Abstract
A novel humic acid and polyquaternium-10 polyelectrolyte complex (PEC) was synthesized utilizing two methods and the solubility and permeability of efavirenz (EFV) were established. Complexation-precipitation and extrusion-spheronization were used to synthesize and compare the drug-loaded PECs. The chemical integrity, thermo-mechanical differences, and morphology between the drug-loaded PECs produced by the two methods were assessed by attenuated total reflectance-Fourier transform infrared, differential scanning calorimetry, and SEM. The extent of drug solubilization was determined using the saturation solubility test while the biocompatibility of both PECs was confirmed by cytotoxicity studies on human adenocarcinoma cells (caco2). Bio-relevant media was used for the solubility and permeability analysis of the optimized PEC formulations for accurate assessment of formulation performance. Ritonavir (RTV) was loaded into the optimized formulation to further corroborate the impact of the PEC on the solubility and permeability properties of a poorly soluble and poorly permeable drug. The optimized EFV-loaded PEC and the RTV-loaded PEC exhibited 14.16 ± 2.81% and 4.39 ± 0.57% increase in solubility, respectively. Both PECs were compared to currently marketed formulations. Intestinal permeation results revealed an enhancement of 61.24 ± 6.92% for EFV and 38.78 ± 0.50% for RTV. Although both fabrication methods produced PECs that enhanced the solubility and permeability of the model Biopharmaceutics Classification System Class II and IV drugs, extrusion-spheronization was selected as most optimal based on the higher solubility and permeability improvement and the impact on caco2 cell viability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The humic acid (HA) and polyquaternium-10 (PQ10) polyelectrolyte complex (PEC) was explored in order to benefit from the synergistic effect of using a polymeric complex contrary to the individual polymers. The novel HA-PQ10 PEC, previously reported (1), was investigated further for the purposes of comparing properties of the complexes synthesized by two different methods. The high affinity for negatively charged groups that is exhibited by PQ10 can be attributed to the presence of the quaternary amino groups. Our previous study revealed that this molecule formed a polyelectrolyte complex with HA, a macromolecule which is classified as an anionic polyelectrolyte. In this study, commercial brown HA and PQ10 of 656.1 g/mol were utilized (1). It is essential to identify a simplified and cost-effective method of PEC fabrication applicable to contemporary dosage form design. Solid dispersions were synthesized using the complexation-precipitation (C-P) and extrusion-spheronization (E-S) approaches. Solid dispersions have been known to enhance the bioavailability of drugs (2). Dispersion of the drug within a matrix prevents agglomeration of the drug while encouraging its solubility. These dispersions are also responsible for increasing the surface area of the drug, therefore enhancing the wettability of the bioactive (3). However, the main drawback of solid dispersions is the tendency of the drug to recrystallize due to the metastable properties of the amorphous formulation (4,5).
The aqueous solubility and intestinal tissue permeability of efavirenz (EFV) were measured and any differences to a selected marketed formulation were noted. Poor solubility occurs when the drug solubilization rate is slower than the rate of its passage in the gastrointestinal tract (2). Various methods of improving the hydrosolubility of drugs have been employed and these include, but are not limited to, the addition of surfactants into formulations, the reduction of drug particle size, delivery of the drug in emulsion vehicles, and the use of solid dispersions (6,7). The permeability of such drugs can also be enhanced by utilizing polymeric delivery systems that increase the residence time of the drugs or by encapsulating the drugs in microspheres and/or nanoparticles that have a high surface area to volume ratio.
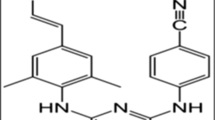
The model anti-HIV drug, EFV, was loaded into HA-PQ10 PEC fabricated by the complexation-precipitation and the extrusion-spheronization techniques. According to the Biopharmaceutics Classification System (BCS), EFV is categorized in Class II (8). It is a non-nucleoside reverse transcriptase inhibitor (NNRTI) with a bioavailability estimated to be between 40 and 45% and a solubility of 3–9 μg/mL (9,10). Any drug with a solubility <10 μg/mL is regarded as poorly soluble. NNRTIs bind to the reverse transcriptase in a non-competitive manner. RTV was added to the optimized PEC in order to validate applicability of the complex to BCS Class IV drugs (11) which exhibit both poor solubility and poor permeability. RTV is classified as a PI which inhibits the formation of mature infectious virions. It possesses a bioavailability of 5% and an aqueous solubility of 1.26 μg/mL (11). Low aqueous solubility and permeability also results in low and variable bioavailability in vivo, and consequentially, higher drug doses have to be dosed in order to achieve optimal biological effects (12).
MATERIALS AND METHODS
Materials
Humic acid (HA) (brown), polyquaternium-10 (PQ10), microcrystalline cellulose (Avicel PH-101) (MCC), and sodium lauryl sulfate (SLS) were procured from Sigma-Aldrich Co. (St. Louis, MO, USA). Pure efavirenz (EFV) and ritonavir (RTV) powder (99.99% purity) were purchased from Xingcheng Chemphar Co., Ltd. (Taizhou, Zhejiang, China) and used as received. The commercially available comparator products were Sonke-Efavirenz® 200 mg and soft elastic capsules of Norvir® 100 mg (ritonavir). All other reagents used were of analytical grade.
Synthesis of the Drug-Loaded Polyelectrolyte Complex (PEC)
Table I is a list of the abbreviations used in the synthesis of the drug-loaded polyelectrolyte complex (PEC).
Complexation-Precipitation (C-P) via Solution-Blending of HA and PQ10
Synthesis of the drug-loaded PEC by C-P involved dissolving EFV (200 mg) in methanol (20 mL) prior to titrating one polymer solution into the other. The EFV solution was added to the HA solution (100 mL of 5.75 mg/mL) and agitated for 5 min. Thereafter, an aqueous solution of PQ10 (235 mL of 2.4 mg/mL) was slowly titrated into the HA/EFV solution to facilitate self-assembly of the drug-loaded PEC (PEC-E(C-P)) within seconds. The precipitate was filtered and dried in a vacuum oven at 40°C for 12 h after which it was milled and filled into hard gelatin capsules.
Extrusion-Spheronization (E-S) via Benchtop Extruder and Spheronizer
A combination of HA (1400 mg), PQ10 (1410 mg), EFV (650 mg), and microcrystalline cellulose (MCC) (1000 mg) were blended in a homogenizer for 5 min before synthesis of the EFV-loaded PEC (PEC-E(E-S)). The same amount of HA and PQ10 used in the C-P method was employed in E-S as well. Deionized water was added to form wet granules of the powder mass. The homogenous wet granules were transferred into the barrel of a mini-screw extruder (Caleva Ltd., Sturminster Newton, Dorset, UK) for extrusion at 100 rpm. MCC absorbed the granulating fluid (water) which was then compressed onto the outer surface of the extrudates, thus further lubricating the surface to render the extrusion process feasible. The extrudates were then collected and immediately spheronized in a multi-bowl spheronizer (Caleva Ltd., Sturminster Newton, Dorset, UK).
Physicochemical and Mechanical Characterization of the Drug-Free HA-PQ10 PEC
Assessment of the Surface Morphology
SEM (FEI Nova NanoLab™ 600 DualBeam FIB/SEM, Hillsboro, OR, USA) was utilized for determining the surface morphology of the PEC samples. Analysis was conducted at 20 kV. Prior to mounting samples onto the SEM stage, they were thoroughly dried and sputter coated with chromium for 2 min. All images were taken at ×2000 magnification.
Complexation Validation via PEC Solubility Testing in Varying Solvents
Complete synthesis of the PECs was evaluated utilizing the solvent test. PECs were dispersed separately in ethyl acetate, dimethyl sulfoxide (DMSO), formic acid, ethanol, acetone, dichloromethane (DCM), and water at 80°C for 24 h under constant stirring. The achromatic solutions were observed for any color changes which would indicate the solubility of PECs in the solvent.
Characterization of EFV-Loaded HA-PQ10 PECs Produced by E-S and C-P
Influence of PEC Synthesis Method on the Interaction of HA and PQ10
Attenuated total reflectance-Fourier transform infrared, ATR-FTIR, with a single reflection diamond MIRTGS detector (PerkinElmer® Spectrum 100 Series FTIR Spectrometer; PerkinElmer Ltd., Beaconsfield, UK) was employed to assess the interactions between the PEC, pure drug, and native polymers. This was conducted over a wavenumber of 6000 and 450 cm−1 using 25 wavescans per spectrum and a force of 115 N. A resolution of 4 cm−1 was utilized.
Determination of the Thermal Properties of PECs and Physical Mixture
Melting points of dry powder samples were comparatively deduced by using differential scanning calorimetry (DSC) (Mettler Toledo DSC-1 STARe System, Schwerzenback, ZH, Switzerland) over a temperature range of 20–300°C with a heating rate of 10°C/min. Nitrogen was used as the purge gas to avoid oxidation. A maximum of 10 mg sample was placed in a 40-μL aluminum pan and the lid was crimped and perforated. Analysis of the thermograms revealed the specific temperature that the samples melted at and the curves were plotted as heat flow (mW) against temperature.
Determining the Extent of Supersaturation and In Vitro EFV Release
The percentage drug-loading (%DL) was ascertained by adding finely ground PEC powder (100 mg) into simulated intestinal fluid (SIF) (40 mL) at pH 6.8 containing SLS (1%) (according to USP monograph, USP 32-NF 27). The powders were dispersed in the SIF. After incubation in an orbital shaker bath, samples were filtered with a 0.22-μm filter and drug concentrations determined spectrophotometrically (Lambda 25 UV/Vis spectrophotometer; PerkinElmer, MA, USA) at 247 nm for EFV (R 2 = 0.9926) and 241 nm for RTV (R 2 = 0.9998). The saturation solubilities were determined by adding formulations containing 2.5 mg/mL of EFV in 10 mL SIF. This was incubated in an orbital shaker bath for 36 h and drug concentrations were determined via UV absorbance measurements (13). Sampling was conducted every 12 h and the highest quantity of drug concentration determined was considered the supersaturation amount. The EFV release rate from the capsules was determined using the USP Apparatus II paddle stirrer at 50 rpm (USP 33) (Erweka DT 700; Erweka GmbH, Heusenstamm, Germany). Formulations were placed under a ring-mesh assembly to prevent flotation that may lead to unsteady and biased drug release profiles (14). The temperature was maintained at 37°C and sample aliquots (5 mL) were collected at predetermined times over a 24-h test period. Sink conditions in the dissolution fluid (900 mL) were maintained by replacing the samples withdrawn immediately with drug-free media. The dissolution medium consisted of SIF, which was necessary to perform the studies under physiologically relevant conditions since the solubility of poorly aqueous soluble drugs is affected by the presence of surfactants. Saturation solubility, EFV release, and permeation studies for the optimized PECs were undertaken in the fed state simulated intestinal fluid (FeSSIF). Preparation of FeSSIF comprised dissolving NaOH (20.2 g), glacial acetic acid (43.25 g), and NaCl (59.37 g) in deionized water (5 L) and thereafter adjusting the pH to 5.0. Native FeSSIF (500 mL) was used to dissolve sodium taurocholate (59.08 g) and 59.08 mL of lecithin dissolved in methylene chloride (100 mg/mL) was added to the blank FeSSIF. The methylene chloride was eliminated from the emulsion by evaporation and the volume of the media was adjusted to 2 L (15). Collected sample aliquots were immediately filtered using a 0.22-μm filter and diluted appropriately. Equation 1 was employed to calculate the %DL.
Ex Vivo Permeability Studies Employing Porcine Small Intestinal Tissue
A Franz Diffusion Cell apparatus (PermeGear Inc., Bethlehem, PA, USA) was utilized to measure the drug permeation across porcine small intestinal tissue from the PECs across a concentration gradient of 4 mg/mL. PBS (12 mL) at pH 7.4 was added to the acceptor compartment. An emulsion of capsule contents (4 mg/mL) in SIF and FeSSIF for the optimized PECs was added to the donor compartment. The two compartments were separated by a portion of excised porcine small intestinal tissue (ileum). The intestinal tissue was freshly obtained from a Large White pig and immediately snap-frozen for preservation. Prior to the permeation experiment, the tissue was thawed and allowed to equilibrate on the acceptor compartment to 37°C for 20 min. The adipose and muscular layers were carefully excised. The study was performed over a 24-h period at 37°C. Sample aliquots (0.5 mL) were withdrawn and replaced by PBS of equal volume. The mean values of the quantity of EFV that permeated were used to compute the flux (J) and permeability coefficient (P) of the drug according to Eq. 2 and Eq. 3. Flux is the quantity of drug transported across the membrane over a certain period of time and was determined employing Eq. 2.
where M is the mass of EFV present in the acceptor compartment at time t and A is the area available for permeation (16). Permeability can be defined as the rate of EFV permeation per unit concentration (17) and the permeability coefficient was calculated according to Fick’s first law.
where P (cm/h) is the permeability coefficient, V (mL) is the volume of the acceptor compartment (12 mL), A (cm2) is the effective permeation area (1.767 cm2), t (h) is the time interval, C 1 (mg/mL) is the concentration in donor compartment, and C 2 (mg/mL) is the concentration in the acceptor compartment (18). The transepithelial electrical resistance (TEER) of the porcine tissue was measured using a SevenMulti™ dual meter pH/conductivity (Mettler Toledo, Zurich, Switzerland) at 25°C to confirm tissue integrity.
PEC Optimization via a Design of Experiments (DoE) Approach
Implementation of a Randomized Box-Behnken Experimental Design
A series of PEC formulations were synthesized using a two-factor, two-tier Box-Behnken statistical design. Minitab® V16 software (Minitab Inc., PA, USA) was used to generate the experimental design template constituting 13 formulations, 5 center-points, and 1 replicate with the ratio of HA/PQ10 and the quantity of granulation fluid (deionized water) being the independent variables as shown in Table II. The mean dissolution time (MDT) from the drug release studies and the drug flux from porcine intestinal tissue permeability studies over 24 h were the responses that were considered and maximized to obtain an optimized PEC formulation with enhanced solubility and permeability of the encapsulated drug. Maximum speeds of extrusion and spheronization were set as constants based on results from preliminary studies that showed that maximum speeds were ideal to facilitate the required extrusion and spheronization. EFV was used as the model drug for the experimental design phase of this study.
Evaluation of Extrudate Flow Properties of the HA-PQ10 PEC
The Hausner ratio (HR) was calculated to determine the flow properties of the extrudates that were then encapsulated into gelatin capsules. In order to measure the bulk and tapped densities, initial and final volumes of specific extrudate weight were recorded. The volume was at least 60% of the cylinder volume [Method 1—section 2.9.34 European Pharmacopoeia (EP8.0, 2014)]. The final volume was measured after tapping a 50-mL glass cylinder onto a hard wood surface from a 20 mm height (19). Bulk density, tapped density, and the HR were calculated according to Eqs. 4, 5, and 6.
where w is the weight of the extrudates (g), v 0 is the initial volume of extrudates (mL), and v f is the final volume of the extrudates (mL).
Establishing the Surface Morphology and Porosity of the Optimized PEC
Surface morphologies of the optimized formulations were determined by SEM as explained in 2.3.1. The porosity was measured using BET isotherms of nitrogen on a porosity analyzer (ASAP 2020; Micromeritics Instrument Co., GA, USA) (20). The extrudates were degassed to remove surface moisture and impurities. In order to measure the surface area, pore size, and pore volume of the samples, unspheronized pieces of extrudates which were at least 100 mg in weight were analyzed. Analysis involved two phases, the evacuation phase and the heating phase. Parameters for the evacuation phase included a temperature ramp rate of 10°C/min with a target temperature of 40°C and an evacuation rate of 50 mmHg/s. The temperature ramp rate for the heating phase was 10°C/min with a 30°C hold time. The analyzer generated the Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) nitrogen adsorption and desorption analysis. BET analysis provided surface area analysis while BJH provided pore size and pore volume analysis. Equations 7 and 8 were implemented for BET surface area determination.
where, A s (BET) is the total surface area and a s (BET) is the specific surface area of the adsorbent mass (m). L is the Avogadro constant (20).
RESULTS AND DISCUSSION
Outcome of PEC Synthesis and Physical Variances Between PEC(E-S) and PEC(C-P)
Synthesis of the PECs was successful using either the C-P or E-S approaches. With the C-P technique, complexation occurred instantaneously when the PQ10 solution was added to the HA drug solution. This was evidenced by the formation of strand-like precipitates as a result of the complexation process. Self-assembly of the polymers produced a dense supernatant that presented a challenge in filtration. For the E-S technique, it was imperative to add excipients that facilitated extrusion and subsequent spheronization.
Analysis of Surface Morphology of the PEC in Compacted and Powdered Form
Scanning electron microscopy (SEM) images of the PECs synthesized by the two methods revealed that they notably differed morphologically (Fig. 1). The compact PEC(E-S) (Fig. 1b) displayed an irregular surface devoid of the large pores associated with the PEC(C-P) fibers. Powdered PEC(C-P) maintained its fibrous nature while the surface of PEC(E-S) was tightly packed together in the powdered form. Surface morphology may be utilized in predicting drug dissolution properties which are dependent on surface area (21). From the SEM images, the pores on the PEC(C-P) may facilitate faster interaction of the PEC with the dissolution fluid, thus enhancing solubility and the rate of drug release. However, PEC(E-S) would be expected to have a more extended drug release pattern.
Validation of Drug-Free PEC Complexation via Solubility Testing in Varying Solvents Media
Stoichiometric PECs are generally insoluble in any known solvent (22). The PECs were insoluble in all the representative solvents: ethyl acetate (electron donor), DMSO (electron acceptor), formic acid (proton donor), ethanol (proton donor), acetone (proton acceptor), DCM (dipole-dipole interaction), and deionized water (neutral). The slight solubility of PEC(E-S) in water was attributed to the partial complexation from E-S which resulted in the presence of uncomplexed polymers that were readily soluble in the water. Formic acid was included to validate the typical behavior of PECs containing cellulose-based polymers which dissolve completely in the acid (23). Both formulations completely dissolved in formic acid.
Characterization of EFV-Loaded PEC Synthesized by C-P and E-S
The Influence of Complexation Method on EFV, HA, and PQ10 Interactions
The FTIR spectra (Fig. 2) revealed that the dominant bond (C=O cyclic, 1700 cm−1) from EFV was present in both PECs and the physical mixture (pE), with its position remaining constant in all formulations. However, its intensity was diminished significantly in the PECs and pE compared to the pure drug. Another EFV bond expressed in PEC-E(E-S), PEC-E(C-P), and pE was the exocyclic triple bond stretching (2248 cm−1). These characteristic peaks are consistent with the ones reported by da Costa and co-workers (24). Exhibition of these bonds in the drug-loaded formulations confirmed the presence of the unaltered drug in the drug/polymer complex or physical blend. The tertiary amide bond in EFV (1600 cm−1) overlaps with the new bond that is expected to form in PECs as previously reported (1). This is the point of complexation between NMe3 + and COO−. Most of the groups in EFV are not available for bond formation with the polymers except for the N–H bond. This is detected at 3500–3100 cm−1 and would overlap with the polymeric O–H stretch detection at the same wavenumber. The interaction of the N–H bonds with hydrogen donor groups on the polymer chains may result in drug/polymer interaction which may improve drug entrapment and overall enhance drug solubility as the bond would easily break in aqueous medium. The shape of the bond at 3500–3100 cm−1 was sharper in the drug-loaded PECs than the drug-free PECs and pE. The resultant band around 3300 cm−1 had a lower intensity than that of the drug-free PEC signifying drug/polymer interaction (25,26). FTIR is a quantitative analysis of the bonds found in the test sample; therefore, the diminished absorbance of EFV bands in the formulations could have resulted from the masking effect of the polymers on the drug due to the high polymer/drug ratio.
Elucidation of the Thermodynamic Properties of the EFV-Loaded PEC Compared to Drug-Free PEC and Physical Drug/Polymer Mixtures
The DSC thermograms in Fig. 3 revealed the differences in thermal properties of the PECs produced by the two methods, pure EFV and physical blends. The physical blend shows various T m values, some of which correspond to the T m of PEC(E-S), PEC(C-P), and EFV. As expected, multiple endothermic events were noted for pE and one of them coincides with the melting point of pure EFV. The endotherm is reduced compared to that of the pure drug due to the high polymer/drug ratio. There was no sharp melting endotherm corresponding with that of the crystalline API in both drug-loaded PECs, thus signifying the good miscibility of the API into the polymeric matrix. According to literature, the disappearance or shifting of the endotherm of a pure drug is indicative of lack of defined crystalline arrangements. This was probably a result of the API being partially miscible in the matrix and therefore forming a mixed solid dispersion system of amorphous and crystalline drug (27). This is consistent with the FTIR results that revealed that drug/polymer interactions occurred. Ideal drug–polymer interactions are vital in order to prevent clumping of drug which is responsible for drug polymorphism. It can be inferred that the drug crystallinity was reduced upon dispersion into the HA-PQ10 and therefore led to enhanced drug solubility. A similar conclusion was reached by Mabrouk and co-workers (13) who concluded that increase in solubility of efavirenz loaded into physically crosslinked hydroxyethylcellulose and polyacrylic acid hydrogel was a result of a decrease in crystallinity of the drug as well as drug–polymer interactions. The endothermic peaks from 50 to 100°C were attributed to loss of moisture from the samples. The peaks (around 150°C) adjacent to the one for EFV were attributed to the polymer thermal properties; this same peak is exhibited in the drug-free extruded sample. This peak becomes broader in both complexes, thus it can be concluded that the PECs are more amorphous in nature compared to the native polymers. PEC(C-P) was expected to undergo complete complexation during synthesis in aqueous medium and hence would be more amorphous than PEC(E-S). Complexation of PEC(E-S) was probably partial after synthesis and only completed in situ during drug release and permeation when the system got in contact with the aqueous medium. PECs form in water or under temperatures which would cause the polymers to melt, and neither of these conditions were met during the E-S process (1). The presence of the drug in the PEC resulted in remarkable changes in PEC-E(C-P) which expressed significant endothermic events unlike PEC(C-P) which hardly showed any changes during heat absorption. The behavior of PEC-E(E-S) was identical to its drug-free counterpart.
Evaluation of the Extent of Supersaturation and EFV Release
Both PECs exhibited %DL >14% with PEC-E(C-P) possessing higher EFV-loading (20.82 ± 2.03% w/w) than PEC-E(E-S) (14.19 ± 0.49% w/w). This was due to the presence of excipients that were pertinent for the E-S process therefore lowering the %DL. The amount of MCC required to facilitate the E-S process was directly proportional to the amount of drug in the formulation (28). Caution was exercised in determining the ideal drug loading in order to avoid leaching of drug from the extrudates. PEC-E(E-S) exhibited superior solubility properties (200 ± 10.78 μg/mL) to PEC-E(C-P) (110 ± 2.96 μg/mL) and pE (40 ± 1.02 μg/mL). The incorporation of EFV into the HA-PQ10 PEC greatly increased the wettability of EFV. Solubility enhancement was attributed to the partial conversion of the crystalline drug to the amorphous form which provided better interaction between the drug and dissolution medium due to the negation of lattice energy in the system. The PEC, due to the nature of the native polymers (HA and PQ10), was able to attract more dissolution medium which was trapped in the matrix for prolonged periods of time thus allowing sufficient contact between the drug and medium, leading to increase in wettability and consequently higher solubility. For PEC-E(E-S), the extrusion process led to the dispersion and possible hydrogen bond interactions of EFV with the polymeric matrix thus enhancing drug solubility. After 36 h, PEC-E(E-S) still provided a saturated system and this demonstrated that a considerable degree of stability in protecting EFV from recrystallization within the dissolution time period was present. PEC-E(C-P) did not maintain this stability since the highest EFV concentration was detected at the 24-h sampling point. Supersaturation of the drug may not occur if an amorphous system rapidly reverts to the crystalline state (29). It was imperative to implement the additional step of complexation since the physical blend did not result in notable solubility amplification of EFV. Figure 4a depicts the EFV release profiles over a 24-h period. PEC-E(E-S) and PEC-E(C-P) both exhibited controlled release of EFV due to the entrapment of the drug in the novel complex. For satisfactory comparison of the drug-loaded PECs, MCC was added to the PEC-E(C-P) before encapsulation into the hard gelatin capsules The formulation that yielded superior solubility and permeability enhancement was selected for optimization and loading of the additional BCS class IV drug, RTV. Since the PECs do not easily dissolve in SIF, they were responsible for slowly releasing the drug from the system, thus providing a controlled release system. Drug release from pE was poor (<40%), and this validated the need for using a PEC instead of the physical blend for optimal drug delivery. With the physical blend, upon dissolution of the capsule shell, the exposed blend formed a PEC in situ. However, the PEC formation occurred on the surface and was not uniformly distributed within all the drug/polymer blend. Drug release from PECs is affected by the specific chemical structures of the native polymers, hence different release profiles are expected from different polymer combinations (30). The amphiphilic HA was responsible for lowering the solid/liquid interfacial tension therefore increasing the wettability of the lipophilic EFV which was trapped in the hydrophobic core of the polymer. This further prevented the agglomeration of EFV particles.
Assessment of Ex Vivo Intestinal Tissue Permeability of EFV from the PEC
Transmembrane permeability profiles of the PECs were superior to those of the comparator products as illustrated in Fig. 4b. The constant EFV permeation from the PECs revealed that there was no saturation in the intestinal membrane. The permeability coefficient for PEC-E(E-S) (0.163 ± 0.043 cm2 h−1) and PEC-E(C-P) (0.232 ± 0.008 cm2 h−1) was elevated by the presence of larger amounts of free drug due to supersaturation. EFV loading into the PEC system was responsible for this behavior. The consistent permeation coupled with higher permeability coefficients and flux values observed in the PEC-E(C-P) were due to faster EFV release. PEC-E(C-P) exhibited the highest flux of 0.054 ± 0.024 mg cm−2 h−1 while PEC-E(E-S) had a flux of 0.033 ± 0.052 mg cm−2 h−1. The least flux was recorded for pE (0.008 ± 0.017 mg cm−2 h−1). Figure 5 shows the TEER of the intestinal tissue before and after the permeation studies were undertaken. Results demonstrated any changes that might have occurred to the integrity of the tissue as a result of drug permeation. Both PECs had an increase in membrane charge contrary to pE which exhibited a decrease in membrane charge when EFV crossed the small intestinal tissue. It can be deduced that the EFV-loaded PECs did not cause any irreversible membrane damage and neither were there any leakages since EFV passage was constant. Controlled drug release was also responsible for the constant concentration gradient on the membrane. The improved permeation did not compromise the tissue viability as evidenced by the reversibility of TEER value after placement in distilled water at the end of the study. Permeation enhancement can be attributed to the interaction of the mucoadhesive PEC with the intestinal tissue. This led to prolonged duration of the drug at the absorption site in high concentrations due to enhanced solubility of the drug. The presence of mucus could have enhanced effectiveness of the complex. PEC-E(c-p) did not have a stronger interaction with intestinal mucosa compared to the EFV-loaded extrudates. Permeation was affected by the PEC delivery system and not the physical mixture implying that the mechanism was not tight junction controlled but largely dependent on the resulting complex.
Analysis of the Experimental Design and Optimized PEC Formulations
Analysis of the Physical Properties of the Experimental Design Formulations
PEC synthesis by E-S was chosen for optimization purposes since the complex showed superior solubility over 24 h. All design formulations were effectively extruded and spheronized. At least 95 ± 0.39% of the extrudates fell within the ideal size range of 500–1000 μm as determined by Singh and co-workers (31). The optimized PEC formulation had a composite desirability of 0.939. Loading of an alternative drug RTV into the optimized formulation (resulting in PEC-R(E-S)) did not alter the E-S properties and RTV was also soluble in and compatible with the optimized polymer ratio. The HR value measures the interparticulate friction between extrudates and the test was implemented to determine suitability of the samples for encapsulation in a hard gelatin shell (32). The HR value was <1.25 (Table II) for all formulations; therefore, F1–F13 had good flow properties. Generally, the smaller the HR value, the better the flow properties of the test sample (33,34). The small difference between the bulk (D a) and tapped (D c) densities further demonstrated that the extrudates had good flow properties.
Rapid contact of the extrudates with the aqueous medium facilitated the formation of the rate-controlling PEC due to in situ complexation. The highest MDTs were recorded for F4, F5, and F8, and these formulations contained the most PQ10 (1:3). This finding was due to the formation of a hydrated viscous layer around the extrudates as they absorbed the dissolution medium, and this was consistent with the finding noted by Charoenthai and co-workers (35). High molecular weight polymers are associated with sustained drug release from a system (35). The excess PQ10 provided the charge required for interaction with the aqueous environment thus attracting more aqueous fluid around the extrudates. It was observed that the extrudates from the formulations containing 5:3 polymeric ratios (F1–F3, F6, F9, F12, and F13) dissolved as the drug release progressed while the rest of the formulations remained intact during the study. For the three groups of polymeric ratios, the highest MDTs were noted for the formulations synthesized with four drops of distilled water for 446 mg of drug/polymer powder mass; therefore, this was the ideal amount of granulating fluid for maximizing MDT. The presence of excess HA, a macromolecule, resulted in the drug release retardation seen in F7, F10, and F11. The amount of drug that permeated across the intestinal tissue was enhanced by the increase in the amount of HA in the formulation. The highest amount was recorded for F11 (Table III), which was optimal.
Analysis of PEC Formulation Variables on MDT and Ex Vivo Drug Permeability
The optimal PEC formulation (having the composition of F11) consisted of a 3:1 HA/PQ10 polymeric ratio and synthesized with four drops of granulation fluid. Selection of the optimized system was based on the formulation that yielded both maximized MDT and flux values. Supplemental 1 is a schematic of the optimization desirability plots as determined by the Box-Behnken experimental design.
Analysis of the regression coefficients for MDT revealed that p values were significant (<0.05), therefore depicting good interaction of the polymers and granulating fluid. An R 2 of 97.8% was noted. Similarly, regression coefficients for flux showed significance for the interaction of HA and PQ10 with water and an R 2 value of 79.7%. The range for the p values was 0.023–0.299. The residual plots for MDT and flux further validated a good fit of the model for the data. The plots indicated that there was a correlation between the observed and predicted data. The 2D response contour plots generated by Minitab® (Supplemental 2) revealed that utilizing a formulation with either high- or low-level HA results in high MDT values. On the contrary, the use of the mid-level granulating fluid would be recommended to enhance the MDT. The contour plot for drug flux depicts that an increase of HA and water causes a simultaneous increase in amount of drug that permeated the intestinal tissue.
Characterization of the Optimized PEC Formulation
Analysis of Surface Morphology and Quantification of Porosity of the Extrudates
Spherical extrudates were obtained after spheronization as depicted in Fig. 6. The technique was responsible for the smoothing of the extrudates (Fig. 6a, d) devoid of the rough surface observed in the cross-sectional images of both PECs (Fig. 6b, e). PEC-E(E-S) had larger, interconnected, and more numerous pores which were not observed in PEC-R(E-s). Figure 6c and f are surface morphologies of the extrudates after 24-h drug release studies. Both PECs exhibited a porous surface with PEC-R(E-S) revealing larger pores than PEC-E(E-S). It can be inferred that drug release from PEC-E(E-S) occurred by diffusion while release from PEC-R(E-S) involved erosion of the matrix.
Porosity is a measure of the quantity of N2 gas adsorbed onto the sample pores. Pore diameter may impact the rate of drug release due to the interaction of the drug-loaded matrix with the aqueous dissolution medium (36). PEC-E(E-S) showed a typical type IV isotherm on the adsorption isotherm (Supplemental 3) and a negative curvature attributed to a decrease in adsorption as the pores filled up. PEC-R(E-S) exhibited a type V isotherm due to the mesoporous material and a low energy of adsorption. Evaluation of the BET results (Table IV) revealed that PEC-E(E-S) had a significantly larger surface area (−249.7811) than PEC-R(E-S) (−6.5412). This finding may be correlated with the SEM images (Fig. 6b, e). The BJH findings on pore sizes and volumes also maintained a similar trend of PEC-E(E-s) exhibiting a larger intraparticular porosity. Both formulations possessed mesopores since their pore sizes fell in the 2–50 nm range.
Evaluation of Saturation Solubility, Drug Release, and Permeability of the Optimized PEC Formulation
The solubility results revealed that both PECs exhibited more superior solubility properties to the commercially available comparator products [cE (EFV) and cR (RTV)]. PEC-E possessed a much greater increase in supersaturation (14.16 ± 2.81%) than PEC-R (4.39 ± 0.57%). cR is a lipid-based formulation and such formulations have been the most popular for enhancing drug bioavailability over the last two decades (37). In vitro dissolution studies were conducted in FeSSIF. The small intestine was selected as the target area of interest because most absorption occurs at this point. The upper intestine contains more bile salts and lecithin in the fed state therefore making it an important site for dissolution of poorly soluble drugs (14). Figure 7a depicts profiles of the fractional drug release against time for PEC-E(E-S) and PEC-R(E-S) over 24 h. The comparator brands exhibited immediate drug release within 2 h and the amount of drug released remained constant for the remainder of the 24 h. Drug release for cE was incomplete (<80%) and cR exhibited a total drug release within 2 h. Solid dispersions have been known to prevent crystal growth of drugs as well as reduce the drug particle size and this property is responsible for the increase in concentration of the drug that undergoes dissolution (38). Solid dispersions are responsible for creating a microenvironment that promotes supersaturation of the drug in the dissolution medium (39). These findings may be correlated to the Noyes Whitney equation (Eq. 9) which is used to demonstrate how the dissolution rate of poorly soluble compounds may be improved so as to enhance oral bioavailability.
where dC/dt is the rate of dissolution, A is the surface area available for dissolution, D is the diffusion coefficient of the compound, C s is the saturation solubility of the compound in the dissolution medium, C is the concentration of drug in the medium at time t, and h is the thickness of the diffusion boundary layer (40).
Loading the drugs into the PECs was beneficial in delaying the occurrence of any precipitation which is common in supersaturated solutions. This stability was achieved by the physical interaction of the drug with the polymers as validated by the FTIR results. Figure 7b highlights the permeability profiles of PEC-E(E-S) and PEC-R(E-S) and their respective comparator products. Permeation enhancement for PEC-E(E-S) and PEC-R(E-S) in comparison to the marketed formulations was 61.24 ± 6.92% and 38.78 ± 0.50%, respectively. According to the collated data, there was a tremendous permeation flux and permeability enhancement of the BCS class II/IV drugs which were loaded in the novel PEC when measured against cE and cR. This enhancement was more pronounced in PEC-E(E-S) and this may be attributed to EFV’s ability to cross the intestinal tissue. The use of HA and PQ10 had a contributory effect on the permeation of EFV and RTV as both polymers have previously been reported to possess good mucoadhesive properties (41,42). A study by Mirza and co-workers (43) revealed that the permeation of carbamazepine complexed to HA improved significantly compared to the permeation of the pure drug. This enhancement was attributed to HA being amphiphilic and more hydrophobic in aqueous media.
CONCLUSIONS
Extrusion-spheronization and complexation-precipitation techniques were successfully implemented for both drug-free and drug-loaded polyelectrolyte complex fabrication. Although they enhanced the delivery of the bioactive, synthesis by extrusion-spheronization provided more desirable qualities in the resultant solid dispersions. The use of solid dispersions has been widely implemented as a solubilization technique, though there are limited studies on their implementation to enhance the solubility and permeability of anti-HIV drugs in BCS Class II/IV. Solid dispersions of EFV and RTV in the optimized HA-PQ10 PEC showed promising results for targeted release to the small intestine and accelerated drug solubility and permeability. However, there is a need to further understand the physicochemical stability of the drug-loaded polyelectrolyte complex. The formulation constituting the novel HA-PQ10 PEC may also be applied for targeted release of highly water-soluble drugs. The quantity of HA used in this study was below the permissible 512 mg/kg reported previously by Mirza and co-workers (43). Therefore, the PEC can be safely utilized for drug delivery purposes and the extruded PEC formulation was selected for optimization. Overall, these results indicated that the PEC enhanced the solubility and permeability of BCS Class II/IV drugs that may have a positive impact on their bioavailability in a suitable in vivo model.
References
Siyawamwaya M, Choonara YE, Kumar P, Kondiah PPD, du Toit LC, Pillay V. A humic acid-polyquaternium-10 stoichiometric self-assembled fibrilla polyelectrolyte complex: effect of pH on synthesis, characterization, and drug release. Int J Polym Mater Polym Biomater. 2016;65(11):550–60.
Kim JY, Zaoutis T, Chu J, Zhao H, Rutstein R. Effects of highly active antiretroviral therapy (HAART) on cholesterol in HIV-1 infected children: a retrospective cohort study. Pharmacoepidemiol Drug Saf. 2009;18(7):589–94.
Singh G, Sharma S, Gupta GD. Extensive diminution of particle size and amorphization of a crystalline drug attained by eminent technology of solid dispersion: a comparative study. AAPS PharmSciTech. 2016:1–5.
Serajuddin A. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88(10):1058–66.
Shergill M, Patel M, Khan S, Bashir A, McConville C. Development and characterisation of sustained release solid dispersion oral tablets containing the poorly water soluble drug disulfiram. Int J Pharm. 2016;497(1):3–11.
Usach I, Melis V, Peris JE. Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability. J Int AIDS Soc. 2013;16(1):18567.
Siyawamwaya M, Choonara YE, Bijukumar D, Kumar P, du Toit LC. Pillay V a review: overview of novel polyelectrolyte complexes as prospective drug bioavailability enhancers. Int J Polym Mater Polym Biomater. 2015;65(11):955–68.
Fandaruff C, Segatto Silva MA, Galindo Bedor DC, de Santana DP, Rocha HVA, Rebuffi L, Azanza Ricardo CL, Scardi P, Cuffini SL. Correlation between microstructure and bioequivalence in anti-HIV drug efavirenz. Eur J Pharm Biopharm. 2015;91:52–8.
Rao MR, Shirsath C. Enhancement of bioavailability of non-nucleoside reverse transciptase inhibitor using nanosponges. AAPS Pharm Sci Tech. 2016;1–1.
Six K, Verreck G, Peeters J, Brewster M, den Mooter GV. Increased physical stability and improved dissolution properties of itraconazole, a class II drug, by solid dispersions that combine fast- and slow-dissolving polymers. J Pharm Sci. 2004;93(1):124–31.
Dengale SJ, Hussen SS, Krishna BSM, Musmade PB, Gautham Shenoy G, Bhat K. Fabrication, solid state characterization and bioavailability assessment of stable binary amorphous phases of ritonavir with quercetin. Eur J Pharm Biopharm. 2015;89:329–38.
Jones E, Ojewole E, Pillay V, Kumar P, Rambharose S, Govender T. Monolayered multipolymeric buccal films with drug and polymers of opposing solubilities for ARV therapy: physico-mechanical evaluation and molecular mechanics modelling. Int J Pharm. 2013;455(1):197–212.
Mabrouk M, Chejara DR, Mulla JAS, Badhe RV, Choonara YE, Kumar P, Pillay V. Design of a novel crosslinked HEC-PAA porous hydrogel composite for dissolution rate and solubility enhancement of efavirenz. Int J Pharm. 2015;490(1):429–37.
Viral S, Dhiren P, Mane S, Umesh U. Solubility and dissolution rate enhancement of licofelone by using modified guar gum. Int J PharmTech Res. 2010;2:1847–54.
Sinha S, Ali M, Baboota S, Ahuja A, Kumar A, Ali J. Solid dispersion as an approach for bioavailability enhancement of poorly water-soluble drug ritonavir. AAPS PharmSciTech. 2010;11(2):518–27.
Tang M, Dettmar P, Batchelor H. Bioadhesive oesophageal bandages: protection against acid and pepsin injury. Int J Pharm. 2005;292(1):169–77.
Dezani AB, Pereira TM, Caffaro AM, Reis JM, Serra CH, dos Reis Serra CH. Determination of lamivudine and zidovudine permeability using a different ex vivo method in Franz cells. J Pharmacol Toxicol Methods. 2013;67(3):194–202.
Lavon I, Grossman N, Kost J. The nature of ultrasound–SLS synergism during enhanced transdermal transport. J Control Release. 2005;107(3):484–94.
Jan K, Riar CS, Saxena DC. Engineering and functional properties of biodegradable pellets developed from various agro-industrial wastes using extrusion technology. Int J Food Sci Tech. 2015;52(12):7625–39.
Kondiah PP, Tomar LK, Tyagi C, Choonara YE, Modi G, du Toit LC, Pillay V. A novel pH-sensitive interferon-β (INF-β) oral delivery system for application in multiple sclerosis. Int J Pharm. 2013;456(2):459–72.
DiNunzio JC, Miller DA, Yang W, McGinity JW, Williams III RO. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol Pharm. 2008;5(6):968–80.
Shovsky A, Varga I, Makuska R, Claesson PM. Formation and stability of water-soluble, molecular polyelectrolyte complexes: effects of charge density, mixing ratio, and polyelectrolyte concentration. Langmuir. 2009;25(11):6113–21.
Ito H, Miyamoto T, Inagaki H, Noishiki Y, Iwata H, Matsuda T. In vivo and in vitro blood compatibility of polyelectrolyte complexes formed between cellulose derivatives. J Appl Polym Sci. 1986;32(2):3413–21.
da Costa MA, Seiceira RC, Rodrigues CR, Hoffmeister CRD, Cabral LM, Rocha HVA. Efavirenz dissolution enhancement I: co-micronization. Pharmaceutics. 2012;5(1):1–22.
Sathigari SK, Radhakrishnan VK, Davis VA, Parsons DL, Babu RJ. Amorphous-state characterization of efavirenz—polymer hot-melt extrusion systems for dissolution enhancement. J Pharm Sci. 2012;101(9):3456–64.
Pawar J, Tayade A, Gangurde A, Moravkar K, Amin P. Solubility and dissolution enhancement of efavirenz hot melt extruded amorphous solid dispersions using combination of polymeric blends: a QbD approach. Eur J Pharm Sci. 2016;88:37–49.
Koh PT, Chuah JN, Talekar M, Gorajana A, Garg S. Formulation development and dissolution rate enhancement of efavirenz by solid dispersion systems. Indian J Pharm Sci. 2013;75(3):291.
Kleinebudde P. The crystallite-gel-model for microcrystalline cellulose in wet-granulation, extrusion, and spheronization. Pharm Res. 1997;14(6):804–9.
Prasad D, Chauhan H, Atef E. Amorphous stabilization and dissolution enhancement of amorphous ternary solid dispersions: combination of polymers showing drug–polymer interaction for synergistic effects. J Pharm Sci. 2014;103(11):3511–23.
Shao Y, Li L, Gu X, Wang L, Mao S. Evaluation of chitosan–anionic polymers based tablets for extended-release of highly water-soluble drugs. Asian J Pharm Sci. 2015;10(1):24–30.
Singh G, Pai RS, Devi VK. Response surface methodology and process optimization of sustained release pellets using Taguchi orthogonal array design and central composite design. J Adv Pharm Tech Res. 2012;3(1):30.
Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008;9(1):250–8.
Shravani D, Lakshmi PK, Balasubramaniam J. Preparation and optimization of various parameters of enteric coated pellets using the Taguchi L9 orthogonal array design and their characterization. Acta Pharm Sin B. 2011;1(1):56–63.
Singh I, Kumar P. Preformulation studies for direct compression suitability of cefuroxime axetil and paracetamol: a graphical representation using SeDeM diagram. Acta Pol Pharm. 2012;69(1):87e93.
Charoenthai N, Kleinebudde P, Puttipipatkhachorn S. Use of chitosan-alginate as alternative pelletization aid to microcrystalline cellulose in extrusion/spheronization. J Pharm Sci. 2007;96(9):2469–84.
Berger J, Reist M, Mayer JM, Felt O, Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):35–52.
Jannin V, Chevrier S, Michenaud M, Dumont C, Belotti S, Chavant Y, Demarne F. Development of self emulsifying lipid formulations of BCS class II drugs with low to medium lipophilicity. Int J Pharm. 2015;495(1):385–92.
Betageri GV, Makarla KR. Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int J Pharm. 1995;126(1):155–60.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72.
Dash S, Padala NM, Lilakanta N, Prasanta C. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
Zhang X, Bai R. Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules. J Colloid Interface Sci. 2003;264(1):30–8.
Mazoniene E, Joceviciute S, Kazlauske J, Niemeyer B, Liesiene J. Interaction of cellulose-based cationic polyelectrolytes with mucin. Colloids Surf B Biointerfaces. 2011;83(1):160–4.
Mirza MA, Ahmad N, Agarwal SP, Mahmood D, Anwer MK, Iqbal Z. Comparative evaluation of humic substances in oral drug delivery. Results Pharma Sci. 2011;1(1):16–26.
Acknowledgements
This work was funded by the National Research Foundation (NRF) of South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Siyawamwaya, M., Choonara, Y.E., Kumar, P. et al. Synthesis, Comparison, and Optimization of a Humic Acid-Quat10 Polyelectrolyte Complex by Complexation-Precipitation versus Extrusion-Spheronization. AAPS PharmSciTech 18, 3116–3128 (2017). https://doi.org/10.1208/s12249-017-0803-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0803-4