Abstract
This review presents scientific and regulatory considerations for the development of solid oral modified release (MR) drug products. It includes a rationale for patient-focused development based on Quality-by-Design (QbD) principles. Product and process understanding of MR products includes identification and risk-based evaluation of critical material attributes (CMAs), critical process parameters (CPPs), and their impact on critical quality attributes (CQAs) that affect the clinical performance. The use of various biopharmaceutics tools that link the CQAs to a predictable and reproducible clinical performance for patient benefit is emphasized. Product and process understanding lead to a more comprehensive control strategy that can maintain product quality through the shelf life and the lifecycle of the drug product. The overall goal is to develop MR products that consistently meet the clinical objectives while mitigating the risks to patients by reducing the probability and increasing the detectability of CQA failures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Despite numerous advances in drug delivery technology, approximately 50% of the novel drug approvals by the FDA in the recent years have been for solid oral dosage forms (1). From a patient perspective, the simplicity and ease of administration are the biggest advantages of solid oral formulations. While most solid oral dosage forms are immediate release (IR) formulations, modification from an IR profile is often necessary to achieve desired therapeutic objectives and benefits for the patient. These include reduced dosing frequency for better patient compliance, better controlled plasma drug levels for the given therapeutic application with reduced overdose risk or lower incidence of side effects, and in some cases, an enhanced bioavailability to reduce total drug intake (2).
The most common modified release (MR) dosage forms are extended release (ERFootnote 1) and delayed release (DR) formulations. ER dosage forms are designed to prolong drug release in a predictable and reproducible manner into the gastrointestinal (GI) tract. DR dosage forms are typically enteric coated formulations that release the drug at once, in a specified location in the small intestine or colon, (a) to reduce drug degradation in acidic environment of the stomach, thereby providing enhanced bioavailability,(b) to minimize the irritation to the mucosal cells of the stomach caused by certain drugs such as aspirin, and (c) to ensure site-specific drug delivery for improved local or systemic therapeutic effects, such as products targeting the colon indicated for inflammatory bowel disease. In some cases, a solid oral dosage form may contain IR and DR or ER components within the same tablet or capsule, either as a single ingredient or as a fixed dose combination of ingredients. Overall, the design of MR drug delivery intends to alter the timing and control of drug release in a predetermined fashion or to target selective sites in the GI tract using formulation technologies for patient benefit.
As regulatory agencies are advocating quality-by-design (QbD) implementation in pharmaceutical development and life cycle management, regulatory decisions are increasingly based upon product and process understanding derived from scientific and quality risk management principles. One of the primary objectives of the Office of Pharmaceutical Quality in the Center for Drug Evaluation and Research at FDA is to assure that all human drug products meet the same quality standards to safeguard clinical performance (3). More patient-centered risk assessments will be the focus of regulatory evaluations with the ultimate goal to assure desired consistent efficacy and safety over the lifecycle of drug products (4). Under the QbD paradigm, the target product profile (TPP) is predetermined to achieve the desired clinical objectives for MR drug product development (5). The factors that influence the clinical objectives in the TPP necessitating the development of MR drug product include the therapeutic indication, physicochemical properties, biopharmaceutics, and clinical pharmacology of the drug.
The clinical objectives in the TPP lead to a quality target product profile (QTPP) which is the prospective summary of the quality attributes of a drug product that are ideally achieved to ensure the desired quality, taking into account safety and efficacy of the drug product (6). Biopharmaceutics plays an important role in linking product quality attributes with clinical performance. Therefore, effective integration of biopharmaceutics in product development and lifecycle management based on QbD principles is important, especially for MR products (7). Biopharmaceutics tools such as bioavailability (BA)/bioequivalence (BE) measurements, in vitro dissolution testing, clinically relevant specifications based on In vitro-In vivo Correlations/Relationships (IVIVC/R), and the risk assessment based on biopharmaceutics principles are frequently used in drug development to investigate the effect of quality attributes on product clinical outcomes.
This article is intended to present a high-level overview of scientific and regulatory considerations in solid oral MR drug product development with the aim of maximizing benefit and minimizing risk to the patient. The concepts of product and process understanding including the importance of biopharmaceutics tools, establishing comprehensive control strategy, lifecycle management considerations, and applicability of the relevant FDA guidance will be discussed. The relevant FDA guidance and documents are listed in Table 1.
PRODUCT AND PROCESS UNDERSTANDING
An understanding of the effect of formulation and manufacturing process on pharmacokinetic and clinical performance is important for MR product development and lifecycle risk management. The drug delivery technologies for modifying the release rate of drug have been extensively discussed (8–12). For ER dosage forms, commonly utilized technologies include polymeric systems such as matrix, coated reservoir, and osmotic pump systems that provide prolonged drug release through diffusion and dissolution as a result of swelling or erosion of polymeric system, across insoluble membrane barriers, or osmotic pressure-induced release (8, 11–13). DR dosage forms frequently employ coatings as barriers to prevent drug release in the stomach or provide site-specific drug release (14). The selection of modified drug delivery technology depends on the predefined clinical objectives along with the manufacturers’ preference.
Key Considerations in Product Design
The most challenging part of MR drug development is to design delivery systems that elicit predictable and reproducible release rate of the therapeutic agent to meet the clinical objectives (15). It is worth mentioning that advanced modeling and simulation approaches are increasingly being employed to aid the MR drug product development (16–19). For instance, the required in vivo release rate or dissolution profile can be obtained by deconvolution of the predefined drug exposure time-course profile required for the clinical target in the TPP. The techniques used to assess regional absorption characteristics of a compound including in vitro or in situ models and site-specific delivery in animal models and human subjects are also employed (18–22). Thus in vitro, in situ, in silico, and in vivo models can be used to determine the appropriate drug release profile for product development.
The general concepts of product and process understanding based on QbD principles are described in detail previously (6). In addition, ICH Q8 (R2) provides the framework for QbD in the development of pharmaceutical dosage forms to assure safety and efficacy of commercial drug products for the patient with reproducible quality. With the prospective QTPP, the critical quality attributes (CQAs) of the drug product can be identified. Criticality depends on the severity of harm to patients should that attribute fall outside acceptable range, and not on the probability or detectability of failure. A comprehensive knowledge of drug substance properties, including physicochemical, biopharmaceutical, pharmacokinetic (PK), and pharmacodynamic (PD) properties is crucial for MR drug product design. These properties should be given full consideration when designing or selecting delivery technologies, along with the properties of the excipients, specifically the polymeric excipients used to modify the release rate. In MR formulation development, the rate-controlling excipients should be appropriately selected based on the selected release mechanism desired as a performance outcome of the drug product. Material attributes of the rate controlling polymers such as amount, molecular weight distribution, viscosity in solution phase, type, and extent of substitutions (e.g., HPMC) can significantly affect the release rate that in turn can affect in vivo exposure. The inherent high variability in physicochemical properties associated with polymeric excipients between different manufacturers, manufacturing sites, or lots may need to be investigated because of their potential impact on drug release and absorption. Selection of appropriate type and amount of plasticizer for coated formulations may be important in terms of producing films with adequate mechanical properties. The critical ratios in the formulation composition (such as the ratio of drug substance to rate controlling polymer or to soluble components in a matrix tablet, IR:ER pellet ratio in capsules, drug substance/osmotic agent ratio in osmotic MR dosage form) can also be important in achieving the target in vitro dissolution profiles. Many of these attributes of drug substance and excipients may be critical material attributes (CMAs).
CMAs are those attributes of input material whose variability can affect the CQAs and hence should be within appropriate limits, ranges, or distribution (6, 23). Moreover, CMAs not only include those of the drug substance and excipients used in the manufacturing process but also include attributes of the in-process material as “input” for subsequent process steps. For example, particle size distribution, moisture or residual solvent, assay, and in vitro dissolution which can be considered as CQAs of coated pellets (i.e., an in-process material) may be considered as CMAs of the subsequent unit operation such as blending, encapsulation, or compression. The example in Fig. 1 illustrates the effect of IR:ER ratio and release rate of the ER component on the clinical profiles of acetaminophen MR formulation (based on PK simulation) (24). It can be seen that these CMAs can affect Cmax, Tmax, and AUC of the MR product and may lead to different therapeutic outcomes in patients. Therefore, it is important to assess the impact of product design and CMAs on clinical profile of the MR product.
The simulated in vivo profiles for acetaminophen to show the interplay between the release rate and the ratios of IR:ER. It assumes a first-order and zero-order dissolution for the IR and ER portions, respectively. The red solid lines represent the concentration profiles for the IR/ER final product, which is synthesized by the simulated PK profiles from the IR portion (blue dotted lines) and the ER portion (green dashed lines). The PK parameters used for simulations are provided in each panel. The percentages of the IR portion of the total dose are (from top to bottom): 0, 10, 20, 30, and 40%. The release rates of ER component are (from left to right): 0.04, 0.07, 0.1, 0.13, and 0.16%/h. Presented at the 2012 AAPS Annual Meeting and Exposition (24)
A potential risk that may be unique to MR drug products is dose dumping. Dose dumping has been defined as unintended, rapid drug release in a short period of time of the entire amount or a significant fraction of the drug contained in the MR drug product (25). As per 21 CFR 320.25(f)(1)(ii), the in vivo bioavailability profile of a drug product with an ER claim should rule out dose dumping. Depending upon the indication and the therapeutic index of the drug, dose dumping can pose a significant risk to patients due to safety issues or diminished efficacy. For example, the safety risk posed by alcohol-induced dose dumping for an oral opioid analgesic MR product has resulted in product withdrawal from market (26). Evaluation of the potential for alcohol-induced dose dumping using in vitro dissolution is therefore important for further assessment of safety and efficacy risks of solid oral MR products (27) across multiple therapeutic areas.
Interdependence of Product and Process Design and Impact on CQAs
It is well known that product and process design cannot be decoupled from each other. A pharmaceutical formulation becomes a candidate for a commercial drug product only when it can be successfully scaled up and manufactured at commercial scale. The commercial drug product is expected to have the same quality attributes and clinical performance as the drug product used in pivotal clinical trials or BE studies for both brand and generic drugs. Successful scale up depends on process understanding that includes identifying critical process parameters (CPPs) for each unit operation. CPPs are those parameters whose variability can impact CQAs and hence should be monitored or controlled (5).
One of the major goals of product and process understanding is to minimize the risk of clinical failure to the patient by identifying the likely failure modes of the identified CQAs. Appropriate formal or informal risk assessment tools can be used to identify high-risk material attributes or process parameters during product and process design. ICH Q9 provides the general guidelines for quality risk management for the pharmaceutical industry (4). Risk assessments aid in focusing development efforts and resources on high and medium risk CMAs and CPPs to reduce the development time while appropriately addressing the risk of product failure to the patient. Because in vitro dissolution profile may be directly linked to clinical performance of the solid oral MR products, it is important to understand the product- and process-related failure modes to achieve and maintain the target in vitro dissolution profile consistently upon scale up, over the shelf life, and throughout the product’s life cycle. There have been multiple reports of product recalls due to failure of the marketed MR products to adhere to the in vitro dissolution specifications through shelf life, which may have direct impact on clinical safety and efficacy, therefore risk to patients (28–30). In addition, lack of process understanding may also prevent the manufacturers from successfully scaling up their products, thereby preventing the entry into the market.
Simpler MR formulations such as single layer matrix tablets typically require conventional unit operations such as blending, granulation, and compression which may be easier to scale up due to significant experience and prior knowledge. More complex unit operations such as extrusion-spheronization, drug layering on placebo pellets, polymer coating of drug-loaded pellets, blending/encapsulation of pellets with different release profiles, laser drilling of orifices for osmotic systems, and bilayer tablet compression involve many high-risk process parameters that need to be identified using the appropriate risk assessment tools. In order to evaluate formulation and process robustness, experiments can be designed to evaluate the impact of levels or ranges of CMAs and CPPs on CQAs, especially the in vitro dissolution. Design of experiments (DoEs) may be employed to limit the number of experiments when a large number of attributes need to be investigated. Interactions between the attributes can also be identified using DoE approach. In general, product and process understanding aim to answer the following questions during the development program:
-
1.
How can the CMAs of selected drug substance, excipients, and in process material that affect drug product CQAs be identified and controlled to avoid product failure?
-
2.
How can the CPPs of the selected manufacturing process that affect drug product CQAs be identified and controlled within proven and acceptable ranges or the design space to avoid failure?
-
3.
How can the interactions between and within different CMAs and CPPs affect the CQAs?
Because the formulation and process are interdependent, appropriate experiments can be designed to generate product and process understanding at the same time (6,23,31,32). Such experiments will also capture the interactions between the CMAs and CPPs. As an example, for a coating unit operation, the scale independent process state variables such as product temperature, exhaust temperature, and humidity are controlled by the scale-dependent operating parameters such as spray rate, nozzle pressure, inlet airflow, and temperature. The interaction between CMAs such as polymer type, viscosity grade, and molecular weight distribution along with the CPPs like spray rate, airflow, temperature, and humidity during coating and curing can be studied using carefully designed experiments to ensure uniform and reproducible coating properties which do not change significantly through shelf life, thereby assuring a stable in vitro dissolution profile of the MR product. If appropriate multivariate experiments are conducted, a “Design Space” within which product and process variables do not impact the CQAs of the solid oral MR products may be established with concurrence from the regulatory agencies (5).
Stability Considerations
In addition to CMAs and CPPs, it is important to understand the effect of environmental factors on the CQAs early in the development program. The properties of polymeric excipients can significantly be affected by temperature and humidity on storage, during drug product manufacture, as well as afterwards on stability. It can lead to change in dissolution through the shelf life, thereby potentially altering clinical performance of the MR drug product and increasing the risk of harm to the patient. Identification of critical temperature and humidity thresholds for drug product stability can help identify the suitable container closure system and storage conditions for the commercial product. While the reproducibility and stability of dissolution profile as desired is critical, other standard performance targets such as assay, degradation products, content uniformity, moisture content, and polymorphic form of drug substance in the final product are also important to assure that the product performs as mentioned on the label throughout its shelf life and during the in-use period.
Evaluation of in-use performance of the drug product may be necessary if hydrophilic or hygroscopic polymeric excipients are used in the formulation to control drug release. The products stored in the pharmacy in large count high-density polyethylene (HDPE) bottles or dispensed to patients in polypropylene pharmacy vials may be exposed to transient high humidity conditions until the container is exhausted. The ICH stability conditions do not capture the performance of the drug product during the in-use period. Therefore, demonstration of satisfactory product performance under simulated in-use conditions can mitigate the risk of CQA failures during patient in-use period.
Additional Considerations for Generic Solid Oral MR Products
For generic MR drug product development, the applicants that file abbreviated new drug applications (ANDAs) referencing safety and efficacy of a particular reference listed drug (RLD) have the responsibility to carefully analyze the RLD product design before establishing a QTPP for their generic product. While generic applicants have the freedom to design their MR products using different technologies as compared to the RLD, it is incumbent on them to prove that the design differences do not translate into therapeutic inequivalence. For oral MR drug products, the product-specific BE criteria along with the provided BE metrics published by the FDA are a good reference. Moreover, additional meaningful PK parameters beyond AUC and Cmax may be useful to evaluate BE of certain MR products. For example, equivalence of therapeutic exposures at a certain time point or period as denoted by partial AUCs, either earlier (e.g., insomnia) or later (e.g., attention deficit hyperactivity disorder), as needed for the indication and therapeutic effect. When a different release mechanism than that of the RLD is employed, sufficient justification may be necessary to assure no adverse effect on clinical performance due to the change of the drug release kinetics such as first-order release vs. zero-order release.
Based on the above discussion, it is evident that in vitro dissolution plays a very important role in characterizing, controlling, and assuring MR drug product quality over shelf life for the life cycle of the drug product. It can also be an important surrogate for clinical performance of these products. Therefore, biopharmaceutics tools such as appropriate in vitro dissolution method development and establishing clinically relevant specifications based on concepts of IVIVC/R are especially important for MR dosage forms.
IN VITRO DISSOLUTION METHODS FOR MR DRUG PRODUCTS
Due to the critical role of biopharmaceutics linking product quality attributes with clinical performance, effective integration of biopharmaceutics in the QbD framework is an important aspect for successful MR product development and approval. For a new drug application (NDA), BA evaluation is an important component for the exploration of the efficacy and safety of the drug products, while BE studies are often necessary for bridging formulations and evaluating the impact of manufacturing and process changes on the clinical outcomes for both new and generic products. However, because of the high-cost and long timeline of clinical studies, BA/BE study at every stage of product development is unnecessary, impractical, and inefficient to be used in the QbD paradigm for studying the influence of formulation and manufacturing process changes on clinical performance. As dissolution plays an important role in defining a drug’s PK profile, especially for MR products, appropriate in vitro dissolution testing can be very valuable throughout the product life cycle and may effectively serve as a bridge between CMAs, CPPs and the in vivo performance.
General Considerations in Dissolution Method Development
In vitro dissolution can be employed during the preclinical and early clinical development phase for drug product prototype selection and proof of concept clinical studies. More importantly, it is also a critical control strategy element during clinical, scale-up, and commercial stages to confirm that validation and commercial batches are equivalent to the biobatches that have acceptable efficacy and safety profiles (and to justify post-approval changes). In general, a specific dissolution testing method is included for a MR product in an NDA and ANDA, which at a minimum serves a routine quality control purpose. It could be a brand-new method (generally for NDAs) or available methods listed in the USP monograph or FDA dissolution database (generally for ANDAs). It is worth noting that a dissolution method is formulation specific, especially for MR products. The methods listed in the USP and the FDA dissolution database may not necessarily be appropriate for drug products with the same active ingredient (and different excipients/formulation), because of the differences in release mechanisms, composition, and delivery system design.
Although drug release of MR products may be much more complex than IR, the general principles for dissolution characterization of both types of formulation are the same: to assure consistent product quality and performance with a robust discriminating method to signal potential problems with in vivo PK profile. The guidelines for in vitro dissolution method development and validation provided in the FDA guidance and the USP general chapters (<711>, <1092>, and <1088>) may serve as a starting point for method development. The choice of apparatus for MR products should be based on knowledge of the formulation. The sampling scheme is generally set at multiple time points (33) to provide more time-specific dissolution information, due to the drug product being designed to release over an extended time period or within a certain timeframe. Notably, the current compendial approaches may not be biorelevant with respect to volumes, composition, and physicochemical properties of the test media and also may not take into consideration the mechanical and hydrodynamic forces that can influence dosage-form behavior during passage through the GI tract. As a result, the compendial methodologies may not necessarily be able to identify a formulation or batch with problematic in vivo performance, which raises a challenge for product quality control.
Biopredictive Dissolution Method Development
For promoting QbD implementation in the pharmaceutical industry, there is a critical need for new techniques and approaches for biorelevant or biopredictive dissolution method development as a means to gain information that may be more meaningful from a clinical perspective. As the in vivo performance of oral MR dosage forms is determined by the interplay of various physiological and dosage-form-derived parameters, dissolution method development should be based on the comprehensive understanding on physicochemical properties of the drug substance and product, the biological environment that the product will be exposed in patients, and drug dissolution and absorption mechanisms. In this regard, using biorelevant media could be the first step in the attempt to mimic the condition of in vivo dissolution from a theoretical or developmental standpoint. Various biorelevant media simulating conditions in the fasted or fed stomach, small intestine, and colon have been summarized previously (15). Moreover, the advantages of USP Apparatus 3 (BioDis®) and Apparatus 4 (Flow though cell) were emphasized for biopredictive dissolution testing, considering the possibilities of these apparatus to mimic the changes in physicochemical conditions and, to some extent, the mechanical forces experienced by MR formulations in the GI tract (15).
Dissolution test equipment could also be customized, such as an in vitro dissolution/permeation system development by Kataoka et al. which has been successfully utilized for predicting the oral absorption of poorly water-soluble basic drugs (34,35). However, the overall utility of such approaches in terms of feasibility of use (validation, analytical support, etc.) in quality control environments and general utility throughout the regulated industry (cost, reproducibility, etc.) has not yet been realized.
To aid biopredictive testing method development, advanced modeling approaches can be employed, such as physiologically based pharmacokinetic (PBPK) absorption modeling, which integrates anatomical and physiological parameters of the GI tract with the physicochemical properties of drug substances and products to simulate and predict the drug in vivo exposure (36–38). Using this approach, the in vivo drug dissolution in the GI tract can be deconvoluted from GI transit, drug permeation, gut wall metabolism or first-pass metabolism, and drug disposition, thereby providing more realistic information for adjusting the in vitro testing conditions to mimic the in vivo drug dissolution. This modeling approach has been increasingly used as an important biopharmaceutics tool in drug development and regulatory review (36–38) and will be further discussed in the following section for clinically relevant specification setting.
Dissolution Method Development Report in Regulatory Submission
To support and justify the selection of a proposed dissolution method, a report for method development along with method validation can be included in the regulatory submission. The method development report generally includes the following: (a) solubility over the physiological pH range and other physicochemical properties of the drug substance; (b) release mechanisms to help the selection of an appropriate medium; (c) detailed description of the dissolution method; (d) investigations of dissolution conditions (i.e., selection of the equipment/apparatus, agitation/rotation speed, in vitro dissolution/release media, media pH, assay, sink conditions, use of sinker and enzyme if applicable); and (e) supporting data to demonstrate the discriminating power of the method for CMAs and CPPs.
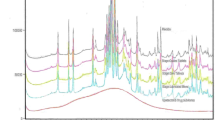
For MR products, the choice of apparatus could be based on knowledge of the formulation and actual dosage-form performance in the in vitro test system and sampling at multiple time points. The dissolution profile should be complete and cover at least 85% of drug release of the labeled amount or whenever a plateau (i.e., no increase over three consecutive time-points) is reached (33). A systematic approach such as DoE is recommended for selecting dissolution conditions, which considers various factors simultaneously, not only for the main effects of the individual factors but also for the interactions among the factors. For demonstrating the method discriminating ability, the dissolution profiles of the reference (target) product and the test products that are intentionally manufactured with meaningful variations for the most relevant critical manufacturing variables (i.e., ±10–20% change to the specification ranges of these variables; including CMAs and CPPs) are typically compared. Furthermore, providing supportive data is encouraged to show the selected dissolution method is able to reject batches that are not bioequivalent to the reference batches, as demonstrated in Fig. 2, where distinct dissolution profile shapes obtained from the two batches that showed differences in efficacy performance in a comparative clinical trial.
Rationale for Setting Dissolution Specification
The setting of dissolution specifications for MR products should use multiple-time-point criteria covering the entire profile of drug release process, which differs from that for IR products where a single time-point acceptance criterion may be sufficient. Nevertheless, dissolution specification establishment for MR products should follow the same principles recommended in the FDA dissolution guidance for IR oral dosage forms (39). First, setting dissolution specifications should take a risk-based approach, which focuses on the batch(es) used in the pivotal clinical studies, because the clinical performance of the product is the most important consideration for quality control. When there are insufficient in vitro data from pivotal clinical trials to adequately represent the real variability of the commercial batches, taking the batches from all the relevant clinical studies into consideration is recommended, along with the manufacturing information of those batches (number, date, site, and size of the batch) to evaluate the factors causing the quality variability. Second, the acceptance criteria should be set based on the average in vitro dissolution data with multiple time points (e.g., at least three time points for ER products covering the initial, middle, and terminal phases of the complete dissolution profile data), which encompass the timeframe where at least 80% of the drug is dissolved or where a plateau of drug dissolved is reached if incomplete dissolution is occurring. Third, the selection of the specification ranges is based on mean target value ±10% and NLT 80% for the last specification time-point. Note that this practice takes the mean at each time point into consideration, equivalent to USP stage 2 testing (n=12), for an adequate control of product quality. Specification set based on the mean data using only six dosage units has often been too permissive and may lead to release of products non-bioequivalent to the pivotal biobatches. Wider specification ranges could be acceptable provided adequate justification is given (e.g., supported by an approved IVIVC/R model). Lastly, the time points and corresponding acceptance criteria should be appropriately selected to discriminate batches that have inadequate quality. The time points should be representative and encompass the whole process of dissolution, at which the maximum discriminating potential to identify quality differences are evident. Ideally, the specification should be set with clinical relevance to identify non-bioequivalent drug product to the pivotal biobatches which have demonstrated acceptable efficacy and safety profiles. It can be achieved by a valid IVIVC or IVIVR as discussed in the following section. If there is no such IVIVC or IVIVR established, clinically relevant dissolution specifications are also possible, if the in vivo data are available to verify the limits of dissolution specification. Overall, the adequacy of the proposed dissolution acceptance criteria for a product is evaluated based on the totality of dissolution data provided in the application.
IVIVC/R AND CLINICALLY RELEVANT PRODUCT SPECIFICATIONS
Current Status of IVIVC/R in Regulatory Submissions
One of the most efficient approaches to set clinically relevant dissolution specifications for MR products is through IVIVC/R. IVIVC development for MR products has been advocated for decades by regulatory agencies in the expectation that the information will be useful in establishing dissolution specifications and will permit certain formulation and manufacturing changes without an in vivo BE study (40). Nevertheless, IVIVC activities still remain in an underutilized state in the pharmaceutical industry, as indicated by the low submission percentage in regulatory applications, possibly due to the low success rate for IVIVCs included in drug submissions (about 40%) (41). Seemingly, IVIVCs have not been actively employed to facilitate decision-making during drug development, such as for formulation selection and manufacturing and process optimization. The usages of IVIVC seem to primarily focus on biowaiver requests to justify pre-approval (bridging formulations) and post-approval changes (manufacturing site and process/equipment changes) without BA/BE studies.
Specifically for pre-approval biowaivers in NDAs, IVIVC can be used to bridge formulations in the clinical studies and reduce the number of BE studies prior to drug approval. An IVIVC established in the early phase of drug development will aid in the formulation and process optimization by providing clinical evidence with the predicted PK data from the in vitro dissolution. Meanwhile, based on the IVIVC prediction, the commercial batch could be easily bridged with development batches and pilot batches, using in vitro dissolution as an adequate surrogate. Clearly, drug product development process can be highly streamlined with largely reduced cost and time for clinical studies by an IVIVC. During post-approval stages, a validated IVIVC (mainly level A) can be used to request biowaivers for significant changes in manufacturing sites and process, non-release controlling excipient and release controlling excipients with certain prerequisites, which otherwise need a BA/BE study.
An IVIVC as well as IVIVR can also be used for dissolution specification setting. Generally, IVIVC/R can be employed to evaluate the proposed acceptance criteria to assure that all lots that have dissolution profiles within the upper and lower limits of the specifications are bioequivalent, or less optimally but still possible, lots exhibiting dissolution profiles at the upper and lower dissolution limits are bioequivalent to the clinical/bioavailability lots or to an appropriate reference standard. More details regarding the applicability of IVIVC/R for MR products are outlined in the FDA IVIVC guidance and SUPAC MR Guidance.
Role of IVIVC/R in Bridging Quality and Clinical Performance
In the QbD approach for pharmaceutical development, IVIVC/R is emphasized as an important biopharmaceutics tool for enhancing MR drug product and process understanding with the ultimate goal of ensuring consistent efficacy and safety throughout the drug product’s life cycle, via clinically relevant product specification establishment. Clinically relevant product specifications may be defined as those that can ensure the delivery of the intended dose at a consistent rate to patients to guarantee consistent safety and efficacy profiles for the marketed product relative to those achieved by the clinical trial formulations. By linking product quality to the patients for product life cycle management and continual improvement, clinically relevant product specifications such as for CQAs as well as CMAs and CPPs embedded in QbD are expected to warrant enhanced product quality with consistent safety and efficacy profile desired in the life cycle of drug products.
As a bridge between in vitro testing and in vivo exposure, IVIVC can be effectively utilized for understanding the impact of the drug product CQAs on in vivo performance. Through IVIVC/R, product quality specifications can be established optimally with assured clinical outcomes using in vitro dissolution testing as an endpoint as shown in Fig. 3. Briefly, the risk assessment of an ER matrix tablet identified three high risk CMAs: particle size (PS) of the active pharmaceutical ingredient (API), viscosity of a matrix forming agent (MFA), and the PS of a matrix forming enhancing agent (MEA). Their impacts on dissolution were investigated by the DoE approach. The data were analyzed by partial least square (PLS) regressions and validated by the comparisons between the model predicted and the observed dissolution values, along with a “leave one out” cross validation. The validated model was then used to predict dissolution at various values of MFA viscosity, MEA PS, and the PS of the API, followed by the prediction of the AUC and Cmax based on a multiple level C IVIVC. The AUC and Cmax were compared to those of the clinical batches (which have demonstrated adequate efficacy and safety), and the ratios of AUC and Cmax (predicted to the clinical batches) were calculated and plotted against the three high-risk material attributes as shown in Fig. 3. The rectangular box encloses the region of selected specifications, which are well within the BE range (0.8–1.25) and therefore considered to be appropriate with greater confidence (42).
AUC ratios for different combinations of three CMAs. The combinations of MFA viscosity and MEA PS were evaluated under four distinct values of PS of API, expressed as the ratios of percentage of larger particle size to that of smaller particle size. Presented at the 2013 AAPS Annual Meeting and Exposition (42)
Strategies to Promote IVIVC/R in Regulatory Decision-Making
Considering the low success rate of IVIVC studies in regulatory submissions, there is an unmet need for more research on new approaches to increase IVIVC activities and aid in clinically relevant specification establishment. Based on a survey conducted to investigate the current state of IVIVC in the submissions, the common deficiencies leading to the low acceptance rate include insufficient number of release rates used for IVIVC construction, lack of rank order in dissolution rates and in vivo exposure, inconclusive model predictability, inappropriate data analysis or modeling, among other factors (41). The root causes of IVIVC failures may be due to (1) planning IVIVC as a post hoc event that prevents the use of robust/appropriate data analysis, (2) lack of biorelevance of compendial dissolution methods to predict the in vivo drug release, (3) the limitations of conventional approaches for IVIVC development.
To help increase IVIVC-based submissions in regulatory decision-making, a potential path forward could in principle be to conduct IVIVC studies in the early phase of drug development and plan with an appropriate study design and data analysis. Moreover, new approaches or technologies in model development could also be helpful for increasing the success rate, such as mechanistic IVIVC/R based on PBPK modeling approach. In general, PBPK models could be developed based on the prior knowledge on drug physicochemical and PK properties and verified by the available BA/BE data from clinical trials. PBPK approach shows promising advantages for designing IVIVC in a mechanistic framework, considering its ability to incorporate the different mechanisms influencing drug disposition (e.g., drug release, GI transition, metabolism, and elimination) and provide more realistic perspectives regarding the effect of formulation and manufacturing changes on in vivo performance. As such, this approach could be advantageous for IVIVC/R establishment by overcoming the fundamental limitation of the conventional two-stage and one-stage approaches that take insufficient considerations on drug in vivo dissolution and absorption mechanisms under physiological state for the modeling. By this approach, the in vivo dissolution can be separately deconvoluted from GI transit, permeation, gut wall metabolism, or first-pass metabolism, providing more straightforward correlation between the in vitro dissolution and the in vivo drug release (rather than the absorption which is complicated by other processes such as GI transition, chemical and metabolic degradation in the GI tract, etc.).
PBPK models can also be utilized to aid in biopredictive dissolution method development, using the deconvolution function to delineate the in vivo dissolution profile (37) and adjust dissolution condition to reflect the in vivo drug release. With biopredictive dissolution testing, it is expected that the likelihood of a successful IVIVC would greatly increase. Using the available in vitro and in vivo data, an IVIVC may be attempted using either conventional- or physiologically based approaches. If an IVIVC is not achievable, an IVIVR providing a semi-quantitative or qualitative (e.g., rank order) agreement between the in vitro dissolution and in vivo absorption or exposure may be an alternative. With an established IVIVC or IVIVR, the dissolution testing can be adequately used as an endpoint for the DoE studies to define design spaces of CMAs and CPPs for assured product safety and efficacy in the patients.
CONTROL STRATEGY
The failure of CQAs including in vitro dissolution of MR drug products occurs either due to lack of robust formulation design, lack of control of the CMAs, insufficient process understanding or lack of control of CPPs, and sometimes inadequate test methods. Evaluation and understanding of these critical variables is therefore necessary to assess risks and failure modes of the MR drug product performance. A comprehensive control strategy can be established based on the product and process understanding in order to mitigate the probability of occurrence or increase the detectability of potential failure of the CQAs, thereby decreasing risk to the patient.
ICH Q8 (R2) defines a control strategy as a planned set of controls, derived from current product and process understanding that ensures process performance and product quality (5). QbD approaches in development lead to greater product and process understanding which can be leveraged to decrease probability of product failures. Without sufficient product and process understanding, the control strategy will rely on extensive end product testing along with narrow limits on material attributes and process parameters studied during development (6). However, such extensive end product testing and narrow controls may not be able to prevent scale up, stability, or product failures in the market because of lack of product and process understanding which may have resulted in inappropriate or inadequate controls. For example, the manufacturers may not be able to achieve the target dissolution profile of the MR product that was used for pivotal batches when they scale up the drug product to commercial scale. Conversely, extensive product and process understanding may result in a flexible control strategy within the established design space that can adapt to variability in the input material attributes and process parameters (6). Such an approach may involve real time release (RTR) testing. The examples include continuous monitoring of ER coating thickness and uniformity on pellets or tablets using process analytical technologies (PAT) such as near infrared (NIR) and Raman spectroscopy (43–46), or other spectroscopic imaging techniques (47). In these cases, process parameters may be automatically adjusted using the feedback loop to achieve the desired CQA. If the clear correlation of the attributes such as polymer type, molecular weight distribution, viscosity in solution, and coating thickness with in vitro dissolution through carefully designed multivariate experiments is demonstrated, in theory, the end product testing for this particular CQA may not be required. In reality, reduced end product testing may be possible if appropriate upstream controls are implemented based on product and process understanding.
It is to be noted that not all of the attributes or parameters need to be controlled. The type and level of control depends on the risk of failure of the CQAs of the final product due to the variability in the CMA or CPP under investigation. Appropriate controls on CMAs and CPPs, environmental and packaging controls, all derived from product and process understanding, will ensure predictable and reproducible performance of final drug product reducing the probability of product failure. In addition to decreasing the probability of failure of CQAs, increasing detectability of failure is an important aspect of the comprehensive control strategy. Therefore, the integration of biopharmaceutics tools such as predictive dissolution methods and clinically relevant specifications derived from IVIVC/R are useful in linking the CMAs and CPPs with the in vivo performance of the MR drug product. A representative example, a comprehensive control strategy for an MR product incorporating material controls, process controls, environmental controls, and end product controls, is given in the ANDA QbD example for MR drug product published by FDA (48).
LIFE CYCLE MANAGEMENT AND CONTINUOUS IMPROVEMENT
Any post-approval change in formulation, manufacturing process, or controls may bring uncertainty to the quality, clinical performance, and risk profile of the MR drug products. However, post-approval changes during product life cycle are unavoidable and sometimes even desirable as the quality standards and analytical methods evolve and improve. Most post-approval changes relate to changes in raw material sources, manufacturing, and testing sites, batch sizes, and analytical methods. The regulatory requirements for post-approval changes for MR drug products have traditionally been guided by the SUPAC MR guidance (49). SUPAC assesses post-approval changes in risk level categories 1 to 3 requiring stability, dissolution, and BE data as applicable. However, a thorough understanding of product and process gained in the QbD paradigm along with the associated control strategy including clinically relevant specifications mitigates the risk of product failure and potential harm to the patient. A key to successful life cycle management of MR products is this knowledge base created during the product development. In addition, product and process knowledge continues to be gathered throughout the life cycle with evaluation of batch trends, process capability and stability, and necessary corrective actions in order to provide consistent quality of drug product to each individual patient (50). Effective leveraging of the knowledge base provided by product and process understanding gained throughout the life cycle benefits both industry and regulatory agencies. It can result in more efficient change management processes and increased opportunities for innovation and continuous improvement for the industry. From a regulatory agency perspective, it increases the confidence in applicants’ understanding of product and process and may decrease their burden in evaluating the changes.
CONCLUSION
This review provides an overview of product and process understanding considerations for solid oral MR product development that integrates biopharmaceutics principles as illustrated in Fig. 4. The rationale for the development of solid oral MR product is based on clinical requirements mentioned in the TPP of the proposed product. A patient-focused structured development program that demonstrates product and process understanding avoids potential failures of the commercial drug product. Application of appropriate biopharmaceutics tools including clinically relevant specifications based on IVIVC/R assures predictable and reproducible clinical performance of the MR product. A comprehensive control strategy that reduces probability and increases detectability of failure of the CQAs mitigates the risk of harm to the patient. The use of QbD principles in the development of MR products may decrease regulatory burden and provide increased opportunities for innovation and continuous improvement for the industry.
Notes
ER dosage forms are sometimes also referred to as XR, sustained release (SR), or controlled release (CR) products. This article uses ER.
References
New Drugs at FDA: CDER’s new molecular entities and new therapeutic biological products. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/. Accessed 06 May 2016.
Shargel L, Wu-Pong S, Yu A. Chapter 17. Modified-release drug products. In: Shargel LW-P, S; Yu AC, eds. Applied Biopharmaceutics & Pharmacokinetics. New York, NY: McGraw-Hill; 2012. http://accesspharmacy.mhmedical.com/content.aspx?bookid=513&Sectionid=41488035. Accessed 03 May 2016
Office of Pharmaceutical Quality, FDA Pharmaceutical Quality Oversight, One Quality Voice. 2015. http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM442666.pdf. Accessed 06 May 2016.
U.S. Food and Drug Administration. Guidance for industry Q9 quality risk management. 2006.
U.S. Food and Drug Administration. Guidance for industry Q8(R2) pharmaceutical development. 2009.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83. doi:10.1208/s12248-014-9598-3.
Selen A, Cruanes MT, Mullertz A, Dickinson PA, Cook JA, Polli JE, et al. Meeting report: applied biopharmaceutics and quality by design for dissolution/release specification setting: product quality for patient benefit. AAPS J. 2010;12(3):465–72. doi:10.1208/s12248-010-9206-0.
Leucuta SE. Drug delivery systems with modified release for systemic and biophase bioavailability. Curr Clin Pharmacol. 2012;7(4):282–317.
Reza MS, Quadir MA, Haider SS. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J Pharm Pharm Sci. 2003;6(2):282–91.
Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin Biol Ther. 2004;4(1):35–51. doi:10.1517/14712598.4.1.35.
Verma RK, Arora S, Garg S. Osmotic pumps in drug delivery. Crit Rev Ther Drug Carrier Syst. 2004;21(6):477–520.
Zema L, Maroni A, Foppoli A, Palugan L, Sangalli ME, Gazzaniga A. Different HPMC viscosity grades as coating agents for an oral time and/or site-controlled delivery system: an investigation into the mechanisms governing drug release. J Pharm Sci. 2007;96(6):1527–36. doi:10.1002/jps.20802.
Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol. 2005;57(5):533–46. doi:10.1211/0022357055957.
Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6(1):33–66.
Garbacz G, Klein S. Dissolution testing of oral modified-release dosage forms. J Pharm Pharmacol. 2012;64(7):944–68. doi:10.1111/j.2042-7158.2012.01477.x.
Kesisoglou F, Balakrishnan A, Manser K. Utility of PBPK Absorption modeling to guide modified release formulation development of Gaboxadol, a highly soluble compound with region-dependent absorption. J Pharm Sci. 2015;105(2):722–28. doi:10.1002/jps.24674.
Kesisoglou F, Xia B, Agrawal NG. Comparison of deconvolution-based and absorption modeling IVIVC for extended release formulations of a BCS III drug development candidate. AAPS J. 2015;17(6):1492–500. doi:10.1208/s12248-015-9816-7.
Olivares-Morales A, Kamiyama Y, Darwich AS, Aarons L, Rostami-Hodjegan A. Analysis of the impact of controlled release formulations on oral drug absorption, gut wall metabolism and relative bioavailability of CYP3A substrates using a physiologically-based pharmacokinetic model. Eur J Pharm Sci. 2015;67:32–44. doi:10.1016/j.ejps.2014.10.018.
Wang B, Liu Z, Li D, Yang S, Hu J, Chen H, et al. Application of physiologically based pharmacokinetic modeling in the prediction of pharmacokinetics of bicyclol controlled-release formulation in human. Eur J Pharm Sci. 2015;77:265–72. doi:10.1016/j.ejps.2015.06.020.
Kuentz M, Nick S, Parrott N, Rothlisberger D. A strategy for preclinical formulation development using GastroPlus as pharmacokinetic simulation tool and a statistical screening design applied to a dog study. Eur J Pharm Sci. 2006;27(1):91–9. doi:10.1016/j.ejps.2005.08.011.
Sjogren E, Westergren J, Grant I, Hanisch G, Lindfors L, Lennernas H, et al. In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: application of the mechanistic absorption model GI-Sim. Eur J Pharm Sci. 2013;49(4):679–98. doi:10.1016/j.ejps.2013.05.019.
Takano R, Sugano K, Higashida A, Hayashi Y, Machida M, Aso Y, et al. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm Res. 2006;23(6):1144–56. doi:10.1007/s11095-006-0162-4.
Lionberger RA, Lee SL, Lee L, Raw A, Yu LX. Quality by design: concepts for ANDAs. AAPS J. 2008;10(2):268–76. doi:10.1208/s12248-008-9026-7.
Duan J, Qiu Y. Using IVIVC to guide the design of complex modified-release dosage forms consisting of IR (immediate release) and ER (extended release) components. Chicago: AAPS Annual Meeting; 2012.
Friebe TP, Asgharzadeh F, Gray A, Hughes K, Hebestreit J-P, Rosiaux Y, et al. Regulatory considerations for alcohol-induced dose dumping of oral modified-release formulations. Pharm Tech. 2015;38(10):40–6.
FDA news release. 2005. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108460.htm
Anand O, Yu LX, Conner DP, Davit BM. Dissolution testing for generic drugs: an FDA perspective. AAPS J. 2011;13(3):328–35.
Enforcement report—week of June 18, 2014. http://www.accessdata.fda.gov/scripts/enforcement/enforce_rpt-Product-Tabs.cfm?action=select&recall_number=D-1381-2014&w=06182014. Accessed 06 May 2016.
Enforcement report—week of July 29, 2015. http://www.accessdata.fda.gov/scripts/enforcement/enforce_rpt-Product-Tabs.cfm?action=select&recall_number=D-1271-2015&w=07292015&lang=eng. Accessed 06 May 2016.
Enforcement report—week of July 9, 2014. http://www.accessdata.fda.gov/scripts/enforcement/enforce_rpt-Product-Tabs.cfm?action=select&recall_number=D-1415-2014&w=07092014&lang=eng. Accessed 06 May 2016.
Jethara SI, Patel MR. Optimizing oral controlled release drug delivery systems using experimental designs. Intel Prop Rights. 2015;3:135. doi:10.4172/2375-4516.1000135.
Naelapää K, Veski P, Kristensen HG, Rantanen J, Bertelsen P. Building quality into a coating process. Pharm Dev Technol. 2010;15:35–45. doi:10.3109/10837450902882377.
USP38-NF33 (United States Pharmacopeial Convention). General Chapter: <1092> The dissolution procedure: development and validation. Rockville; 2015.
Kataoka M, Itsubata S, Masaoka Y, Sakuma S, Yamashita S. In vitro dissolution/permeation system to predict the oral absorption of poorly water-soluble drugs: effect of food and dose strength on it. Biol Pharm Bull. 2011;34(3):401–7.
Kataoka M, Masaoka Y, Yamazaki Y, Sakane T, Sezaki H, Yamashita S. In vitro system to evaluate oral absorption of poorly water-soluble drugs: simultaneous analysis on dissolution and permeation of drugs. Pharm Res. 2003;20(10):1674–80.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:300–21. doi:10.1016/j.ejps.2013.09.008.
Wei H, Lobenberg R. Biorelevant dissolution media as a predictive tool for glyburide a class II drug. Eur J Pharm Sci. 2006;29(1):45–52. doi:10.1016/j.ejps.2006.05.004.
Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiologically based absorption modeling in implementing Quality by Design in drug development. AAPS J. 2011;13(1):59–71. doi:10.1208/s12248-010-9250-9.
U.S. Food and Drug Administration. Guidance for industry: dissolution testing of immediate release solid oral dosage forms. 1997.
U.S. Food and Drug Administration. Guidance for industry extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations. 1997.
Suarez SS. FDA’s experience on IVIVC—new drug products, presentation at the PQRI workshop co-sponsored by FDA, AAPS, USP, and FIP. Rockville; 2012 http://pqri.org/wp-content/uploads/2015/08/pdf/Suarez.pdf
Duan J et al. The application of IVIVC in setting the clinical relevant manufacturing specifications. San Antonio: AAPS Annual Meeting; 2013.
Hohl R, Scheibelhofer O, Stocker E, Behzadi SS, Haack D, Koch K, et al. Monitoring of a hot melt coating process via a novel multipoint near-infrared spectrometer. AAPS PharmSciTech. 2016. doi: 10.1208/s12249-016-0504-4
Knop K, Kleinebudde P. PAT-tools for process control in pharmaceutical film coating applications. Int J Pharm. 2013;457(2):527–36. doi:10.1016/j.ijpharm.2013.01.062.
De Beer T, Burggraeve A, Fonteyne M, Saerens L, Remon JP, Vervaet C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int J Pharm. 2011;417(1-2):32–47. doi:10.1016/j.ijpharm.2010.12.012.
De Beer TR, Bodson C, Dejaegher B, Walczak B, Vercruysse P, Burggraeve A, et al. Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. J Pharm Biomed Anal. 2008;48(3):772–9. doi:10.1016/j.jpba.2008.07.023.
Haaser M, Karrout Y, Velghe C, Cuppok Y, Gordon KC, Pepper M, et al. Application of terahertz pulsed imaging to analyse film coating characteristics of sustained-release coated pellets. Int J Pharm. 2013;457(2):521–6. doi:10.1016/j.ijpharm.2013.05.039.
U. S. Food and Drug Administration. Quality by design for ANDAs: an example for modified-release dosage forms. 2011. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM286595.pdf. Accessed 06 May 2016.
U.S. Food and Drug Administration. Guidance for industry SUPAC-MR: modified release solid oral dosage forms. 1997.
U.S. Food and Drug Administration. Guidance for industry Q10 pharmaceutical quality system. 2009.
U.S. Food and Drug Administration. Guidance for industry Q8, Q9, and Q10 questions and answers (R4). 2011.
Acknowledgements
The authors thank Richard Lostritto and Ashley Boam for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Duxin Sun and Simon Zhou
Rights and permissions
About this article
Cite this article
Li, M., Sander, S., Duan, J. et al. Scientific and Regulatory Considerations in Solid Oral Modified Release Drug Product Development. AAPS J 18, 1406–1417 (2016). https://doi.org/10.1208/s12248-016-9974-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9974-2