs are shown in Fig. 1. The diameter of nanoparticles seen in TEM image (Fig. 1) was in accordance with the result obtained through size analysis. The images revealed the discrete and spherical shape of particle without aggregation. A large negative zeta potential obtained was advantageous for achieving lower-sized particles, owing to the particles remaining discrete without agglomeration.
In Vitro Drug Release
Figure 2 shows the in vitro release profiles of darunavir-loaded nanoparticles in comparison to plain drug suspension. Plain drug suspension showed 11.2% drug release in 8 h reaching a plateau. Higher drug release was found with nanoparticle formulation as compared to drug release from plain drug suspension. For SLNs, cumulative percent drug released was only 4.4% in SGF during 2 h followed by 14.4% in SIF by end of 12 h. The drug release from peptide-grafted and non-peptide-grafted SLNs was similar with no significant difference.
Qualitative Determination of Surface-Grafted Peptide
SDS-PAGE study was used to verify the presence of peptide on nanoparticle. Dithiothreitol (DTT) was used to break the sulfate bond present in the peptide. In absence of DTT (Fig. 3a non-reducing PAGE), standard peptide solution gave sharp band while no band was seen with formulation sample. On treatment with DTT (Fig. 3b reducing PAGE), there was breakage of sulfate band, and sharp band was seen in the formulation samples. These bands were similar to that of the standard peptide band thus confirming the attachment of peptide on SLNs surface.
Quantitative Determination of Surface-Grafted Peptide
The calibration plot of BSA was prepared in order to estimate the amount of peptide attached to nanoparticles. Linearity was obtained in beer’s range of 10–50 μg/mL, and the regression equation obtained was y = 0.004x + 0.1325 with R 2 = 0.9988. Percent conjugation obtained was 73.12% indicating a significant binding affinity of the peptide to the lipid.
Cell Line Studies
Identification of Internalization Pathway
The results of endocytic uptake of formulated nanoparticles in presence or absence of specific endocytic inhibitors are shown in Table III. The relative uptake efficiency of darunavir in presence of endocytic inhibitors is graphically depicted in Fig. 4. Cellular uptake of SLNs was reduced by 29.35% in presence of chlorpromazine, decreased over 41.58% in presence of nystatin while there was no significant difference in cell uptake in presence of amiloride. It is reported that chlorpromazine and nystatin inhibit clathrin- and caveole-mediated endocytosis [29]. Hence, the decrease in cellular uptake of SLNs in presence of these inhibitors indicated that the Caco-2 uptake of SLN was mediated via clathrin- and caveoli-mediated endocytosis and not via macropinocytosis since amiloride (which inhibits macropnocytosis) presence had no significant effect on uptake efficiency of SLNs. Among the three endocytic pathways explored in the above study, the highest decrease in cellular uptake was obtained by use of caveole-mediated endocytic inhibitor—nystatin. Thus, it could be deduced that, among these pathways, SLNs preferably use caveole-dependent endocytic pathways. This is supported by the reported data that the anionic nanoparticles are more likely to use caveole-dependent endocytosis [30], and our developed nanoparticles are anionic in nature. It is also reported that majorly more than one pathways have been utilized by different nanoparticles for the endocytic uptake [29, 31].
Intestinal Permeability Study
The gastrointestinal permeability of prepared nanoparticles was assessed by calculating the in vitro permeability coefficients in Caco-2 cell model of gastrointestinal membrane. The results are shown in Table IV. The permeability coefficient Papp for plain darunavir solution was found to be 3.44 × 10−6 cm/s. Even in presence of DMSO which is a known permeation enhancer, the Papp of plain darunavir solution was significantly lower than that of the nanoparticles [32]. The permeability coefficient for darunavir-loaded SLNs was found to be 1.39 × 10−5 cm/s which was 4.04 times more than plain drug solution. The lower permeability for plain drug solution was because of poor permeability characteristics of darunavir. The permeability of darunavir was enhanced by loading drug in SLNs which could be attributed to higher uptake of SLNs by endocytosis in Caco-2 cells.
Confocal Microscopy
The uptake of peptide-grafted and non-peptide-grafted SLNs was studied in two cell lines—Molt-4 and Caco-2 cell line in order to study the binding effect due to grafting of SLNs with peptide having CD4+ receptor affinity. The nuclei were stained using DAPI. After 4 h of incubation, the nanoparticles were mainly observed in cytoplasm. The result of uptake in Molt-4 cell line (CD4 positive cell line) is shown in Fig. 5 while uptake in Caco-2 cell line (CD4 negative cell line) is shown in Fig. 6. The images showed a higher fluorescence obtained using Pept-D-SLNs in Molt-4 cell line in comparison to non-peptide-grafted SLNs (D-SLNs) indicating the greater binding of peptide-grafted SLNs to CD4 positive Molt-4 cell line. In contrast, both the nanoparticle formulations showed similar fluorescence in CD4 negative Caco-2 cell line indicating no effect of peptide on binding with these cells. Moreover, for peptide-grafted SLNs, fluorescence was lesser as compared to that obtained in Molt-4 cells which showed that the peptide-grafted SLNs have higher affinity for CD4 positive Molt-4 cell line. Hence, the peptide-grafted SLNs had higher affinity to bind with the HIV host cells compared to non-grafted SLNs. This would lead to enhanced exposure of drug to the HIV host cells.
In Vivo Studies
Pharmacokinetic Studies
The plasma concentration of darunavir was estimated by LC-MS method. The regression equation of area ratio (A) to concentration (C) in linear range of 25–100 ng/mL was as follows: A = 567 C + 6200 (r 2 = 0.9982), limit of detection was 5 ng/mL indicating that it is a sensitive method for detection of darunavir at low concentrations. The plasma drug concentration versus time profile is depicted in Fig. 7, and the results of various pharmacokinetic parameters are given in Table V. Cmax increased by 2.68 fold upon nanoparticle administration (D-SLNs) in comparison to plain drug suspension. Similarly, there was a significant increase in AUC0-inf for nanoparticle formulation (P < 0.05) in comparison to plain drug suspension. The enterocyte formation and absorption of lipids via intestinal lymphatics may be the reason for enhanced oral bioavailability from nanoparticle formulations, since the Tmax for nanoparticles was delayed. Another reason might be the presence of long chain fatty acid (hydrogenated castor oil) in nanoparticles which is reported to stimulate lipoprotein formation and bypass portal circulation through intestinal lymph uptake [12]. The plasma concentration of darunavir in rats after oral administration of SLNs and peptide-grafted SLNs showed no significant difference (P > 0.5). It indicated that the grafting of peptide had no effect on its pharmacokinetic profile.
In Situ Absorption from Stomach and Small Intestine
The results of absorption of darunavir suspension and darunavir-loaded nanoparticles after intragastric and intraduodenal administration are shown in Fig. 8. AUC0-t for drug suspension and D-SLNs were obtained as 22.75 ± 3.10 and 56.98 ± 2.89 ng h/mL, respectively after intra gastric administration and 190.92 ± 11.81 and 479.35 ± 24.04 ng h/mL after intra duodenal administration. There was significant enhancement in plasma concentration upon administration of nanoparticles in comparison to free drug suspension. After intra gastric and intra duodenal administration of D-SLNs, AUC0-t increased by 2.50 and 2.51 fold, respectively, in comparison to AUC0-t for suspension which might be due to the enhanced solubilization of drug.
Organ Biodistribution Study
The distribution pattern of darunavir in the different organs upon administration of plain drug suspension and its ligand-attached SLNs (Pept-D-SLNs) were studied and the results obtained are shown in Fig. 9. Pept-D-SLNs showed a higher distribution of darunavir than from plain drug suspension as demonstrated by an increased impregnation of virtually all investigated tissues except the liver. An initial higher uptake was found in the intestine (due to rapid uptake by GITract upon administration) followed by a subsequent decrease indicating distribution into other organs. Major HIV reservoir organs namely the spleen and brain showing higher darunavir concentration upon nanoparticle administration indicate the superiority of nanoparticle systems in increasing the accumulation of drug in various target organs. The transport of most protease inhibitors including darunavir across the blood brain barrier is reported to be very poor [33]. The results showed a 1.5–3.5 fold higher accumulation of darunavir in the brain when administered through solid lipid nanoparticles compared to suspension. This might be due to the inherent ability of nanoparticles to cross blood brain barrier by opening the tight junction between endothelial cells [34]. This would lead to increased retention of nanoparticles in the brain capillaries combined with absorption through capillary walls by receptor-mediated endocytosis [35, 36]. Since darunavir is metabolized in the liver [37], its lower concentration upon nanoparticle administration is of great therapeutic benefit. Moreover, the kidney showed decreased drug concentration in initial hour in nanoparticle formulation indicating a delayed clearance of drug from the body. This data is supported by the pharmacokinetic study where mean residence time was found to be higher in nanoparticle formulation (6.69 h) compared to plain drug suspension (4.39 h).
The results of biodistribution study in PP and non-PP region are shown in Fig. 10. There was significant difference between drug concentrations in both the regions (P < 0.05). It was observed that darunavir amount in PP region was 4.12 fold higher as compared to non-PP region. A higher concentration in PP region observed might be due to the size of nanoparticles. This is corroborated by one study that stated that 100-nm polymeric nanoparticles were taken by PP region in significantly higher amount in comparison to nanoparticles of size 500 nm, 1 μm, and 10 μm [27]. It is also hypothesized by Florence et al. [38] that nanoparticles because of their smaller size would have an efficient uptake via Peyer’s patch leading to higher transport to lymphatic organs such as the mesenteric lymph nodes and spleen.
CONCLUSION
We have demonstrated that the nanoparticle formulation greatly improved the bioavailability of poorly soluble darunavir. Peptide having affinity for HIV host cells was successfully grafted on the surface of nanoparticles. The resulting formulation gave increased binding with the HIV host cells and thus could be promising carrier in active targeting of the drugs to the HIV reservoir. Thus, the present research work led to a development of formulations for bioavailability enhancement of protease inhibitors and their targeting to HIV reservoirs. However, extended research involving pharmacodynamic studies could yield a clearer picture of the efficiency of the formulation in reduction of viral loads.
References
Rittweger M. Clinical pharmacokinetics of darunavir. Clin Pharmacokinet. 2007;46(9):739–56.
McKeage K, Perry CM, Keam SJ. Darunavir Drugs. 2009;69(4):477–503.
Haubrich R, Berger D, Chiliade P, Colson A, Conant M, Gallant J, et al. Week 24 efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients. AIDS. 2007;21(6):F11–8.
Santos JR, Llibre JM, Bravo I, Garcia-Rosado D, Canadas MP, Perez-Alvarez N, et al. Short communication: efficacy and safety of treatment simplification to lopinavir/ritonavir or darunavir/ritonavir monotherapy: a randomized clinical trial. AIDS Res Hum Retrovir. 2016 May;32(5):452–5.
Sosnik A, Chiappetta DA, Carcaboso ÁM. Drug delivery systems in HIV pharmacotherapy: what has been done and the challenges standing ahead. J Control Release. 2009;138(1):2–15.
Race E, Dam E, Obry V, Paulous S, Clavel F. Analysis of HIV cross-resistance to protease inhibitors using a rapid single-cycle recombinant virus assay for patients failing on combination therapies. AIDS. 1999;13(15):2061–8.
Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356(9239):1423–30.
Thommes M, Baert L, van’t Klooster G, Geldof M, Schueller L, Rosier J, et al. Improved bioavailability of darunavir by use of κ-carrageenan versus microcrystalline cellulose as pelletisation aid. Eur J Pharm Biopharm. 2009;72(3):614–20.
Inugala S, Eedara BB, Sunkavalli S, Dhurke R, Kandadi P, Jukanti R, et al. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) of darunavir for improved dissolution and oral bioavailability: in vitro and in vivo evaluation. Eur J Pharm Sci. 2015 Apr 3;74:1–10.
Thommes M, Baert L, Rosier J. 800 mg darunavir tablets prepared by hot melt extrusion. Pharm Dev Technol. 2011;16(6):645–50.
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76.
Chakraborty S, Shukla D, Mishra B, Singh S. Lipid—an emerging platform for oral delivery of drugs with poor bioavailability. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2009 Sep;73(1):1–15.
Wagner D, Spahn-Langguth H, Hanafy A, Koggel A, Langguth P. Intestinal drug efflux: formulation and food effects. Adv Drug Deliv Rev. 2001;50:S13–31.
Aji Alex MR, Chacko AJ, Jose S, Souto EB. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci. 2011;42(1–2):11–8.
Saksena NK, Haddad DN. Viral reservoirs an impediment to HAART: new strategies to eliminate HIV-1. Current drug targets Infectious disorders. 2003;3(2):179–206.
Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–93.
Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997 Nov 14;278(5341):1291–5.
Kovacevic A, Savic S, Vuleta G, Muller RH, Keck CM. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): effects on size, physical stability and particle matrix structure. Int J Pharm. 2011;406(1–2):163–72.
Desai J, Thakkar H. Effect of particle size on oral bioavailability of darunavir-loaded solid lipid nanoparticles. J Microencapsul. 2016;33(7):669–78.
Endsley AN, Ho RJ. Design and characterization of novel peptide-coated lipid nanoparticles for targeting anti-HIV drug to CD4 expressing cells. AAPS J. 2012;14(2):225–35.
Life_technologies. Available from: https://tools.lifetechnologies.com/content/sfs/brochures/1601925-Electrophoresis-Handbook.pdf Accessed Jan 2014.
Life_technologies. Available from: https://www.lifetechnologies.com/in/en/home/references/protocols/proteins-expression-isolation-and-analysis/sds-page-protocol.html Accessed Jan 2014.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–75.
Roger E, Lagarce F, Garcion E, Benoit J-P. Lipid nanocarriers improve paclitaxel transport throughout human intestinal epithelial cells by using vesicle-mediated transcytosis. J Control Release. 2009;140(2):174–81.
Hafner A, Lovric J, Voinovich D, Filipovic-Grcic J. Melatonin-loaded lecithin/chitosan nanoparticles: physicochemical characterisation and permeability through Caco-2 cell monolayers. Int J Pharm. 2009 Nov 3;381(2):205–13.
Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–44.
Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996 Dec;13(12):1838–45.
Patravale V, Dandekar P, Jain R. Nanoparticulate drug delivery: perspectives on the transition from laboratory to market: Elsevier; 2012.
Zhang Z, Gao F, Bu H, Xiao J, Li Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: in vitro characteristics and absorption mechanism in rats. Nanomedicine : Nanotechnol, Biol Med. 2012;8(5):740–7.
Kou L, Sun J, Zhai Y, He Z. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J Pharm Sci. 2013;8(1):1–10.
Mo R, Jin X, Li N, Ju C, Sun M, Zhang C, et al. The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials. 2011 Jul;32(20):4609–20.
Marren K. Dimethyl sulfoxide: an effective penetration enhancer for topical administration of NSAIDs. Physician Sportsmedicine. 2011;39(3):75–82.
Wong HL, Chattopadhyay N, Wu XY, Bendayan R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv Drug Deliv Rev. 2010;62(4–5):503–17.
Kaur A, Jain S, Tiwary AK. Mannan-coated gelatin nanoparticles for sustained and targeted delivery of didanosine: in vitro and in vivo evaluation. Acta Pharma. 2008;58(1):61–74.
Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001 Mar 23;47(1):65–81.
Ahsan F, Rivas IP, Khan MA, Torres Suarez AI. Targeting to macrophages: role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J Controlled Release : Off J Control Release Soc. 2002;79(1–3):29–40.
Fabbiani M, Di Giambenedetto S, Ragazzoni E, D'Ettorre G, Parruti G, Prosperi M, et al. Darunavir/ritonavir and raltegravir coadministered in routine clinical practice: potential role for an unexpected drug interaction. Pharmacol Res. 2011 Mar;63(3):249–53.
O'Hagan DT, Palin KJ, Davis SS. Intestinal absorption of proteins and macromolecules and the immunological response. Crit Rev Ther Drug Carrier Syst. 1988;4(3):197–220.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Desai, J., Thakkar, H. Darunavir-Loaded Lipid Nanoparticles for Targeting to HIV Reservoirs. AAPS PharmSciTech 19, 648–660 (2018). https://doi.org/10.1208/s12249-017-0876-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0876-0