Abstract
Tablet subdivision is a common practice used mainly for dose adjustment. The aim of this study was to investigate how the technical aspects of production as well as the method of tablets subdivision (employing a tablet splitter or a kitchen knife) influence the accuracy of this practice. Five drugs commonly used as subdivided tablets were selected. For each drug, the innovator drug product, a scored-generic and a non-scored generic were investigated totalizing fifteen drug products. Mechanical and physical tests, including image analysis, were performed. Additionally, comparisons were made between tablet subdivision method, score, shape, diluent composition and coating. Image analysis based on surface area was a useful tool as an alternative assay to evaluate the accuracy of tablet subdivision. The tablet splitter demonstrates an advantage relative to a knife as it showed better results in weight loss and friability tests. Oblong, coated and scored tablets had better results after subdivision than round, uncoated and non-scored tablets. The presence of elastic diluents such as starch and dibasic phosphate dehydrate conferred a more appropriate behaviour for the subdivision process than plastic materials such as microcrystalline cellulose and lactose. Finally, differences were observed between generics and their innovator products in all selected drugs with regard the quality control assays in divided tablet, which highlights the necessity of health regulations to consider subdivision performance at least in marketing authorization of generic products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The division of oral tablets into two or more parts before intake is a fairly common practice (1). This procedure is performed many times by patient’s own initiative or following physician or pharmacist recommendations for dose adjustments, dose titration, swallowing facilitation or even treatment cost reduction (2–4).
The main problem related to this practice is the wide dosage variation of the tablet fragments, which could result either in a subtherapeutic or toxic dose, particularly in cases of drugs with narrow therapeutic index (4–9). Additionally, formulations with modified pharmaceutical performance can be impaired by the subdivision process, leading to hazardous outcomes (10,11). Elderly and paediatric tablet consumers are especially affected by tablet subdivision due to the high frequency with which they use this procedure and the commonly vulnerable health condition of these target groups (5,10,12).
Although scored tablets imply the possibility of subdivision, such characteristic is currently not regulated in many countries. As so, mechanical behaviour after subdivision is not considered for registration, and generic drug products have not been required to have similarity with the innovator one with regard to this aspect (13).
The available literature is not sufficient to precisely determine which production technical aspects impact most on tablet subdivision, although relevant differences have been observed between different types of tablet splitters. In fact, influence of shape, surface, composition or coating on tablet subdivision is discordant, and whether the presence of scoring is a favourable factor for the accuracy of tablet subdivision is still a controversial issue (8,14–17). Consensus is also not reached regarding the best procedure to subdivide tablets. Although, in daily practice, breaking by hand or using of a tablet splitter are still the most common subdivision methods, other means for tablet fraction have been described as the use of scissors and kitchen knife (4,14,18).
Considering this scenario and the relevance of the subject, this study was designed to determine the key technical aspects of tablet production (i.e. tablet shape, the presence of score and coating and composition) in the subdivision accuracy of five different drugs and three drug products of each drug (innovator, scored generic, non-scored generic) using two different methods for tablet subdivision (a commercial tablet splitter and a kitchen knife).
MATERIAL AND METHODS
Material
Immediate-release oral tablets of five different drugs, which are often subdivided in clinical practice by elderly patients, were selected—atenolol 50 mg, captopril 25 mg, hydrochlorothiazide 25 mg, losartan 50 mg and sertraline 50 mg. For each of these drugs, three sorts of drug products marketed in Brazil were chosen: the innovator drug product and two generics randomly selected (one scored and another non-scored), totalling 15 different drug products (Table I). The studies were conducted using the same batch for each product for all tests.
Study Protocol
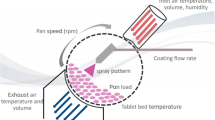
Tablets were subdivided using a commercial tablet splitter (Inconterm, Brazil) and a kitchen knife (Fig. 1). New tablet splitters were acquired for this study and used within the usage limit established by Van Riet-Nales and col. of up to hundred times without changes in its performance (19). The different products were submitted to image analysis followed by mechanical and physical tests to assess the subdivision impact on weight, hardness, friability and disintegration. Comparisons were built based on method for tablet subdivision (knife and tablet splitter), score (scored and non-scored tablets), shape (round and oblong) and coating (uncoated and coated tablets). A qualitative analysis of the tablet diluents starch, lactose monohydrate, microcrystalline cellulose (MCC) and dibasic phosphate dehydrate (DPD) (presence and absence) was also performed. The study protocol is outlined in Fig. 2.
Mechanical and Physical Characterization of Tablets
Weight
Twenty tablets of each drug product were individually weighed using an analytical balance Shimadzu model AUY 220, before and after subdivision. Weight variation was measured by the difference between the experimental weight of half tablets and the theoretical value, which was the whole tablet weight divided by two. Weight loss was calculated as the difference between the weight of the whole tablet and the sum of their half tablets.
Hardness
The hardness of ten whole tablets or halves of each drug product was obtained using a durometer Nova Etica model 298-AT. The results were expressed as hardness variation, which was calculated by the difference between the hardness of whole tablets and half tablets. Halves were measured in durometer in a way the force was applied parallel to the direction of division.
Friability
Tablet friability was measured as the percentage of weight loss of twenty whole tablets or halves of each drug product tumbled in a friabilometer Nova Etica model 300 operating at 25 rpm for 4 min. The results were expressed as friability variation of whole and half tablets.
Disintegration Time
Tablet disintegration time was measured in water at 37°C in a disintegration tester Nova Etica model 301-6. For each variable of the study, six randomly selected tablets were tested. The results were expressed as disintegration time variation of whole and half tablets.
Image Analysis
Ten tablets from each sample were analysed using a stereomicroscope Stereo Zoom Microscope XTL connected to a video camera. The images were captured with software ISCapture version 2.5.1 and processed with software Image-Pro Plus version 4.5.0.29. Tablet surface area was measured and compared.
Statistical Analysis
Statistical analysis was performed taking into account the subdivision tablet method, differences between generics and innovator and information related to the technical characteristics of tablets such as drug, shape, surface, presence of score, presence of coating and excipients. Statistical analysis was performed using SPSS® version 20.0 and Prism version 5.0 software. Mechanical and physical characterization of tablet data was expressed as the mean ± standard error of the mean, and p < 0.05 was considered statistically significant. Quantitative variables were tested for normal distribution with the Shapiro-Wilk test. Possible differences among groups were investigated by performing an ANOVA or Kruskal-Wallis test, followed by Tukey’s or Dunn’s multiple comparison tests. When two groups were compared, we used Student’s t test or Mann-Whitney U test. All correlations between the characterizations of tablet data were determined using Pearson product-moment estimates (r). Reference values for each quantitative variable were 7.49 for weight variation, 0.76 for weight loss, 54.94 for hardness variation, 0.37 for friability variation, 12.52 for disintegration time variation and 10.85 for surface area variation.
RESULTS AND DISCUSSION
In general, the subdivision process compromised the mechanical strength of tablets (Table II). A reduction of subdivided tablets hardness (approximately 50%) was noticed in comparison to whole tablets, which may be, at least in part, influenced by the size and shape of the tablets (20). The practical implication of such result is that once a tablet is divided, the sparing half should be kept and handled with even more care, as the risk of disintegration and partial losses is increased. Indeed, tablet halves were 0.7% more friable than whole tablets, which is consistent with a previous study (5). This is because tablet subdivision weakens the dosage form structure by generating sharp corners that are easily eroded by the mechanical friction during the disintegration test. For ordinary tablets, the maximum value accepted by US pharmacopoeia for the friability assay is 1.0% (21). In the present study, several drug products remained outside this limit after the subdivision, namely hydrochlorothiazide (innovator, scored, subdivided by knife) with 3.1%, captopril (generic, scored, subdivided by knife) with 2.3%, captopril (innovator, scored, subdivided by knife) with 2.3%, hydrochlorothiazide (generic, non-scored, both subdivided by knife and tablet splitter) with 1.6%, sertraline (generic, non-scored, subdivided by knife) with 1.6% and captopril (innovator, scored, subdivided by tablet splitter) with 1.0%. Based on the difficulty of keeping the pharmacopoeia limits after subdivision, US health agency (FDA) has recommended the extension of the friability limit to 3% for tablets after subdivision (22). However, there is no scientific evidence to support the safety of changing the acceptable limit for this assay.
Tablet halves disintegration was approximately 20% faster to whole tablets (Table II). This could be explained by the irregular distribution of lubricants in tablets (23), which might be concentrated on the tablet surface and, due to its lipophilic characteristics, hinder tablet disintegration. Tablet subdivision creates a new face without lubricant in the dosage form, exposing the tablet core, which accelerates the disintegration of tablet halves. Disruption of tablet aesthetic coat, in addition to specific surface increase, can also justify the fast disintegration of subdivided tablets (24). In absolute terms, however, changes in these parameters represent a maximum of 4.5 min (in the case of atenolol—generic, scored, subdivided by splitter tablet), which might have little impact on the dissolution and bioavailability of most products.
The weight loss related to tablet fragmentation and crumbling caused by subdivision was less than 2% (Table II). These data seem to be compatible with other studies that have found values of average weight loss ranging from 0.2 to 3.8% (7,9,17,25,26). Still, the high coefficient of variation for this test is noteworthy. Some studies noted individual weight loss as high as 23.5 and 19.4% (7). In the present study, the highest values were found for sertraline (generic, non-scored, subdivided by knife) with a weight loss of 38.9% and hydrochlorothiazide (generic, non-scored, subdivided by knife) with 19.1% of weight lost. The reduction in tablet mechanical strength after subdivision, observed by the decrease in hardness and the increase in friability, is probably the main cause of weight loss variation. As might be expected (16), there was a strong positive correlation between this response (weight loss) and the friability variation (r = 0.432; p = 0.001).
Weight variation is one of the most important variables to set the security of a subdivision process because it is directly related to dose when the active substance is uniformly distributed within the tablet mass. Our data showed a mean weight variation of 9.9% ± 10.0. Some drug products present weight variations of nearly 50%. These data are in accordance with others in the literature, which identified an average weight variation of 7%, with some products having a weight variation of up to 40% when evaluating different techniques for tablet division (27). Other studies have described variations higher than 10% on the expected weight of halved tablets on a portion of tested drug products ranging from 16 to 41% (7,8,17,24). Weight variations greater than 20% are described for approximately 12% of the tested tablets in two of these studies (7,8). Except for drugs with a wide therapeutic index, such magnitude of dose variation can lead to serious consequences for the health of consumers.
Image analysis quantified variations in the specific surface of subdivided tablets (15.2%; Table II) and related them to the weight variation (Fig. 3). As expected, there was a statistical correlation between these parameters (r = 0.169; p = 0.001). Considering the lack of specific quality control tests to evaluate the tablet subdivision process, the image analysis used in this work proved to be a simple and useful analytical tool in the evaluation of the subdivision process.
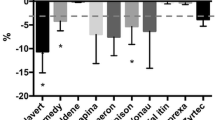
The Brazilian health agency (ANVISA) follows international parameters similar to the USA (FDA) and European member states (EMA) health agencies concerning the regulation of generic drugs. Generic drugs must be bioequivalent to the innovator drug product (27). The five drugs studied showed significant differences between the innovator and their generics in at least three control assays (Table II). With regard to the subdivision performance, innovator drug products are not equivalents to their generics. Additionally, the two generics evaluated for each drug also presented a different performance from each other. These differences were associated not only with the presence of scoring, as all five drugs showed significant differences considering only the scored tablets (innovator drug product and scored generic). This issue was also reported by Wilson and col., who did not find equivalence in subdivision for generic and innovator glyburide tablets (28).
In this sense, the concept of functional score established by the FDA and the European Pharmacopoeia (22,29) could solve this problem. Those guidelines for tablets containing a score with subdivision purpose require that the behaviour in the subdivision process is assessed. Nevertheless, the recommended tests are only for tablets divided by hand and do not cover the use of tablet splitter and knife, which are very used especially to subdivide small tablets or tablets without break mark (19).
In our studies, the most pronounced differences between innovator and generic drug product occurred with sertraline (Table II), which showed significant differences in all evaluated parameters (p < 0.05). In the specific case of this antidepressant drug, side effects such as nausea, insomnia and diarrhoea could be exacerbated due the subdivision process (30). Hence, a better understanding of subdivision tablets is the first step in designing a more suitable tablet for this propose.
According to Fig. 4a, Mann-Whitney U test noted that the splitter tablet produced lower weight loss and friability variation than a knife (p < 0.001 and p = 0.002, respectively). In theory, a tablet splitter helps centralize the tablet and allow a section in a most appropriate place. The literature shows contradictory conclusions about this issue. Some researchers have indicated better performance using a tablet splitter than a knife (4,18,31). Nonetheless, Boggie and col. found no difference between manual breaking and a tablet splitter, whereas Teng and col. showed superior results in subdivide tablets using a razor blade instead of manual subdivision (14,32). A recent study showed that hand breaking presented superior results in tablet subdivision than tablet splitter. In addition, the authors pointed out that subdivision process is highly influenced by the type of tablet splitter (19).
Figure 4b shows the responses obtained from subdivision in scored and non-scored tablets. Scored tablets presented a lower weight variation (8.6% ± 0.4, p = 0.000) than non-scored tablets (12.6% ± 0.7), which is in accordance with the findings of other studies (8,16,17,33). The score delimits the region to be sectioned, providing a more precise division of the tablet. Moreover, this element reduces the thickness of the tablet in the cutting region, facilitating the process.
Following the weight variation results, Fig. 4b also shows a higher variation in the surface area of non-scored tablets (18.6 ± 1.3%, p = 0.000) than scored ones (13.4 ± 1.0%). Hardness variation was also lower for scored tablets (52.0% ± 1.2) than non-scored tablets (55.9% ± 14.6, p = 0.031). A possible reason for this behaviour may be the more regular forms of split scored tablets. The statistical relationship between surface area variation and hardness variation support this inference (r = 0.101; p = 0.013).
Tablet shape is usually chosen considering aesthetics and marketing over technical aspects. However, this variable showed a significant effect in two of the six evaluated parameters (weight loss and surface area variation, p = 0.000 for both) (Fig. 5a). Round tablets exhibited weight loss (2.6% ± 0.2), and surface area variation (17.6% ± 1.0) was noticeably higher than those obtained for oblong tablets (0.7% ± 0.8 and 5.5% ± 0.5, respectively). These results agree with other studies which have also shown facility and better outcomes by subdivide oblong rather than round tablets (7,33). The surface contact area for subdivision, which is smaller in oblong tablets, could explain the better results obtained with oblong tablets (33). Hardness and weight variations showed statistical relevance initially, but this result was attributed to the presence of coating. There were no differences for the responses comparing only coated round and coated oblong tablets (p = 0.811 and p = 0.523, respectively).
Coating provides advantages for tablets submitted to subdivision (Fig. 5b). Coated tablets presented lower weight loss (p = 0.000), hardness (p = 0.022) and surface area (p = 0.009) variations with values of 1.4% ± 0.2, 51.5% ± 1.2 and 13.0% ± 1.2, respectively, than uncoated tablets, which presented 2.8% ± 0.3, 54.4% ± 1.3 and 16.6% ± 1.1, respectively. Coating confers inherent strength and elasticity and consequently holds the core together after subdivision, reducing weight loss and hardness variation, which is connected to film properties (7,34,35).
Finally, the qualitative composition of the studied drug products was identified to analyse the possible influence of diluents on tablet subdivision. The following diluents were found in the selected studied tablets: starch, lactose, MCC and DPD. Figure 6 shows the assessment made for the parameters that showed statistical significance.
Regarding hardness, as expected, the presence of materials with plastic behaviour—MCC and lactose—showed better performances (absence of lactose 57.2% ± 1.3, presence of lactose 51.3% ± 1.2, p = 0.010; absence of MCC 57.0% ± 1.7, presence of MCC 51.4% ± 1.1, p = 0.004). However, for important responses, such as weight change and weight loss, lactose and MCC have a negative effect on subdivision, increasing weight variation (absence of lactose 7.6% ± 0.6, presence of lactose 11.1% ± 0.4, p = 0.0001; absence of MCC 7.3% ± 0.5, presence of MCC 11.3% ± 0.5, p = 0.0001) and weight loss (absence of lactose 1.0% ± 0.1, presence of lactose 2.8% ± 0.3, p = 0.0001; absence of MCC 1.5% ± 0.3, presence of MCC 2.6% ± 0.3, p = 0.0056). In contrast, tablets containing starch and DPD have a beneficial effect on weight variation (absence of starch 11.1% ± 0.5, presence of starch 8.3% ± 0.5, p = 0.000) and weight loss (absence of DPD 2.7% ± 0.3, presence of DPD 1.0% ± 0.2, p = 0.000).
Excipient also plays an important influence on compressibility factors and on the consolidation behaviour of each material (36). MCC and lactose predominantly present plastic deformation, whereas starch and DPD show fragmentation and elastic conduct (37–39). In this study, the latter group seems to be more suitable for the subdivision process. It is possible that materials that demonstrate predominantly plastic deformation when subjected to pressure, culminating in the rupture of structure, may collapse and cause major variations in weight compared to materials that can undergo elastic deformation and fragmentation that are able to subdivide without suffering major structural damage (5,26).
CONCLUSION
According to this study, a tablet should have the following desirable characteristics to be securely subdivided—oblong shape, scored and coating. Better subdivision in terms of friability and weight loss considering the drug products studied were achieved using the tablet splitter instead of a kitchen knife. As a significant reduction of hardness and resistance of subdivided tablets has been noticed in all cases, it would be prudent to recommend consumers to immediately use the drug products in halves. In addition, differences in quality control assays found between all generic products and innovator counterparts indicate the necessity of reviewing the health regulations for marketing authorization of generic drug products, at least for new applications. The evaluation of the subdivision capacity of tablets with score, including mechanical assessments of subdivided tablets, currently demanded by the FDA and European Pharmacopoeia, could be an option to solve this issue.
REFERENCES
Quinzler R, Gasse C, Schneider A, Kaufmann-Kolle P, Szecsenyi J, Haefeli WE. The frequency of inappropriate tablet splitting in primary care. Eur J Clin Pharmacol. 2006;62:1065–73. doi:10.1007/s00228-006-0202-3.
Arruabarrena J, Coello J, Maspoch S. Raman spectroscopy as a complementary tool to assess the content uniformity of dosage units in break-scored warfarin tablets. Int J Pharm. 2014;465:299–305. doi:10.1016/j.ijpharm.2014.01.027.
Rodenhuis N, De Smet PAGM, Barends DM. The rationale of scored tablets as dosage form. Eur J Pharm Sci. 2004;21:305–8. doi:10.1016/j.ejps.2003.10.018.
Habib WA, Alanizi AS, Abdelhamid MM, Alanizi FK. Accuracy of tablet splitting: comparison study between hand splitting and tablet cutter. Saudi Pharm J. 2014;22:454–9. doi:10.1016/j.jsps.2013.12.014.
Shah RB, Collier JS, Sayeed VA, Bryant A, Habib MJ, Khan MA. Tablet splitting of a narrow therapeutic index drug: a case with levothyroxine sodium. AAPS PharmSciTech. 2010;11:1359–67. doi:10.1208/s12249-010-9515-8.
Weissman EM, Dellenbaugh C. Impact of splitting risperidone tablets on medication adherence and on clinical outcomes for patients with schizophrenia. Psychiatr Serv. 2007;58:201–6. doi:10.1176/appi.ps.58.2.201.
McDevitt JT, Gurst AH, Chen Y. Accuracy of tablet splitting. Pharmacotherapy. 1998;18:193–7. doi:10.1002/j.1875-9114.1998.tb03838.x.
Tahaineh LM, Gharaibeh SF. Tablet splitting and weight uniformity of half-tablets of four medications in pharmacy practice. J Pharm Pract. 2012;25:471–6. doi:10.1177/0897190012442716.
Elliott I, Mayxay M, Yeuichaixong S, Lee SJ, Newton PN. The practice and clinical implications of tablet splitting in international health. Trop Med Int Health. 2014;19:754–60. doi:10.1111/tmi.12309.
Marriott JL, Nation RL. Splitting tablets. Aust Prescr. 2002;25:133–5.
Mandal TK. Effect of tablet integrity on the dissolution rate of sustained-release preparations. J Clin Pharm Ther. 1996;21:155–7. doi:10.1023/A:1016442514205.
Fischbach MS, Gold JL, Lee M, Dergal JM, Litner GM, Rochon PA. Pill-splitting in a long-term care facility. JAMA. 2001;164:785–6.
Arnet I, Hersberger KE. Misleading score-lines on tablets: facilitated intake or fractional dosing? Swiss Med Wkly. 2010;140:105–10.
Teng J, Song CK, Williams RL, Polli JE. Lack of medication dose uniformity in commonly split tablets. J Am Pharm Assoc. 2002;42:195. doi:10.1331/108658002763508489.
Noviasky J, Lo V, Luft D. Which medications can be split without compromising efficacy and safety? J Fam Pract. 2006;55:707–8.
Ferreira AAA, Prates EC, Fernandes JPS, Ferrarini M. Avaliação do efeito da partição de comprimidos de furosemida sobre a uniformidade da dose. Rev Ciencias Farm Basica e Apl. 2011;32:47–53.
Hill SW, Varker AS, Karlage K, Myrdal PB. Analysis of drug content and weight uniformity for half-tablets of 6 commonly split medications. J Manag Care Pharm. 2009;15:253–61.
Edwards S. Variability in tablet fragment weights when splitting unscored cyclobenzaprine 10 mg tablets. J Am Pharm Assoc. 2004;44:583. doi:10.1331/1544-3191.44.5.583.
Van Riet-Nales DA, Doeve ME, Nicia AE, Teerenstra S, Notenboom K, Hekster YA, et al. The accuracy, precision and sustainability of different techniques for tablet subdivision: breaking by hand and the use of tablet splitters or a kitchen knife. Int J Pharm. 2014;466:44–51. doi:10.1016/j.ijpharm.2014.02.031.
Pitt KG, Heasley MG. Determination of the tensile strength of elongated tablets. Powder Technol. 2013;238:169–75.
USP 2015. General chapters <1216> TABLET FRIABILITY, United States Phamacopeia, 2015.
FDA 2013. Guidance for industry—tablet scoring: nomenclature, labeling, and data for evaluation. U.S. Food and Drug Administration, 2013.
Lakio S, Vajna B, Farkas I, Salokangas H, Marosi G, Yliruusi J. Challenges in detecting magnesium stearate distribution in tablets. AAPS PharmSciTech. 2013;14:435–44. doi:10.1208/s12249-013-9927-3.
Uzunović A, Vranić E. Effect of magnesium stearate concentration on dissolution properties of ranitidine hydrochloride coated tablets. Bosn J Basic Med Sci. 2007;7:279–83.
Helmy SA. Tablet splitting: is it worthwhile? Analysis of drug content and weight uniformity for half tablets of 16 commonly used medications in the outpatient setting. J Manag Care Spec Pharm. 2015;21:76–86.
Gupta A, Hunt RL, Khan MA. Influence of tablet characteristics on weight variability and weight loss in split tablets. Am J Heal Pharm. 2008;65:2326–8. doi:10.2146/ajhp080371.
FDA 2014. U.S. Food and Drug Administration, understanding generic drugs, U.S. Food Drug Adm. 2014. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingGenericDrugs. Accessed July 10, 2015.
Wilson MG, Kaiser FE, Morley JE. Tablet-breaking ability of older persons with type 2 diabetes mellitus. Diabetes Educ. 2001;27:530–40.
European Pharmacopoeia, European Directorate for the Quality of Medicines. Monograph 478 Tablets. 8.0 ed. 2016.
Murdoch D, McTavish D. Sertraline. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depression and obsessive-compulsive disorder. Drugs. 1992;44:604–24. doi:10.2165/00003495-199244040-00007.
Verrue C. Is splitting tablets dangerous? Nurs Times. 2011;107:23.
Boggie DT, DeLattre ML, Schaefer MG, Morreale AP, Plowman BK. Accuracy of splitting unscored valdecoxib tablets. Am J Heal Pharm. 2004;61:1482–3.
Van der Steen KC, Frijlink HW, Schipper CMA, Barends DM. Prediction of the ease of subdivision of scored tablets from their physical parameters. AAPS PharmSciTech. 2010;11:126–32. doi:10.1208/s12249-009-9365-4.
Fell JT, Rowe RC, Newton JM. The mechanical strength of film-coated tablets. J Pharm Pharmacol. 1979;31:69–72.
Sedrati M, Arnaud P, Fontan JE, Brion F. Splitting tablets in half. Am J Hosp Pharm. 1994;51:548–50.
Rojas J, Hernandez S. Effect of the compaction platform on the densification parameters of tableting excipients with different deformation mechanisms. Chem Pharm Bull. 2014;62:281–7. doi:10.1248/cpb.c13-00884.
Ayorinde JO, Itiola OA, Odeniyi MA. Effects of material properties and speed of compression on microbial survival and tensile strength in diclofenac tablet formulations. Arch Pharm Res. 2013;36:273–81. doi:10.1007/s12272-013-0027-4.
Ilić I, Govedarica B, Šibanc R, Dreu R, Srčič S. Deformation properties of pharmaceutical excipients determined using an in-die and out-die method. Int J Pharm. 2013;446:6–15. doi:10.1016/j.ijpharm.2013.02.001.
Rowe RC, Sheskey PJ, Quinn ME. Handbook of Pharmaceutical Excipients, 6th edn. Pharmaceutical Press, 2009.
ACKNOWLEDGMENTS
This research was supported by Brazilian agency FAP-DF project number 0193.001023/2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Teixeira, M.T., Sá-Barreto, L.C.L., Gratieri, T. et al. Key Technical Aspects Influencing the Accuracy of Tablet Subdivision. AAPS PharmSciTech 18, 1393–1401 (2017). https://doi.org/10.1208/s12249-016-0615-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0615-y