ABSTRACT
Beta-carotene is important for fortification of nutritional products while its application is limited by instability. The influence of maltodextrin (MDX) on physicochemical properties and stability of beta-carotene emulsions stabilized by sodium caseinate (SC) was investigated. The emulsions were characterized by dynamic light scattering (DLS), laser diffraction (LD), transmission electron microscopy (TEM), rheometer, and turbiscan lab expert. The effects of pH, ionic strength, and freeze-thaw on stability of emulsions were observed. The emulsions could tolerate up to 2 mol/L NaCl or 10 mmol/L CaCl2 and showed Newtonian behavior. The droplet diameter, polydispersity index, and zeta-potential did not change obviously after 3 months storage at 4°C in dark conditions. The emulsions with MDX showed excellent freeze-thaw stability and gave favorite protection for beta-carotene. The retention ratio of beta-carotene in the emulsions with MDX was above 92.1% after 3 months storage while that in the one without MDX was only 62.7%. The study may provide a promising strategy to improve stability of sensitive nutraceuticals without adding synthetic antioxidants. The findings obtained could provide fundamental basis for rational design of emulsion delivery systems when freeze-thawing is required during manufacturing process or storage period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Vitamin A and its metabolites are essential nutrients for human immune response and cell differentiation. Inadequate intake of vitamin A has been linked to increase risk of night blindness and undermine growth. Vitamin A deficiency (VAD) is a critical public health problem in the developing countries. According to the World Health Organization (WHO), 190 million preschool-aged children and 19 million pregnant women were affected by VAD.
Driven by consumers’ increasing demands for healthy and safety fortified food products, natural nutrients have exerted an explosion of research that overflow into the nutritional supplement industry (1). Beta-carotene, a natural nutrient with pro-vitamin A activity, could be used to lower the prevalence of VAD (2–4). However, there are many challenges to incorporate beta-carotene into nutritional supplements. Beta-carotene, an unsaturated compound, could be easily decomposed by oxygen, heat, and light during manufacturing process and storage period. The application of beta-carotene is limited by its instability (5).
Beta-carotene can be dissolved in the oil phase of emulsions for application in aqueous food products, and oils can facilitate the uptake of beta-carotene (6,7). Incorporating beta-carotene into emulsion delivery system is a promising strategy to protect beta-carotene against degradation and offer a useful technology for scale-up production (8–11).
Depending on the mean droplet diameter, emulsions can be classified into nano-emulsions (10–100 nm), miniemulsions (100–1000 nm), and macroemulsions (0.5–100 μm) (11). Previous studies have focused on the formation, properties, and stability of beta-carotene emulsions. Mao et al. reported that the types of emulsifiers had significant influences on physicochemical stability of beta-carotene emulsions. The degradation rate of beta-carotene was appreciably faster in the emulsions stabilized by small molecule emulsifiers than in the one stabilized by large molecule emulsifiers (12). Yi et al. has evaluated the effect of particle size on physicochemical stability of beta-carotene emulsions stabilized by sodium caseinate (13). Nano-emulsions (14,15) have been attempted for effectively use of beta-carotene; however, the types and concentrations of synthetic surfactants are strictly regulated for application in food products. The potential of toxic effects may be raised due to the use of organic solvents or synthetic surfactants in manufacturing process. Maillard reaction has been introduced to prepare sodium caseinate-maltodextrin conjugates for improving chemical stability of beta-carotene; however, potential safety hazards could be raised because the extent and bonding location of Maillard reaction are uncontrollable (16). The potential of maltodextrin (MDX) to improve chemical stability of beta-carotene and physical stability of sodium caseinate (SC) emulsions have not been reported.
In this study, emulsions were fabricated entirely from natural ingredients which are generally recognized as safe (GRAS). SC has been widely used as an emulsifier for its abilities to be adsorbed at the droplet interface of emulsions. And it exhibits antioxidative characteristics that could be used for encapsulation of sensitive nutraceuticals (17). MDX has been used to improve the emulsion stability via modification of rheological properties in continuous phases (18,19).
The potential of MDX to improve physicochemical stability of beta-carotene emulsions stabilized with SC was investigated. The study may offer a promising strategy to improve stability of sensitive nutraceuticals without adding synthetic antioxidants. Besides, our previous work has reported that emulsion systems emulsified by single food protein could not tolerate extreme environment such as low temperature (16). The findings obtained could provide fundamental basis for rational design of emulsion delivery systems when freeze-thawing is required during manufacturing process or storage period.
MATERIALS AND METHODS
Material
Beta-carotene was purchased from Sigma-Aldrich (Shanghai, China). SC was obtained from Gansu Hualing Casein Co., Ltd (China). Medium chain triglyceride (MCT) was a gift from Gattefosse (Shanghai, China). MDX was purchased from Xiya Regent Co., Ltd (China). Methanol, ethanol, acetonitrile, tetrahydrofuran, and n-hexane were HPLC grade that purchased from Honeywell Co., Ltd (Shanghai, China). All other chemicals and reagents were of analytical grade and used without further purification. Ultrapure water was used in all experiments.
Preparation of Emulsion
SC (1% w/v) was dissolved in ultrapure water, then the solutions as continuous phase were stirred overnight at 4°C to ensure complete hydration. Then MDX was dissolved in SC dispersions at various concentrations (0–10% w/v). An aliquot of 20 mL MCT containing 20 mg beta-carotene as oil phase, which was sonicated for 1 min in a beaker covered with aluminium foil, was heated at 60°C for about 2 min, and then stirred at room temperature for about 5 min until complete solubilization. The samples were flushed with nitrogen during this process to inhibit the degradation of beta-carotene.
The premix containing 5% (w/v) oil phase with 95% (w/v) aqueous phase was first homogenized under high-speed homogenizer (Fluko FA25, Germany) at 10,000 rpm for 2 min to form coarse emulsions. Further homogenization was performed by passing the coarse emulsions through Emulsiflex-C3 High Pressure Homogenizer (Avestin, Inc., Ottawa, Canada) for ten cycles at 600 bar. The formed emulsions were added with sodium azide (0.02% w/v) as an antimicrobial agent, and then transferred to screw-capped amber containers and stored at 4°C in dark condition for 3 months.
Characterization of Emulsions
Droplet Diameter and Zeta-Potential Analysis
The droplet diameter was measured using a Malvern Zetasizer (Nano ZS90, Malvern Instruments Ltd., UK) in the range of 2 nm–3 μm at a fixed detector angle of 90°. Emulsions were diluted 200-folds with ultrapure water in a capillary test tube and equilibrated for about 120 s before measurement. Mie theory was used for the analysis with the refractive index of 1.330 for the oil droplets and absorption index of 0.001 for the dispersion medium. All measurements were made in triplicate and were conducted at 25°C. The droplet diameter and polydispersity index (pdI) were measured by dynamic light scattering (DLS). Total 20 measurements were performed and the mean of each sample was calculated.
The specific surface area was performed by laser diffractometry (LD) using Mastersizer 2000 with a Hydro 2000MU as dispersion unit (Malvern Instruments Ltd., UK) in the range of 20 nm–2000 μm. The pump speed was settled at 1500 rpm. The samples were analyzed by using real refractive index of 1.52 and imaginary refractive index of 0.1.
The zeta-potential (ZP) of samples was measured by Malvern Zetasizer (NanoZS90, Malvern Instruments Ltd., UK). Emulsions were diluted 200-folds with ultrapure water before analysis. More than 20 scans for each record and three measurements were performed on each sample. All measurements were carried out at 25°C in triplicate.
Morphology Measurements
The morphology of emulsions was observed using transmission electron microscopy according to the method have published (13). Ten microliters of diluted emulsions was collected on copper grid, and then dried at room temperature for few minutes. Then 10 g/L phosphotungstic acid was added to dye the particles. Observation was performed at acceleration voltage of 120 kV with JEM-1400 TEM (JEOL Ltd., Japan).
Rheological Measurements
Rheological measurements were performed at 25°C using a dynamic shear rheometer (Kinexus lab+ rotary rheometer, Malvern Instruments Ltd., UK) equipped with a cone and plate test geometry (cone angle 1°, plate diameter 60 mm). Emulsion (0.99 mL) was deposited on the plateau of the rheometer and then equilibrated for 5 min before each measurement. Continuous flow measurements were performed by increasing shear rate from 10 to 1000 s−1, and rheological parameters including shear stress, shear rate, and apparent viscosity were obtained from rSpace software.
Effect of Environmental Stresses on the Physical Stability of Emulsions
A series of emulsion systems with different pH values and ionic strength were prepared to evaluate effects of environmental stresses on physical stability of emulsions. To vary emulsion pH values from pH 2 to 10, 1 mol/L HCl or 1 mol/L NaOH solutions were used, while sodium chloride and calcium chloride were added to adjust ion strength of emulsions at pH 7 condition (20). All formulations with additives were stirred for 30 min and then stored at room temperature overnight to reach a steady state. The droplet diameter, ZP, and pdI of emulsions were measured by DLS and then stored for a week prior to visual observation.
Emulsion Stability Measurements
Storage Stability
The stability of emulsions was tested using a vertical scan analyzer Turbiscan Lab Expert (Formulaction, France), as described by Ji with minor modification (16). Each sample was placed in a cylindrical glass cell, and the instrument scanned transmitting and backscattering intensity along with 45 mm height of glass tube via a laser light source at 880 nm of wavelength.
Freeze-Thaw Stability
The freeze-thaw treatments on physical stability of emulsions were evaluated using Turbiscan Lab Expert (Formulaction, France). Twenty milliliter fresh emulsions was incubated in a −20°C freezer for 48 h, and then thawed completely at room temperature. These freeze-thaw cycles were repeated three times. Subsequently, the emulsions were transferred to a cylindrical glass cell to observe changes of backscattering intensity.
Extraction Method
The beta-carotene in emulsions was extracted using ethanol and n-hexane according to the method of Yuan with minor modification (21); in brief, 0.4 mL emulsions was preliminarily destabilized by mixing with 1 mL ethanol, then 2 mL n-hexane containing 0.1% (w/v) BHT was added immediately and vibrated for 30 s. The yellow-colored organic phase was separated and transferred into a brown volumetric flask. The extraction was performed three times until aqueous layer was clear. The collected supernatant was diluted to 10 mL with n-hexane and then passed a 0.22 μm syringe filter for HPLC assay.
HPLC Assays for Beta-carotene Concentrations
The exact concentration of beta-carotene in emulsions was determined by HPLC system (Shimadzu Corporation, Japan) equipped with variable UV detector set at 450 nm, the separation was carried out with a InertSustain C18 analytical column (RP-18e, 150 mm × 4.6 mm, 5 μm). The mobile phase was a mixture of acetonitrile, methanol, and tetrahydrofuran (50:36:14, V/V/V) containing 0.1% BHT as antioxidant with the flow rate of 1.5 mL/min. The column temperature and automatic sampler temperature were set at 30 and 4°C, respectively. Linear calibration curves (R 2 > 0.999) were plotted for chromatographic peak areas against beta-carotene concentrations over the range of 0.1–10 μg/mL. All samples were diluted to establish suitable concentrations before measurements.
Statistical Analysis
All samples in these experiments were performed in triplicate. The values were expressed as means ± standard deviation. The data were analyzed by one-way ANOVA analysis of variance using the SPSS 21.0 package (IBM INC., New York).
RESULTS AND DISCUSSION
Characterization of Emulsions
Emulsions were prepared by homogenizing 5% oil phase and 95% aqueous phase composed of 1% SC and 10% MDX. The parameters were optimized through single factor test based on our preliminary studies. Studies have been approached to obtain optimization based on the droplet diameter by using various proportions of oil and aqueous phase. Five percent oil phase and 95% aqueous phase was chosen to prepare the emulsions, since the smallest droplet diameter of emulsions was obtained in this condition.
High pressure homogenization was used to prepare emulsions upon the intense disruptive forces when the coarse emulsions passed through the very small homogenization gap with high velocity (22). Increasing homogenizing pressure and cycles in a proper range tend to decrease the droplet diameter (22,23). The droplet diameter had no significant decrease when the homogenization pressure was above 600 bar. A similar phenomenon had occurred when the number of homogenizing cycles was more than ten times. Thus 600 bar and ten homogenizing cycles were chosen to prepare the emulsions in our experiments.
HPLC was used to quantitate beta-carotene in emulsions. Six test solutions of various concentrations ranging from 0.1 to 10 μg/mL of beta-carotene were prepared by dilution of stock solution and tested for linearity test. The detector response at 450 nm was found linear in the whole range of calibration curve. Good linearity (coefficient of correlation r 2 = 0.9998) was achieved in the investigated range for beta-carotene. Linear regression parameters were described by the following equation: A = 120527 C + 3263.1, where A is the peak area at 450 nm and C is the concentration of beta-carotene (Fig. 1).
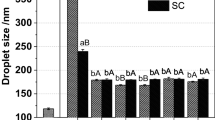
The droplet diameter of emulsions with MDX was 262.8 ± 4.10 nm while the one without MDX was 307.1 ± 5.40 nm, indicating that the emulsions with MDX could form smaller droplets and larger specific surface area compared to the emulsions without MDX (Table I). This result was in good agreement with another study which found the sugar could reduce droplet diameter of SC stabilized emulsions (24). The droplet diameter obtained by Zetasizer is considered to be hydrodynamic diameter. According to the Stokes-Einstein equation, hydrodynamic diameter is related to viscosity of continuous phase. The hydrodynamic diameter will decrease with the increasing of viscosity in continuous phase (25). The results shown in Fig. 2a demonstrated that MDX could increase the viscosity of continuous phase may explain the formation of smaller droplet diameter in emulsions with MDX. The pdI of emulsions SC stabilized emulsions with MDX was 0.12 ± 0.04 (Table I). And a monomodal particle size distribution was obtained immediately after homogenization (26). The result was consistent with the TEM micrograph of droplets (Fig. 3).
ZP values represent an indication of potential physical stability of colloidal systems (27). As shown in Table I, the ZP values of emulsions with MDX were lower than the one without MDX after homogenization. It may be ascribed to the reduction of negative charges between interaction with uncharged MDX and negative charged SC (28).
Rheological properties of nutraceutical products were different depending on their various compositions. Rheological measurements are important for characterization of emulsions because rheological properties could affect the shelf life of products (29). As shown in Fig. 2a, the viscosity of emulsions increased significantly with the addition of MDX at 25°C. Emulsions showed the Newtonian behavior since the shear rate ramps of emulsions represented linear correlation (r 2 > 0.9999) and the intercept of shear stress-shear flow curves was zero (Fig. 2b). The addition of MDX in the emulsions did not change Newtonian behavior of emulsion delivery system.
Effect of Environmental Stresses on the Physical Stability of Emulsions
It has been demonstrated that pH values of continuous phase in emulsions range from acidic to slightly alkaline (20). It is important to maintain the physical stability of emulsions in nutritional products by using suitable pH values. Figure 4 showed a sudden increase of droplet diameter, indicating the emulsions were unstable in pH 3.74 condition. When the pH value of emulsions was very close to the isoelectric point of SC (∼4.6), the emulsion droplets would have small amount of charge (30). The steric stabilization and electrostatic repulsion were insufficient to overcome the van der Waals and hydrophobic attraction resulting in the emulsion destabilization. The results recommended that the emulsions could not be incorporated into dietary supplements that have intermediate pH values (pH 4–5).
The ionic strength is a key point for physical stability of emulsions for their application in nutritional supplements (8). The effects of sodium chloride concentrations (0–4 mol/L NaCl) and calcium ion concentrations (0–50 mmol/L CaCl2) on physical stability of emulsions were shown in Figs. 5 and 6. The ZP values of emulsions decreased from around −45 mV to around −5 mV with the increasing NaCl concentration. The destabilization of emulsions at high NaCl concentrations could be attributed to screening effects of electrostatic repulsion between droplets by sodium ions. Electrostatic repulsion is still sufficiently strong to overcome the van der Waals and hydrophobic attraction at relatively low NaCl concentrations. But electrostatic repulsion is no longer strong enough to lead in destabilization of emulsions above a critical NaCl concentration.
The reason of selecting these salt concentrations was to find a critical sodium chloride concentration that leading to destabilization of emulsions. The droplet diameter of emulsions had a significant increase when the concentration of NaCl was above 2 mol/L. No visible appearances of phase separation were observed after storage at 25°C for 1 week, indicating the steric stabilization played a key role in emulsions.
A sudden increased of droplet diameter was observed when the calcium ion concentration was at 15 mmol/L (Fig. 6). The effects of calcium ions and sodium ions on the destabilization of emulsions were not exactly same. Calcium ions could play the role of screening effects; besides, calcium ions could interact with SC by chelated reaction while sodium ions could not interact with SC (31). So the destabilization was observed in emulsions with relatively low calcium ion concentrations. The emulsions were not recommended to use in dietary supplements containing high calcium ion concentrations.
Emulsions Stability Measurements
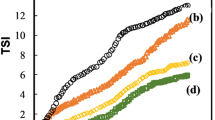
Turbiscan Lab Expert was applied to investigate the stability of emulsions during storage period. The stability of emulsions stabilized by SC and MDX stored in dark conditions at 4°C for 3 months was shown in Fig. 7. The result demonstrated the backscattering and transmitting light intensity profiles of emulsions with MDX kept unchanged after storage period. It was consistent with the phenomenon which droplet diameter, pdI, and ZP did not change considerably after storage period compared to the freshly prepared emulsions. The excellent stability of emulsions may be attributed to four reasons as follows. Firstly, SC could form thick layers on the droplet interface to inhibit droplets flocculation and coalescence (32). MDX may interact with SC at the interface which resulted in forming thicker layers on the droplet interface (28). Secondly, according to stokes law (v = [2(ρ − ρ 0) r 2/9η]·g), the reduction of droplet diameter and the increase of viscosity in continuous phases could prevent emulsions against destabilization. Thirdly, as a rule of thumb, if the absolute ZP values of droplets are above 30 mV, the colloidal systems are stable (33). Emulsions with high ZP values are electrically stable while emulsions with low zeta-potential tend to flocculate. The ZP value of −51.6 mV showed robust stability. Last but not least, MDX in the continuous phases may behave as spacing matrices to inhibit droplet aggregation (34). It can be postulated that the electrostatic charge repulsion and steric hindrance between droplets combined with the depletion stabilization of MDX in continuous phases would help emulsions maintain stable.
Freezing is often used in nutraceutical industry to maintain nutritional values or improve shelf life of nutritional products. When frozen, emulsions may become physically unstable and have a rapid breakdown after thawing. It is necessary for researchers to design rationally dietary supplements which not only exhibit high resistance to freezing process but also maintain their initial properties when thawed. Backscattering (BS) profiles along with the height of glass tube containing emulsions were shown in Fig. 8. After the first curve (t = 0) had been subtracted from the subsequent ones (ΔBS = BS t − BS t=0), the curves are obtained to observe the variations related to the initial emulsions. The ΔBS of emulsions containing MDX were kept at almost zero values that showed excellent freeze-thaw stability. However, the ΔBS of emulsions stabilized by SC changed considerably and visual phase was separated from the emulsions immediately. Additional physicochemical changes lead to the instability in the emulsions stabilized by SC (35). There was insufficient water to hydrate SC molecules in ice crystal state resulting in the rupture of interfacial membranes of droplet. The emulsion droplets may be forced together due to the penetration of the ice crystals into droplets. The good freeze-thaw stability of emulsions with MDX was attributed to cryoprotective effect of polysaccharide. The MDX in continuous phase has abilities to bind with water molecules, and increase viscosity of samples to slow down the diffusion rate of water molecules (36).
Figure 9 showed the chemical stability of beta-carotene in emulsions with or without MDX. The retention ratio of beta-carotene decreased with the increasing of storage time for both formulations. The retention ratio of beta-carotene in emulsions stabilized by SC and MDX was above 92.1% after 3 months stored at 4°C in dark conditions while that stabilized with SC was only 62.7%. It is interesting that the smaller size emulsions with MDX gave more protection for beta-carotene than the ones stabilized with pure SC, this phenomenon seemed to have a conflict with many literatures hypothesis which the small droplet diameter may increase the degradation rate of bioactives encapsulated in emulsions (12). The MDX have made great contributions to inhibit the oxidation of beta-carotene during storage, with the ability to increase viscosity of continuous phase and thicken the SC layer. Additionally, the density improved by MDX at the interface of emulsions may impact diffusion of oxygen, free radicals, and pro-oxidants through the interfacial layer (37). These results indicated the beta-carotene retention had an intimate association with the emulsion stability.
Long-term chemical stability of beta-carotene encapsulated into emulsions stored at 4°C in dark conditions. The retention ratio (relative content C t /C 0 in percent) of beta-carotene emulsions with or without MDX was measured. Where C 0 and C t represent beta-carotene content after preparation or at storage time t (n = 3)
CONCLUSIONS
Emulsions were fabricated entirely from natural ingredients in this study. The potential of MDX for improving physicochemical stability of beta-carotene emulsions stabilized with SC was investigated. Emulsions have shown preferable physical stability and excellent freeze-thaw stability. The addition of MDX reduced carotenoid losses during storage and the retention ratio of beta-carotene was above 92.1% after 3 months stored at 4°C in dark conditions. The beta-carotene emulsions could be used as vitamin A nutritional supplementations for preventing and treating VAD. Based on the results, a promising strategy would be proposed to improve stability of hydrophobic nutraceuticals without adding synthetic antioxidants. The findings obtained could provide fundamental basis for rational design of emulsion delivery systems when freeze-thawing is required during manufacturing process or storage period.
REFERENCES
Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr Opin Colloid Interface Sci. 2009;14(1):3–15. doi:10.1016/j.cocis.2008.01.002.
Palozza P, Muzzalupo R, Trombino S, Valdannini A, Picci N. Solubilization and stabilization of β-carotene in niosomes: delivery to cultured cells. Chem Phys Lipids. 2006;139(1):32–42. doi:10.1016/j.chemphyslip.2005.09.004.
Boon CS, McClements DJ, Weiss J, Decker EA. Factors influencing the chemical stability of carotenoids in foods. Crit Rev Food Sci Nutr. 2010;50(6):515–32.
Brennan C, Brennan M, Derbyshire E, Tiwari BK. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci Technol. 2011;22(10):570–5.
Deming DM, Erdman JW. Mammalian carotenoid absorption and metabolism. Pure Appl Chem. 1999;71(12):2213–23.
Faulks RM, Southon S. Challenges to understanding and measuring carotenoid bioavailability. Biochim Biophys Acta (BBA) Mol Basis Dis. 2005;1740(2):95–100. doi:10.1016/j.bbadis.2004.11.012.
Hornero-Méndez D, Mínguez-Mosquera MI. Bioaccessibility of carotenes from carrots: effect of cooking and addition of oil. Innovative Food Sci Emerg Technol. 2007;8(3):407–12. doi:10.1016/j.ifset.2007.03.014.
Mao Y, Dubot M, Xiao H, McClements DJ. Interfacial engineering using mixed protein systems: emulsion-based delivery systems for encapsulation and stabilization of beta-carotene. J Agric Food Chem. 2013;61(21):5163–9. doi:10.1021/jf401350t.
Ribeiro HS, Guerrero JM, Briviba K, Rechkemmer G, Schuchmann HP, Schubert H. Cellular uptake of carotenoid-loaded oil-in-water emulsions in colon carcinoma cells in vitro. J Agric Food Chem. 2006;54(25):9366–9.
Windhab EJ, Dressler M, Feigl K, Fischer P, Megias-Alguacil D. Emulsion processing—from single-drop deformation to design of complex processes and products. Chem Eng Sci. 2005;60(8–9):2101–13. doi:10.1016/j.ces.2004.12.003.
Perrechil FA, Cunha RL. Oil-in-water emulsions stabilized by sodium caseinate: influence of pH, high-pressure homogenization and locust bean gum addition. J Food Eng. 2010;97(4):441–8. doi:10.1016/j.jfoodeng.2009.10.041.
Mao L, Xu D, Yang J, Yuan F, Gao Y, Zhao J. Effects of small and large molecule emulsifiers on the characteristics of b-carotene nanoemulsions prepared by high pressure homogenization. Food Technol Biotechnol. 2009;47(3):336–42.
Roohinejad S, Oey I, Wen J, Lee SJ, Everett DW, Burritt DJ. Formulation of oil-in-water beta-carotene microemulsions: effect of oil type and fatty acid chain length. Food Chem. 2015;174:270–8. doi:10.1016/j.foodchem.2014.11.056.
Liang R, Shoemaker CF, Yang X, Zhong F, Huang Q. Stability and bioaccessibility of β-carotene in nanoemulsions stabilized by modified starches. J Agric Food Chem. 2013;61(6):1249–57.
Li Y, Zheng J, Xiao H, McClements DJ. Nanoemulsion-based delivery systems for poorly water-soluble bioactive compounds: influence of formulation parameters on polymethoxyflavone crystallization. Food Hydrocoll. 2012;27(2):517–28. doi:10.1016/j.foodhyd.2011.08.017.
Ji J, Zhang J, Chen J, Wang Y, Dong N, Hu C, et al. Preparation and stabilization of emulsions stabilized by mixed sodium caseinate and soy protein isolate. Food Hydrocoll. 2015;51(0):156–65. doi:10.1016/j.foodhyd.2015.05.013.
Gutiérrez FJ, Albillos SM, Casas-Sanz E, Cruz Z, García-Estrada C, García-Guerra A, et al. Methods for the nanoencapsulation of β-carotene in the food sector. Trends Food Sci Technol. 2013;32(2):73–83. doi:10.1016/j.tifs.2013.05.007.
Neirynck N, Van lent K, Dewettinck K, Van der Meeren P. Influence of pH and biopolymer ratio on sodium caseinate−guar gum interactions in aqueous solutions and in O/W emulsions. Food Hydrocoll. 2007;21(5–6):862–9. doi:10.1016/j.foodhyd.2006.10.003.
Dickinson E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003;17(1):25–39. doi:10.1016/S0268-005X(01)00120-5.
Qian C, Decker EA, Xiao H, McClements DJ. Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012;132(3):1221–9. doi:10.1016/j.foodchem.2011.11.091.
Yuan Y, Gao Y, Zhao J, Mao L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res Int. 2008;41(1):61–8. doi:10.1016/j.foodres.2007.09.006.
Sahoo NG, Kakran M, Shaal LA, Li L, Muller RH, Pal M, et al. Preparation and characterization of quercetin nanocrystals. J Pharm Sci. 2011;100(6):2379–90. doi:10.1002/jps.22446.
Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll. 2011;25(5):1000–8. doi:10.1016/j.foodhyd.2010.09.017.
Álvarez Cerimedo MS, Iriart CH, Candal RJ, Herrera ML. Stability of emulsions formulated with high concentrations of sodium caseinate and trehalose. Food Res Int. 2010;43(5):1482–93. doi:10.1016/j.foodres.2010.04.008.
Lee L, Norton IT. Comparing droplet breakup for a high-pressure valve homogeniser and a microfluidizer for the potential production of food-grade nanoemulsions. J Food Eng. 2013;114(2):158–63. doi:10.1016/j.jfoodeng.2012.08.009.
Cornacchia L, Roos YH. Stability of beta-carotene in protein-stabilized oil-in-water delivery systems. J Agric Food Chem. 2011;59(13):7013–20. doi:10.1021/jf200841k.
Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int J Pharm. 2011;420(1):141–6. doi:10.1016/j.ijpharm.2011.08.026.
Klinkesorn U, Sophanodora P, Chinachoti P, McClements DJ. Stability and rheology of corn oil-in-water emulsions containing maltodextrin. Food Res Int. 2004;37(9):851–9. doi:10.1016/j.foodres.2004.05.001.
Dokic-Baucal L, Dokic P, Jakovljevic J. Influence of different maltodextrins on properties of O/W emulsions. Food Hydrocoll. 2004;18(2):233–9. doi:10.1016/s0268-005x(03)00068-7.
Surh J, Decker EA, McClements DJ. Influence of pH and pectin type on properties and stability of sodium-caseinate stabilized oil-in-water emulsions. Food Hydrocoll. 2006;20(5):607–18.
Sosa-Herrera MG, Lozano-Esquivel IE, Ponce de León-Ramírez YR, Martínez-Padilla LP. Effect of added calcium chloride on the physicochemical and rheological properties of aqueous mixtures of sodium caseinate/sodium alginate and respective oil-in-water emulsions. Food Hydrocoll. 2012;29(1):175–84. doi:10.1016/j.foodhyd.2012.02.017.
Liang Y, Gillies G, Patel H, Matia-Merino L, Ye A, Golding M. Physical stability, microstructure and rheology of sodium caseinate-stabilized emulsions as influenced by protein concentration and non-adsorbing polysaccharides. Food Hydrocoll. 2014;36(0):245–55. doi:10.1016/j.foodhyd.2013.10.006.
Kayes J. Pharmaceutical suspensions: relation between zeta potential, sedimentation volume and suspension stability. J Pharm Pharmacol. 1977;29(1):199–204.
Mao L, Roos YH, Miao S. Effect of maltodextrins on the stability and release of volatile compounds of oil-in-water emulsions subjected to freeze–thaw treatment. Food Hydrocoll. 2015;50:219–27. doi:10.1016/j.foodhyd.2015.04.014.
Thanasukarn P, Pongsawatmanit R, McClements D. Influence of emulsifier type on freeze-thaw stability of hydrogenated palm oil-in-water emulsions. Food Hydrocoll. 2004;18(6):1033–43.
Fioramonti SA, Arzeni C, Pilosof AMR, Rubiolo AC, Santiago LG. Influence of freezing temperature and maltodextrin concentration on stability of linseed oil-in-water multilayer emulsions. J Food Eng. 2015;156:31–8. doi:10.1016/j.jfoodeng.2015.01.013.
Dickinson E, Galazka VB. Emulsion stabilization by ionic and covalent complexes of β-lactoglobulin with polysaccharides. Food Hydrocoll. 1991;5(3):281–96.
ACKNOWLEDGMENTS
This research was supported by the following project. Authors gratefully thank College of Chemistry and Chemical Engineering, South China University of Technology, for providing the use of Turbiscan Lab Expert (Formulaction, France). This work was supported by National High-tech R&D Program, namely the 863 Program (No. 2014AA022205), and supported by Science and Technology Foundation Guangzhou, namely “Public Research Platform for Production Technology for Novel Pharmaceutical Formulations” (No. 201509030006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Zhang, X., Wang, X. et al. The Influence of Maltodextrin on the Physicochemical Properties and Stabilization of Beta-carotene Emulsions. AAPS PharmSciTech 18, 821–828 (2017). https://doi.org/10.1208/s12249-016-0572-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0572-5