ABSTRACT
Lovastatin (LOV), an antihyperlipidimic agent, is characterized by low solubility/poor dissolution and, thus, low bioavailability (<5%). A beneficial effect on its bioavailability could result from improving its dissolution. One of the most common methods used to enhance dissolution is the preparation of solid dispersions. Solid dispersions of LOV and silica with different surface areas were prepared. The effects of the type of silica, ratio of drug/silica, incubation period with silica, and the effect of surface area were all studied. Characterization of the prepared formulae for possible interaction between drug and polymer was carried out using differential scanning calorimetery, Fourier transform infrared spectroscopy, powder X-ray diffraction, surface area determination, and scanning electron microscopy. The dissolution profiles of all prepared formulae were constructed and evaluated. It was found that the formula made of LOV and Sylysia 350 FCP in a ratio of 1:5 after an incubation period of 48 h resulted in the best release, and it was stable after 3 months storage at 75% RH and 40°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Oral administration of poorly water-soluble drugs is a challenge to dosage form formulators. This is due to the direct relationship between poor solubility/dissolution and bioavailability and therapeutic effectiveness. Thus, enhancing drug dissolution can solve this problem (1).
Enhancing drug dissolution was previously achieved using various methods including reduction in particle size (micro- or nano-sizing) (2), amorphization (3–5), cyclodextrin solubilization in the absence or presence of different polymers (6,7), salt formation (8), and dispersion of drug in polymeric matrices. The last method gained lots of interest due to its advantages which include simplicity and the presence of drug in the molecular level (9). However, these methods suffer from many disadvantages. For example, the production cost using the cyclodextrin solubilization method is high. Additionally, the stability is poor and the drug loading is low (10) in the other methods. Accordingly, there is a need to develop a method that is devoid of the above-mentioned disadvantages.
Adsorption of drugs onto high surface area carriers like silica is a well-known method for enhancing drug dissolution. It was first described in the early 1970s (11). During the last few years, new carriers were synthesized. These include pharmaceutically porous silicon dioxide (Sylysia 350 FCP®) (12–15), polypropylene foam powder (Accurel®), porous calcium silicate (Florite®) (13,16), magnesium aluminum silicate (Neusilin®) (17–20), and mesoporous silica nanoparticles (MCM-41 and SBA-15). The use of mesoporous silica nanoparticles was promising in the field of peptides, proteins, and gene drug delivery mainly because of their ordered structure, high surface area, large pore volume, tunable pore size, ease of surface functionalization, and their biocompatibility (10,21–25).
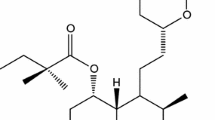
Lovastatin (LOV), belonging to the class statins, is widely used for the treatment of hypercholesterolemia (26,27). It is an inactive lactone that is hydrolyzed to the corresponding β-hydroxy acid form, which is the principal metabolite and the inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (9). According to the Biopharmaceutical Classification System, LOV belongs to class II drugs. It is a highly lipophilic and poorly water-soluble drug (26). Its water solubility is 0.3 μg/ml (28). The rate at which poorly water-soluble drugs dissolve is often the slowest step and therefore exerts a rate-limiting effect on drug bioavailability (29). Absorption of LOV, relative to an intravenous reference dose, is about 30% of the oral dose. This necessitates the administration of an unnecessarily large dose of drug. Also, LOV exhibits low and variable oral bioavailability (<5%) (30) because of the rapid metabolism in the gut and liver; the plasma half-life of oral LOV varies from 1.1 to 1.7 h in adults with normal renal function (30,31). These disadvantages necessitate frequent administration of drug. Thus, a formulation with enhanced dissolution is extremely desirable for LOV to increase the rate of drug absorption and improve the bioavailability and the therapeutic efficacy.
A variety of pharmaceutical formulation technologies were used to enhance the oral bioavailability of LOV, including inclusion in β-cyclodextrins (32), preparation of solid dispersions using different polymers (poloxamer, polyethylene glycol 4000, polyvinylpyrrolidone K30 (33), and modified locust bean gum (29)), superdisintegrants (crospovidone, croscarmellose sodium, and sodium starch glycolate) (34), microemulsions (35,36), microspheres (35,37), nanocrystals (38), solid lipid nanoparticles (39), microparticles prepared by coacervation (40,41), nanostructured lipid carriers (30,39,42), and mesoporous carbon spheres (43). However, these methods have many disadvantages, including recrystallization of the amorphous formed drug in solid dispersions, hygroscopicity or high viscosity of the used carriers, high production cost, poor wetting and poor flow of nanoparticles, limited drug loading, and expulsion of drug during storage. Accordingly, an easier, cost-effective method for enhancing the dissolution on a large scale is needed.
In this research, a relatively simple and well-known formulation technology (preparation of solid dispersions) will be used to enhance drug dissolution. Complexes of LOV with novel newly developed silica polymers (Sylysia 350 FCP, Neusilin US2, Fujicalin, and Aerosil 200) will be prepared and evaluated.
Sylysia 350 FCP is an amorphous silicon dioxide with high specific surface area and porosity, making it a good candidate for efficient drug loading and rapid release. It contains many silanol groups on its surface and is considered safe by the Food and Drug Administration (11,44).
Neusilin US2 is an amorphous magnesium aluminosilicate. It is available as porous granules. It has a neutral pH and a wide range of compatibility. It is similar to Sylysia in having many silanol groups on its surface. The presence of silanol groups on its surface makes it a potential proton donor and acceptor. It is believed that the interaction with the drug and the presence of metal ions prevent recrystallization of the drug (stabilize its amorphous form) (45–47).
Fujicalin is a type of spherical particle containing microcrystals of anhydrous dicalcium phosphate, soluble in acidic media, with high porosity and large specific surface area (48,49).
Aerosil 200 differs from these polymers in that it is a non-porous material made of hydrophilic fumed silica (silicon dioxide) (50).
Accordingly, the aims of this study were: firstly, to prepare solid dispersions of LOV using various silica polymers in different ratios and different incubation periods; secondly, to identify the polymer and drug/polymer ratio that best enhance the dissolution of the drug; and, thirdly, to study the stability of the prepared formula after storage for 3 months at 75% relative humidity (RH) and 40°C.
MATERIALS AND METHODS
Materials
Lovastatin was purchased from Ningbo Tianhong Biotech, China. The three types of silica (Neusilin US2, Fujicalin, and Sylysia 350) were kindly donated by Fuji Chemical Ltd., Japan. Aerosil 200 was obtained from Evonik Industries, Germany. Ethanol (HPLC grade) and sodium hydroxide were supplied by Fisher Chemical, UK. Potassium dihydrogen phosphate (extra pure) was supplied by Scharlau Chemie, Spain. Water used in all experiments was distilled. All chemicals were used as supplied without further modification.
Preparation of Co-evaporates
Fifty milligrams of Neusilin US2 was added to 100 ml ethanolic solution of LOV, resulting in a drug/polymer weight ratio of 1:1. After ultrasonication for 5 min, the suspension was dried using Heidolph rotary evaporator (Laborota 4010 Digital, Germany), the rotation speed of the rotary evaporator was set at 15 rpm, and the solvent was slowly driven off by heating the flask in a hot water bath kept at 75°C and pulling vacuum with an aspirator. Samples were then collected by scraping them from the walls of the flask and drying in a vacuum oven at 40°C (the temperature was predetermined to ensure the stability of LOV) overnight. All the co-evaporates were subsequently passed between sieves of mesh numbers of 80 and 100, further dried, and stored in desiccators over silica gel until further use.
The preparation of the co-evaporate was repeated as mentioned above, but with changing the type of silica (Sylysia 350 FCP, Fujicalin, and Aerosil 200), the drug/polymer ratio (1:2, 1:3, and 1:5), and the time of soaking in ethanol (48 and 24 h).
Preparation of Physical Mixtures
Predetermined amounts of LOV and silica were weighed and mixed using a mortar and a pestle. The same ratios used in the co-evaporation method were used in the preparation of the physical mixtures. Then, the physical mixtures were passed between sieves with mesh numbers 80 and 100 and stored in desiccators for further use.
Characterization of Co-evaporates and the Physical Mixtures
Differential Scanning Calorimetry
Thermal analysis was carried out to assess the thermotropic properties of the drug and the silica polymers and the presence of any interaction between them. Samples of 3–4 mg were heated in aluminum pans at a rate of 5°C/min in the range of 10–400°C. Empty aluminum pans were used as references. Differential scanning calorimetry (DSC) thermograms were recorded using a Shimadzu differential scanning calorimeter (DSC-50, Japan).
Powder X-Ray Diffraction Patterns
Powder X-ray diffraction (PXRD) patterns of LOV, the raw materials, the co-evaporates, and the physical mixtures were obtained for the detection of crystallinity. These were recorded in the range 0–40° using an Ultima IV X-ray diffractometer (Rigaku, Japan) with cobalt radiation at a voltage of 40 kV and a current of 40 mA. The scan step was 0.02°.
Fourier Transform Infrared Spectroscopy
Fourier transform infrared (FTIR) spectroscopy for pure drug, silica polymers, co-evaporates, and the physical mixtures was carried out for additional characterization of polymorphic changes and drug interactions using IRAffinity−1 (Shimadzu). Samples were blended with potassium bromide powder and the test was conducted over a frequency range of 4700–340 and 0.04 cm−1 resolution.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was used to examine the morphological characteristics and surface properties of the drug, silica polymers, the co-evaporates, and the physical mixtures. Samples were mounted on an aluminum stub by a double-sided sticky disc of conductive carbon, then coated with platinum by a sputter coater to render them electrically conductive. The electron beam was scanned over the specimen to produce a digital image using a Philips scanning electron microscope (model Quanta 200, Holland).
In Vitro Release Study
The release rate of LOV from the co-evaporates and the physical mixtures was studied using a USP dissolution apparatus type II (paddle). Twenty milligrams of LOV powder or an equivalent amount of the co-evaporates or the physical mixtures was placed in 900 ml phosphate buffer (pH 7) containing 0.01% (w/v) SLS, at 37 ± 0.5°C and 50 rpm. Five-milliliter samples were withdrawn at predetermined intervals (5, 10, 15, 30, 45, 60, 90, 105, and 120 min), filtered using a 0.45 micro-syringe filter paper, diluted as needed, and assayed spectrophotometrically at λ max (237 nm). The dissolution test was conducted in triplicate and the percentage of drug release was calculated.
Surface Area Determination
A Nov. 2200 multi-speed high gas sorption analyzer (version 6.11, Quantachrome Co., Syosset, NY, USA) was used to obtain nitrogen vapor adsorption isotherms at 77 K. Different silica polymers were degassed by heating in a vacuum oven at 100°C for 24 h prior to use.
Stability Study
The co-evaporates of different polymers were placed in glass vials and stored at 40 ± 2°C and 75 ± 5% relative humidity in a Schutzart-Memmert stability chamber (Germany) according to the ICH guidelines for 3 months. The FTIR spectra and the DSC and PXRD patterns of the stored samples were obtained and compared to those of the freshly prepared samples to detect any changes in crystallinity.
RESULTS AND DISCUSSION
Fourier Transform Infrared Spectroscopy
The FTIR spectra of pure LOV, silica polymers, co-evaporates, and physical mixtures are presented in Fig. 1. Pure LOV showed sharp characteristic peaks at 3532.73, 1706.74, and 1220.66 cm−1. The FTIR spectra of the co-evaporates showed the same peaks of the drug without any changes in their positions, indicating the absence of interaction between the drug and the polymers. The same trend was seen when comparing the FTIR spectra of pure LOV and the physical mixtures prepared using Fujicalin (Fig. 1d). The spectra of the co-evaporates and of the physical mixtures showed both the peaks of the drug and the peaks of the silica polymers, indicating the absence of chemical interaction between drug and polymer.
Differential Scanning Calorimetry
The DSC thermograms of LOV, silica polymers, co-evaporates, and physical mixtures are presented in Fig. 2. The thermogram of pure LOV showed a single sharp endothermic peak corresponding to the melting point of the drug (175°C) and indicating its crystalline nature. Figure 2a showed the effect of Neusilin US2 on the melting point of LOV. The sharp endothermic peak disappeared in the co-evaporate, which might indicate the loss of crystallinity and conversion of the drug to the amorphous form. The same trend was seen in all co-evaporates prepared using Sylysia 350 FCP, Aerosil, and Fujicalin.
By comparing the DSC thermograms of the physical mixtures prepared using Neusilin, Sylysia 350 FCP, Fujicalin, and Aerosil with that of pure LOV, it is clear that the endothermic peak of LOV decreased or almost vanished, indicating a decrease in the crystallinity of the drug. This was expected since, firstly, in the physical mixtures, half the amount of the drug was present compared with pure LOV (drug/polymer ratio, 1:1); secondly, it is proven in the literature that milling using a mortar and a pestle reduces the size of the particles and increases the surface area and surface free energy, converting the drug to the amorphous form. It is worth noting that the decrease in the endothermic peak of the drug in the physical mixture prepared using Fujicalin (Fig. 2d) was less than that in the case of the other silica polymers. This could be due to the difference in the chemical structure between Fujicalin and the other silica polymers. The milling effect on the drug (conversion to the amorphous form) was higher in the case of the physical mixtures prepared using the other silica polymers than in the case of Fujicalin. Fujicalin is composed of microcrystals of dicalcium phosphate and has a lower surface area than the other silica polymers used. Thus, only a small amount of drug was adsorbed onto its surface and the remainder was free (crystalline), resulting in a small decrease in the endothermic peak.
Powder X-Ray Diffraction Patterns
The PXRD pattern of LOV showed sharp characteristic diffraction peaks at angles (2θ) of 9.38, 10.86, 15.66, 16.68, and 18.9, indicating its crystallinity (Fig. 3). These diffraction peaks were still observed in the PXRD patterns of the physical mixtures prepared using different silica polymers, indicating that the drug still retained its crystallinity. On the contrary, no diffraction peaks were observed in the PXRD patterns of the co-evaporates. During processing, LOV was first solubilized in ethanol and then adsorbed and/or entrapped in the pores of the carriers. It was transformed into the amorphous state and was not capable of recrystallization. These results were consistent with those obtained from the thermal analysis experiments.
Scanning Electron Microscopy
The images of pure LOV, the silica polymers, the co-evaporates, and the physical mixtures are shown in Fig. 4. The image of pure LOV showed crystallinity, in contrast to the co-evaporates which showed loss of crystallinity. The images of the physical mixtures showed that some crystallinity was still found. These results were consistent with data obtained by the DSC and PXRD results.
Neusilin US2 and Fujicalin have a spherical characteristic shape, while LOV has a rod-like shape. The SEM images showed that the drug and the polymers retained their shapes in the physical mixtures prepared using Neusilin US2 or Fujicalin, indicating only mixing. The images also showed loss of crystallinity of the drug in the co-evaporates prepared using Neusilin, Aerosil, and Sylysia and a decrease in crystallinity in the co-evaporates prepared using Fujicalin. This indicated the transformation of the drug from the crystalline form to the amorphous form. This was again consistent with the results of the DSC and PXRD experiments.
In Vitro Release Study
The cumulative percentages of LOV released from different silica polymers (different ratios with different soaking times in ethanol) in comparison with pure LOV are shown in Fig. 5. The release of LOV from all polymers was much higher than that from pure LOV. The release from the different polymers was in the following order: Sylysia 350 FCP > Neusilin US2 > Aerosil > Fujicalin.
Comparison of the percent cumulative amount of lovastatin released from solid dispersions prepared using different silica polymers (Neusilin (a), Sylysia (b), Aerosil (c), and Fujicalin (d)) in a drug/polymer ratio of 1:1 and an incubation period of 48 h and raw lovastatin in 900 ml phosphate buffer (pH 7) containing 0.01% (w/v) SLS at 37 ± 0.5°C and 50 rpm (n = 3)
As the ratio of Sylysia 350 FCP polymer/drug increased, the release of the drug increased (1:5 > 1:3 > 1:2 > 1:1; Fig. 5a). This was expected since more carrier was available for trapping the drug. This was consistent with the data obtained from the PXRD, FTIR, DSC, and SEM results.
The same trend was seen with Neusilin US2 (Fig. 5b). It was observed that as the ratio of Neusilin US2 polymer/drug increased, the release of the drug also increased. The increase in the release of the drug could be due to the presence of the drug in the amorphous form and the increase of wetting (51,52).
The release from Aerosil, a non-porous silicon dioxide, was lower than that from both Neusilin US2 and Sylysia 350 FCP (Fig. 5c). This could be attributed to the differences in surface area between the different polymers (Table I). Neusilin US2 had the highest surface area, followed by Sylysia 350 FCP, then Aerosil, and finally Fujicalin.
The same trend concerning the polymer/drug ratio was observed in the case of Fujicalin (Fig. 5d). Increasing the polymer increased drug release (1:5 > 1:3 > 1:2 > 1:1), but the release was lower than that from the other polymers. This might be related to the lower surface area of Fujicalin. Additionally, Fujicalin is insoluble at pH 7 since it is a dibasic calcium phosphate compound (53).
As the soaking time increased, the cumulative amount of the drug released from all polymers for the same ration increased. Soaking for 48 h resulted in a higher release of the drug than soaking for 24 h or for 0 h (instant). This was probably due to more drug uptake by the polymers with longer soaking time. The location of the drug could be at the surface of the polymer or inside the pores. In Table I, it clear that the surface areas of all co-evaporates decreased as compared to those of the pure polymers. The polymers were processed in the same manner as the co-evaporates to rule out the effect of the processing technique. The decrease in the surface area could be due to two reasons. The first reason is the adsorption of the drug onto the surface of the polymer, resulting in increasing its size. The second reason is the entrapment of the drug inside the pores of the polymer, making it inaccessible to the nitrogen molecules during the measurement. This was supported by the SEM images of the co-evaporates which showed that the drug was adsorbed onto the surface of the polymer and entrapped inside the pores (Fig. 4). The second reason was more likely since as the soaking time increased, more drug was entrapped inside the pores of the polymers and the drug release increased.
The release rate from all co-evaporates was much higher than that from the physical mixtures prepared using different silica polymers in the same ratio. This could be due to many reasons. Firstly, the drug in the co-evaporates was dispersed at the molecular level, resulting in better wetting and dissolution. Secondly, as evidenced by the DSC and PXRD patterns, the drug was in the amorphous form inside the co-evaporates compared to the physical mixtures in which it was in the crystalline form. The amorphous form is generally more energetic and water-soluble than the crystalline form. Thirdly, more drug was available in the co-evaporates as compared to the physical mixtures due to differences in the method of preparation. In preparing the co-evaporates, the drug was soaked for different periods of time, resulting in entrapment inside the pores, while in preparing the physical mixtures the drug was added as a solid and mixed immediately with the polymers. Hence, it was only adsorbed onto the surface.
Stability Study
The amorphous form of LOV in the co-evaporates prepared using different polymers remained stable after 3 months of storage at 75% RH and 40°C, as was clear in the DSC and PXRD patterns. For example, LOV in the co-evaporates prepared using Sylysia 350 FCP remained in the amorphous form (Fig. 6) as compared to LOV without Sylysia 350 FCP, which was converted into the crystalline form. Similar results were obtained by other researchers for Tolbutamide (54), Carvedilol (44,55), K-832 (56), Spironolactone (12), and Meloxicam (13).
As suggested in the literature, the stabilization could be due to the porous structure of the silicates and the interaction with the silanol ring on the surface of Neusilin US2 (potential proton donor as well as proton acceptor) (45,57). Unfortunately, co-evaporates prepared using Fujicalin showed some crystallinity after 3 months. This could be due to the lower uptake of the drug in the polymer and lower adsorption onto the surface due to the lower surface area.
CONCLUSIONS
Silica polymers increased the dissolution of LOV possibly by two mechanisms: firstly, the high surface area of silica polymers increased the dispersibility and wetting of LOV, and, secondly, the crystallinity of LOV was decreased by hindering the transformation of the amorphous non-stable form into the crystalline form by physically protecting the drug. The type of polymer and mass transfer from the pores affected the dissolution of LOV entrapped inside the silica. The release of the drug from the different polymers was in the following order: Sylysia 350 FCP > Neusilin US2 > Aerosil > Fujicalin. The formula that resulted in the best release was LOV/Sylysia 350 FCP in a ratio of 1:5 after incubation period of 48 h; it remained stable and amorphous after 3 months storage in 75% RH and 40°C.
REFERENCES
Nagabandi VK, Ramarao T, Jayaveera KN. Liquisolid compacts: a novel approach to enhance bioavailability of poorly soluble drugs. Int J Pharm Biol Sci. 2011;1(3):89–102.
Vogt M, Kunath K, Dressman JB. Dissolution enhancement of fenofibrate by micronization, cogrinding and spray-drying: comparison with commercial preparations. Eur J Pharm Biopharm. 2008;68(2):283–8.
Chawla G, Bansal AK. A comparative assessment of solubility advantage from glassy and crystalline forms of a water-insoluble drug. Eur J Pharm Sci. 2007;32:45–57.
Rasenack N, Hartenhauer H, Müller BW. Microcrystals for dissolution enhancement of poorly water-soluble drugs. Int J Pharm. 2003;254:137–45.
Ochi M, Kimura K, Kanda A, Kawachi T, Matsuda A, Yuminoki K, et al. Physicochemical and pharmacokinetic characterization of amorphous solid dispersion of meloxicam with enhanced dissolution property and storage stability. AAPS PharmSciTech. 2016;17(4):932–9.
Chowdary KPR, Srinivas SV. Influence of hydrophilic polymers on celecoxib complexation with hydroxyl propyl β-cyclodextrin. AAPS PharmSciTech. 2006;7(3):E184–9.
Upadhye SB, Kulkarni SJ, Majumdar S, Avery MA, Gul W, ElSohly MA, et al. Preparation and characterization of inclusion complexes of a hemisuccinate ester prodrug of Δ(9)-tetrahydrocannabinol with modified beta-cyclodextrins. AAPS PharmSciTech. 2010;11(2):509–17.
Serajuddin ATM. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59(7):603–16.
Katare M, Kohli S, Jain A. Evaluation of dissolution enhancement of lovastatin by solid dispersion technique. Int J Pharm Life Sci. 2011;2(7):894–8.
Summerlin N, Qu Z, Pujara N, Sheng Y, Jambhrunkar S, McGuckin M, et al. Colloidal mesoporous silica nanoparticles enhance the biological activity of resveratrol. Colloid Surf B. 2016;144:1–7.
Monkhouse DC, Lach JL. Use of adsorbents in enhancement of drug dissolution. J Pharm Sci. 1972;61(9):1430–5.
Uchino T, Yasuno N, Yanagihara Y, Suzuki H. Solid dispersion of spironolactone with porous silica prepared by the solvent method. Pharmazie. 2007;62(8):599–603.
Sharma S, Sher P, Badve S, Pawar A. Adsorption of meloxicam on porous calcium silicate: characterization and tablet formulation. AAPS PharmSciTech. 2005;305(6):E618–25.
Beg S, Swain S, Singh HP, Patra CN, Rao MEB. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS PharmSciTech. 2012;13(4):1416–27.
Jondhale S, Bhise S, Pore Y. Physicochemical investigations and stability studies of amorphous gliclazide. AAPS PharmSciTech. 2012;13(2):448–59.
Patel PV, Patel HK, Panchal SS, Mehta TA. Self micro-emulsifying drug delivery system of tacrolimus: formulation, in vitro evaluation and stability studies. Int J Pharm Investig. 2013;3:95–104.
Gupta MK, Vanwert A, Bogner RH. Formation of physically stable amorphous drugs by milling with Neusilin. J Pharm Sci. 2003;92:536–51.
Vadher AH, Parikh JR, Parikh RH, Solanki AB. Preparation and characterization of co-grinded mixtures of aceclofenac and Neusilin US(2) for dissolution enhancement of aceclofenac. AAPS PharmSciTech. 2009;10(2):606–14.
Censi R, Gigliobianco MR, Dubbini A, Malaj L, Di Martino P. New nanometric solid dispersions of glibenclamide in Neusilin(R) UFL2. AAPS PharmSciTech. 2016;17(5):1204–12.
Lou H, Liu M, Wang L, Mishra SR, Qu W, Johnson J, et al. Development of a mini-tablet of co-grinded prednisone–Neusilin complex for pediatric use. AAPS PharmSciTech. 2013;14(3):950–8.
Yuting N, Amirali P, Meihua Y, Surajit K, Wenyi G, Chengzhong Y. Recent advances in the rational design of silica-based nanoparticles for gene therapy. Ther Deliv. 2012;3(10):1217–37.
Mody KT, Mahony D, Zhang J, Cavallaro AS, Zhang B, Popat A, et al. Silica vesicles as nanocarriers and adjuvants for generating both antibody and T-cell mediated immune resposes to bovine viral diarrhoea virus E2 protein. Biomaterials. 2014;35(37):9972–83.
Jambhrunkar S, Qu Z, Popat A, Yang J, Noonan O, Acauan L, et al. Effect of surface functionality of silica nanoparticles on cellular uptake and cytotoxicity. Mol Pharm. 2014;11(10):3642–55.
Jambhrunkar S, Qu Z, Popat A, Karmakar S, Xu C, Yu C. Modulating in vitro release and solubility of griseofulvin using functionalized mesoporous silica nanoparticles. J Colloid Interface Sci. 2014;434:218–25.
Abbaraju PL, Meka AK, Jambhrunkar S, Zhang J, Xu C, Popat A, et al. Floating tablets from mesoporous silica nanoparticles. J Mater Chem B. 2014;2(47):8298–302.
Nanjwade B, Derkar G, Bechra H, Nanjwade V, Manvi F. Design and characterization of nanocrystals of lovastatin for solubility and dissolution enhancement. J Nanomedicine Nanotechnol. 2011;2(2):107–13.
Goyal U, Arora R, Aggarwal G. Formulation design and evaluation of a self-microemulsifying drug delivery system of lovastatin. Acta Pharm. 2012;62:357–70.
Prakash K, Jieun R, Hyeongmin K, Ksoo K, Jeong T, Hyunil K, et al. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2015;10(5):363–458.
Patel M, Tekade A, Gattani S, Surana S. Solubility enhancement of lovastatin by modified locust bean gum using solid dispersion techniques. AAPS PharmSciTech. 2008;9(4):1262–9.
Mandal S. Microemulsion drug delivery system: design and development for oral bioavailability enhancement of lovastatin. J Afr Pharm. 2011;78(3):44–50.
Shaikh K, Patwekar S, Payghan S, D’Souza J. Dissolution and stability enhancement of poorly water soluble drug – lovastatin by preparing solid dispersions. Asian J Biomed Pharm Sci. 2011;1(4):24–31.
Patel R, Patel M. Solid-state characterization and dissolution properties of lovastatin hydroxypropyl-β-cyclodextrin inclusion complex. Pharm Technol. 2007;2:72–81.
Patel RP, Patel MM. Physicochemical characterization and dissolution study of solid dispersions of lovastatin with polyethylene glycol 4000 and polyvinylpyrrolidone K30. Pharm Dev Technol. 2007;12(1):21–33.
Vidyadhara S, Ramana Reddy G, Ramu A, Chandana P. Formulation and evaluation of lovastatin fast dissolving tablets using newer super disintegrants. J Pharm Res. 2011;4(6):1762–5.
Kumar S, Nagpal K, Singh S, Mishra D. Improved bioavailability through floating microspheres of lovastatin. DARU. 2011;19(1):57–64.
Singh SK, Verma PRP, Razdan B. Development and characterization of a lovastatin-loaded self-microemulsifying drug delivery system. Pharm Dev Technol. 2010;15(5):469–83.
Jiang T, Wu C, Gao Y, Zhu W, Wan L, Wang Z, et al. Preparation of novel porous starch microsphere foam for loading and release of poorly water soluble drug. Drug Dev Ind Pharm. 2014;40(2):252–9.
Guo M, Fu Q, Wu C, Guo Z, Li M, Sun J, et al. Rod shaped nanocrystals exhibit superior in vitro dissolution and in vivo bioavailability over spherical like nanocrystals: a case study of lovastatin. Colloids Surf B Biointerfaces. 2015;128:410–8.
Suresh G, Manjunath K, Venkateswarlu V, Satyanarayana V. Preparation, characterization and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles. AAPS PharmSciTech. 2007;8(1):24.
Al-Nimry SS, Khanfar MS. Preparation and characterization of lovastatin polymeric microparticles by coacervation-phase separation method for dissolution enhancement. J Appl Polym Sci. 2016;133:43227–36.
Chen C-C, Tsai T-H, Huang Z-R, Fang J-Y. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: physicochemical characterization and pharmacokinetics. Eur J Pharm Biopharm. 2010;74(3):474–82.
Harde H, Das M, Jain S. Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Expert Opin Drug Deliv. 2011;8(11):1407–24.
Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011;415(1–2):232–43.
Kovačič B, Vrečer F, Planinšek O. Solid dispersions of carvedilol with porous silica. Chem Pharm Bull. 2011;59(4):427–33.
Krupa A, Majda D, Jachowicz R, Mozagawa W. Solid-state interaction of ibuprofen and Neusilin US2. Thermochemistry. 2010;509(1–2):12–7.
Mallappa MK, Kesarla R, Banakar S. Calcium alginate-Neusilin US2 nanocomposite microbeads for oral sustained drug delivery of poor water soluble drug aceclofenac sodium. J Drug Deliv. 2015;2015:Article ID 826981.
Qureshi MJ, Mallikarjun C, Kian WG. Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets: a study in diet induced hyperlipidemic rabbits. Asian J Pharm Sci. 2015;10(1):40–56.
Hentzschel CM, Sakmann A, Leopold CS. Comparison of traditional and novel tableting excipients: physical and compaction properties. Pharm Dev Technol. 2012;17:649–53.
Chakma S, Khadka P, Jo K, Kim H, Ro J, Park K, et al. Solubility enhancement of celecoxib using solidified Tween 80 for the formulation of tablet dosage forms. J Pharm Invest. 2015;45(5):449–60.
Fricker G, Wendel ABA, Zirkel JRH, Setzer C, Quinkert R-O, Martin F, et al. Phospholipids and lipid-based formulations in oral drug delivery. Pharm Res. 2010;27:1469–86.
Babu NJ, Nangia A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst Growth Des. 2011;11(7):2662–79.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86:1–12.
Sohn Y, Lee SY, Lee GH, Na Y-J, Kim SY, Seong I, et al. Development of self-microemulsifying bilayer tablets for pH-independent fast release of candesartan cilexetil. Pharmazie. 2012;67:917–24.
Takeuchi H, Nagira S, Yamamoto H, Kawashima Y. Solid dispersion particles of tolbutamide prepared with fine silica particles by spray-drying method. Powder Technol. 2004;141(3):187–95.
Planinšek O, Kovačič B, Vrečer F. Carvedilol dissolution improvement by preparation of solid dispersions with porous silica. Int J Pharm. 2011;406(1–2):41–8.
Miura H, Kanebako M, Shirai H, Nakao H, Inagi T, Terada K. Influence of particle design on oral absorption of poorly water-soluble drug in a silica particle-supercritical fluid system. Chem Pharm Bull. 2011;59(6):686–91.
Sanganwar PG, Gupta RB. Dissolution-rate enhancement of fenofibrate by adsorption onto silica using supercritical carbon dioxide. Int J Pharm. 2008;360(1–2):213–8.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support of the Deanship of Scientific Research at Jordan University of Science and Technology (grant no. 80/2013). Also, they acknowledge Fuji Chemical Ltd. (Japan) and Evonik Industries (Germany) for donating the silica polymers and Aerosil200, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Khanfar, M., Al-Nimry, S. Stabilization and Amorphization of Lovastatin Using Different Types of Silica. AAPS PharmSciTech 18, 2358–2367 (2017). https://doi.org/10.1208/s12249-017-0717-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0717-1