Abstract
Background
Coronary artery disease (CAD) is a leading cause of morbidity and mortality, with a shifting trend towards the younger population. Paraoxonase1 (PON1) is a glycoprotein enzyme associated with high-density lipoprotein (HDL) particles in the blood. It has the ability to protect against lipid oxidation, thereby reducing the risk of atherogenesis. PON1 rs662 gene polymorphism may affect serum PON1 levels as well as its activity and may have a significant role in the pathogenesis of CAD. The present study was conducted to identify the association of PON1 rs662 gene polymorphism with serum PON1 levels in CAD patients in the North Indian population. This case–control study included 71 angiography-proven CAD patients (with > 50% luminal stenosis in one or more coronary arteries) and 71 controls (with < 50% luminal obstruction in angiography). PON1 rs662 gene polymorphism was studied using PCR and RFLP under the standardized protocol. Serum PON1 levels were estimated by ELISA.
Results
The serum PON1 level was significantly lower in the CAD group than in the controls (7.79 ± 3.16 vs. 10.79 ± 3.19 ng/mL; p < 0.0001). Logistic regression analysis showed that homozygous GG genotype of PON1 rs662 SNP has ninefold increased risk of developing CAD in an Indian population (OR = 9.0, 95%CI 2.79–29.06, p = 0.0002). A significantly higher frequency of G allele was also observed in CAD patients than in controls (OR 2.64, 95%CI 1.61–4.33, p = 0.001).
Conclusions
The reduced serum PON1 level is associated with CAD. PON1 rs662 gene polymorphism is significantly associated with CAD susceptibility in the North Indian population.
Similar content being viewed by others
Background
Coronary artery disease (CAD) is a leading cause of morbidity and mortality, contributing to 15.5% of total deaths globally. At present, the phase of epidemiological transition is being observed in which there is an exponential increase in CAD mortality in developing countries as compared to declining drift in developed countries [1]. This may be linked to rapidly increasing industrialization, urbanization, sedentary lifestyle, and unhealthy food habits. According to the World Health Organization, the South Asian region has one of the highest cardiovascular mortality rates in the world [2]. Interactions between various genetic determinants and environmental factors have been implicated in the pathogenesis of premature atherosclerosis in the Indian population [3, 4].
Human Paraoxonase (PON) gene family is located on the long arm of chromosome 7 (7q21.3-q22.1) and includes PON1, PON2, and PON3 [5]. Serum PON1, a 44 kDa Ca2+-dependent glycoprotein enzyme is one of the susceptibility genes which play a significant protective effect on the progression of atherosclerosis. PON1 is produced in the liver and associates with the surface of high-density lipoprotein (HDL) in blood [6]. The antioxidant activity of HDL cholesterol (HDL-C) is primarily due to the PON1 enzyme. It prevents oxidation of low-density lipoprotein cholesterol (LDL-C) and may also help in the metabolism of oxidized LDL-C (ox-LDL) [7,8,9].
PON1 gene has several genetic polymorphisms that can influence the levels and activity of PON1 in circulation [10]. The rs662 gene (or c.575A > G or p. Gln192Arg) polymorphism is located in exon 6 of the PON1 gene that exchanges an arginine (R) for glutamine (Q) at position 192 of the protein (CAA to CGA) [11]. The studies have shown conflicting results on the association between the Q192R gene variation and CAD as this association is not consistent among distinct population groups. In the present study, we examined the association between PON1 rs662 gene polymorphism and serum PON1 levels in CAD patients in Northern India.
Methods
Study population
The present study was conducted in a tertiary care center of Northern India after gaining approval from the institutional ethics review board. The study included randomly selected 142 participants (more than 18 year age) with written informed consent. The diagnosis of CAD was made by a cardiologist on the basis of medical history, clinical examination, and the relevant investigations including angiography (> 50% stenosis in one or more coronary arteries). Out of 142 study participants, 71 were CAD patients and 71 were age and gender-matched controls (with < 50% luminal obstruction on coronary angiography). The exclusion criteria included the recent history of acute coronary syndrome or cerebrovascular event (< 8 weeks), chronic liver and kidney insufficiency, chronic obstructive pulmonary disease, cancer, and repeated blood transfusions.
Sample collection
After overnight fasting, a blood sample was obtained from study participants by venipuncture, of which 2.5 mL each was collected in EDTA vacutainer and plain vacutainer (without anticoagulant). EDTA samples were stored at − 20 °C for the purpose of DNA extraction. The blood in the plain vacutainer was allowed to clot and then centrifuged to obtain serum for further analysis. These samples were stored at − 70 °C until batch analysis of enzyme assay.
Estimation of serum PON1
Estimation of serum PON1 level was done using a commercially available ELISA kit manufactured by Biovendor-Labratorni medicina A.S., following manufacturer’s instructions. This was a two-step enzyme immunoassay for quantitative measurement of serum PON1. The absorbance was read at 450 nm. The measured optical density was directly proportional to the PON1 concentration.
Genotyping
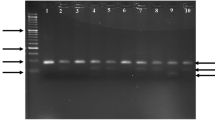
The genomic DNA was extracted from nucleated blood cells using DNA Extraction Kit (Krishgen). This DNA was amplified by PCR using the following primers- forward, 5′-GCTGTGGGACCTGAGCACTT-3′; reverse, 5′-ATACTTGCCATCGGGTGAAATG-3′. The amplification was done in a 20 µL PCR reaction mixture containing 25–30 ng genomic DNA, 50 pmol of each primer, 10 µL Dream Taq green PCR Master-mix (Thermo-scientific) containing 3.2 µM Taq DNA polymerase, 3.2 µM 2X buffer, 32 µM MgCl2, and 3.2 µM of each dNTPs using a thermal cycler (Himedia Eco-96). The PCR conditions used were as follows: initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s; annealing at 60.7 °C for 30 s, then extension at 72 °C for 30 s, with a final extension step at 72 °C for 5 min. The amplified PCR product of 189 bp was incubated with MboI (New England Biolabs) restriction enzyme at 37 °C for 16 h, and digested products were then resolved on 1.1% agarose gel electrophoresis for the Q192R genotypes (AA- 156 bp and 33 bp, AG- 189 bp,156 bp, and 33 bp, GG -189 bp as depicted in Fig. 1).
Digested PCR products of PON1 gene with MboI restriction enzyme. Homozygous AA shows 2 bands at 156 bp and 33 bp, Heterozygous AG shows 3 bands at 189 bp, 156 bp, and 33 bp, and Homozygous GG shows a single undigested band at 189 bp. Lane M is DNA Ladder (50 bp); Lane 3,4,5,6,7 show AA genotype; Lane 8 represents AG genotype; Lane 1,2,9,10,11 are GG genotype
Statistical analysis
The Statistical Package for the Social Sciences for Windows (SPSS), version 25.0 (Chicago, IL, USA), was used to analyze the data. The P value of < 0.05 was considered statistically significant. The Analysis of variance (ANOVA) test was used to determine statistically significant differences between the means of multiple independent groups. Any deviation from Hardy–Weinberg equilibrium was evaluated by the chi-square test. Odds Ratio (OR) was calculated with 95% confidence interval (CI) to estimate the association between genotypes of PON1 gene polymorphism and CAD.
Results
In this study, we evaluated the association between PON1 rs662 (Q192R) gene polymorphism and serum PON1 levels in 142 participants. Of these, 71 patients were categorized as CAD group, and 71 as controls, on the basis of diagnostic coronary angiography. The mean age of CAD patients and controls was 56.5 ± 10.80 and 50.2 ± 9.50 years, respectively. The CAD group consisted of 75% males and 25% females. The case and the control groups were age and gender-matched. The demographic, clinical, and baseline biochemical characteristics of the study population are depicted in Table 1.
There was no significant difference between the frequencies of diabetes mellitus (DM) and hypertension between the groups. The CAD group had a significantly higher number of patients with smoking habits and a family history of CAD. In the lipid profile, serum total cholesterol, LDL-C, and triglycerides were not significantly different between the groups. However, HDL-C was significantly lower and the atherogenic index of plasma (AIP) was found to be significantly higher in the CAD patients. Further, serum levels of both total creatine kinase and its MB fraction were higher in the CAD group.
Serum PON1 levels in CAD patients and controls are shown in Table 2. The serum PON1 levels were significantly lower in the CAD group than in the control group (7.79 ± 3.16 vs. 10.79 ± 3.19 ng/mL, p < 0.0001).
The distribution of genotypes and alleles for the PON1 rs662 (Q192R) polymorphism is shown in Table 3. The frequencies of the AA, AG, and GG genotypes in the CAD patient and control group were 35.2%, 21%, and 35.2% vs. 50.7%, 43.7%, and 4%, respectively. Logistic regression analysis showed that the homozygous GG genotype of PON1 rs662 SNP has ninefold increased risk of developing CAD in the Indian population (odds ratio OR = 9.0, 95% CI 2.79–29.06, p = 0.0002). A significantly higher frequency of the G allele was also observed in CAD patients than in controls (OR 2.64, 95% CI 1.61–4.33, p = 0.001).
The inter-genotypic variation of the serum PON1 levels in the CAD and control group is shown in Table 4. Our results showed that the serum PON1 level in AA, AG, and GG genotypes of the CAD group was 9.8 ± 3.10, 8.04 ± 3.12, and 5.57 ± 1.26 ng/mL, respectively, whereas the respective values in different genotypes were 12.84 ± 2.71, 9.04 ± 1.92 and 5.91 ± 1.18 ng/mL in the control group. The inter-genotypic difference in the serum PON1 values was statistically significant in both the CAD as well as the control group.
Our data also demonstrated that there was no significant difference between the PON1 level of the GG genotype of the CAD group when compared with that of the GG genotype of the control group (Table 5). Moreover, the PON1 level was significantly lower in the CAD group as compared to the controls in individuals with AA or AG genotypes, indicating the role of other factors in influencing the PON1 levels.
In our study, CAD patients were further subdivided into different subgroups on the basis of the degree of coronary vascular stenosis (Table 6), and the number of involved coronary vessels (Table 7). The mean serum PON1 levels significantly decreased as the degree of coronary vascular stenosis increased from mild to severe in the CAD group. When compared within any two groups, the PON1 level was significantly different between the stenosis < 50% and stenosis > 90% groups, and also in the stenosis < 50–69% and stenosis > 90% groups. However, there was no difference between the stenosis < 50% and stenosis 50–69% groups or between the stenosis 50–69% and stenosis 70–90% groups. The PON1 levels did not differ significantly in CAD patients with the different number of involved coronary vessels (p = 0.32).
Discussion
Asian Indians have more susceptibility to develop CAD and more so, a decade earlier than the Caucasian population [12]. Further, a recent study stated that the risk of developing cardiovascular disease among North Indians is higher as compared to other parts of India [13]. It needs to be emphasized that the conventional risk factors have somewhat not been able to justify completely the excess risk of CAD among Indians, raising the possibility of a genetic vulnerability. PON1 Q192R gene polymorphism has been implicated in increased CAD risk in many populations. However, there is a relative paucity of studies on the genotypic and allelic distribution of the PON1 Q192R genotypes in CAD in India. Thus, we examined the implications of this single nucleotide polymorphism (SNP) and its association with PON1 concentration in CAD patients and controls.
Oxidative damage plays an important role in chronic inflammatory diseases such as CAD. PON1 is atheroprotective due to its antioxidant and anti-inflammatory role in the body [14, 15]. It prevents LDL oxidation, thereby reducing the risk of atherosclerosis. [16]. The diverse genetic variations in the PON1 gene may affect its functional role and, thereby, its ability to protect against CAD. Our findings showed that PON1 levels in CAD patients were significantly lower as compared to the controls. Our data also indicated that individuals with GG genotype had a ninefold amplified risk of developing CAD. Furthermore, the serum PON1 level in GG genotypes was significantly lower as compared to PON1 level in AA or AG genotypes in both CAD as well as the control group. This demonstrates that individuals with GG genotype have lower PON1 levels, thereby indicating a significant role of PON1 Q192R gene polymorphism in enhanced CAD risk. Many studies have documented the association between PON1 Q192R gene variants with increased susceptibility to CAD; however, the results have been inconsistent among different population groups. Table 8 lists down some of the relevant studies showing the relationship of this particular polymorphism in distinct ethnic groups.
A meta-analysis study by Saadat [24] reported that RR genotype is significantly associated with increased PON1 enzyme activity in healthy individuals; however, a remarkable heterogeneity between the included studies was also present. As evident from a multitude of studies, the frequency of the polymorphic alleles and haplotypes of PON1 differs among various ethnic groups [24, 25]. The conflicting results in the association of the Q192R polymorphism with the increased risk of CAD may also be due to variations in sample size, genotyping methods, pathological states, and diverse environmental influences [26,27,28].
Chronic inflammation plays a pivotal role in the progression of CAD. Among the plasma lipoproteins, ox-LDL has an extensive role in inflammatory and atherogenic processes [29]. There are several antioxidant enzymes in our body, of which PON1, with its anti-atherogenic properties, has been widely studied. PON1 is a 354 amino acid calcium-dependent protein mainly synthesized in the liver, and present in blood in association with HDL. Reduced PON1 levels may lead to an increase in the circulating levels of ox-LDL, thereby increasing the susceptibility to CAD [30, 31]. Ox-LDL significantly contributes to key processes in the pathophysiology of CAD such as the formation of foam cells [32], endothelial dysfunction [33], oxidative stress [34], and microvascular injury [35].
The serum levels of PON1 along with its enzymatic activity are important determinants of the degree of oxidative stress, thereby playing an important role in diseases characterized by chronic inflammation such as CAD. As with other proteins, the expression and catalytic activity of PON1 are greatly influenced by SNPs [36, 37]. Among these SNPs, PON1 rs662 gene polymorphism has been implicated in modulating the functionality of the PON1 enzyme [38]. A recent study reported that Q alloenzyme has more ability to hydrolyze oxidized lipids, and also protects LDL particles against the oxidative processes more efficiently than R alloenzyme [39]. The R allele variant has been associated with a less active isoform of PON1 against lipoprotein oxidation, resulting in an enhanced risk of vascular disease [40]. A meta-analysis study found that the R allele gene variant is significantly associated with increased levels of ox-LDL, triglycerides, total cholesterol, and LDL-C, which may lead to a higher risk of CAD [22].
In our study, the aggregate allele frequency in the total study population was 61.3% for wild allele and 38.7% for polymorphic allele, which corroborated with global frequency. Our study demonstrated that within the GG genotype, PON1 levels did not differ significantly between CAD and control group, indicating that GG genotype is a major genetic determinant for decreased PON1 levels. However, the PON1 level was significantly lower in the CAD group as compared to the controls within AA or AG genotypes. Thus, it can be interpreted that although the serum PON1 level was affected by PON1 rs662 gene polymorphism and is significantly lower in individuals with GG genotype, however, there are other exogenous factors also including environmental modulators, which affect PON1 levels in CAD patients. It is well known that a multitude of environmental factors (such as diet, lifestyle, smoking, and alcohol consumption) have a significant effect on PON1 activity. Some pathological states also influence the enzyme levels as well as antioxidant activity of PON1 independently from the genotype, resulting in a state of oxidative stress, and accelerated development of atherosclerosis [26,27,28, 41]. Nevertheless, a recent study stated that the genetic determinants of the PON1 phenotype have stronger influences than the contributions of the lifestyle risk factors [42]. Thus, it is suggested that evaluation of genotype, as well as serum PON1, might be potential markers of CAD [43].
Our study showed that the PON1 levels significantly decreased in severe (> 90%) luminal stenosis, irrespective of the number of involved coronary vessels. A recent study found a positive association between low PON1 activity and CAD severity, particularly in smokers and diabetes patients [44]. Thus, serum PON1 may have a prognostic value in determining the severity of CAD.
Conclusions
Our findings support the association between PON1 rs662 (Q192R) gene polymorphism and low PON1 levels in CAD patients. Individuals with GG genotype have low PON1 levels compared to AA and AG genotypes. Polymorphic G allele may be responsible for altering the functionality of the PON1 enzyme, leading to an increased risk of CAD. Moreover, as CAD is a multifactorial disease due to interaction between various environmental and genetic influences, there may be other modulators also, that alter the serum PON1 levels in these individuals. Moreover, PON1 may be of prognostic importance in severe coronary vascular stenosis.
The limitation of our study was the relatively small sample size, which represented only a fraction of CAD patients admitted to a tertiary care hospital in the given time period. However, our study reveals the importance of additional risk factors, including genetic polymorphisms, and valuable markers such as PON1 levels, which may be useful to predict CAD in the Indian population. Prospective studies with a larger population are warranted to have a better understanding of the role of PON1 in enhanced CAD susceptibility. PON1 as a potential therapeutic candidate is also a point of interest for further studies.
Abbreviations
- AIP:
-
atherogenic index of plasma
- ANOVA:
-
analysis of variance
- CI:
-
confidence interval
- CAD:
-
coronary artery disease
- DM:
-
diabetes mellitus
- ELISA:
-
enzyme-linked immunosorbent assay
- EDTA:
-
ethylenediaminetetraacetic acid
- HDL:
-
high-density lipoprotein
- HDL-C:
-
high-density lipoprotein cholesterol
- LDL:
-
low-density lipoprotein
- LDL-C:
-
low-density lipoprotein cholesterol
- OR:
-
odds ratio
- Ox-LDL:
-
oxidized LDL
- PON:
-
paraoxonase
- PCR:
-
polymerase chain reaction
- RFLP:
-
restriction fragment length polymorphism
- SNP:
-
single nucleotide polymorphism
- SPSS:
-
statistical package for the social sciences
References
Bodkhe S, Jajoo SU, Jajoo UN, Ingle S, Gupta SS, Taksande BA (2019) Epidemiology of confirmed coronary heart disease among population older than 60 years in rural central India—a community-based cross-sectional study. Indian Heart J 71:39–44. https://doi.org/10.1016/j.ihj.2019.01.002
World Health Organization. Global status report on noncommunicable diseases. 2014. https://www.who.int/nmh/publications/ncd-status-report-2014/en/. Accessed 2014.
D’Agostino RB, Grundy S, Sullivan LM, Wilson P (2001) Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 286:180–187. https://doi.org/10.1001/jama.286.2.180
Group DP (2002) Prediction of mortality from coronary heart disease among diverse populations: is there a common predictive function? Heart 88:222. https://doi.org/10.1136/heart.88.3.222
Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN (1996) The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 33:498–507. https://doi.org/10.1006/geno.1996.0225
Deakin S, Leviev I, Gomaraschi M, Calabre-si L, Franceschini G, James RW (2002) Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J Biol Chem 277(276):4301–4308. https://doi.org/10.1074/jbc.M107440200
Eren E, Yilmaz N, Aydin O (2013) Functionally defective high-density lipoprotein and paraoxonase: a couple for endothelial dysfunction in atherosclerosis. Cholesterol 2013:92090. https://doi.org/10.1155/2013/792090
Eren E, Ellidag HY, Aydin O, Yılmaz N (2014) Homocysteine, paraoxonase-1 and vascular endothelial dysfunction: Omnibus viis Romam Pervenitur. J Clin Diagn Res 8:CE01–CE04. https://doi.org/10.7860/JCDR/2014/7827.4773
Toth PP, Barylski M, Nikolic D, Rizzo M, Montalto G, Banach M (2014) Should low high-density lipoprotein cholesterol (HDL-C) be treated? Best Pract Res Clin Endocrinol Metab 28:353–368. https://doi.org/10.1016/j.beem.2013.11.002
Qing-Yang H, Meng-Rong E, Sen-Lin J (2006) Linkage and association studies of the susceptibility genes for type 2 diabetes. Acta Genet Sin 33:573–589. https://doi.org/10.1016/S0379-4172(06)60087-5
Luo Z, Pu L, Muhammad I et al (2018) Associations of the PON1 rs662 polymorphism with circulating oxidized low-density lipoprotein and lipid levels: a systematic review and meta-analysis. Lipids Health Dis 17:281. https://doi.org/10.1186/s12944-018-0937-8
Joshi P, Islam S, Pais P, Reddy S, Dorairaj P, Kazmi K et al (2007) Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 297(3):286–294. https://doi.org/10.1001/jama.297.3.286
Geldsetzer P, Manne-Goehler J, Theilmann M, Davies JI, Awasthi A, Danaei G et al (2018) Geographic and sociodemographic variation of cardiovascular disease risk in India: a cross-sectional study of 797,540 adults. PLoS Med 15(6):e1002581. https://doi.org/10.1371/journal.pmed.1002581
Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW et al (2002) Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 106(4):484–490. https://doi.org/10.1161/01.cir.0000023623.87083.4f
Gupta N, Singh S, Maturu VN, Sharma YP, Gill KD (2011) Paraoxonase 1 (PON1) polymorphisms, haplotypes and activity in predicting cad risk in North-West Indian Punjabis. PLoS ONE 6(5):e17805. https://doi.org/10.1371/journal.pone.0017805
Litvinov D, Mahini H, Garelnabi M (2012) Antioxidant and anti-inflammatory role of paraoxonase 1: implication in arteriosclerosis diseases. N Am J Med Sci 4(11):523–532. https://doi.org/10.4103/1947-2714.103310
Matam K, Khan IA, Hasan Q, Rao P (2015) Coro- nary artery disease and the frequencies of MTHFR and PON1 gene polymorphism studies in a varied population of Hyderabad, Telangana region in south India. J King Saud Univ Sci 27(2):143–150. https://doi.org/10.1016/j.jksus.2014.09.002
Hassan MA, Al-Attas OS, Hussain T, Al- Daghri NM, Alokail MS, Mohammed AK et al (2013) The Q192R polymorphism of the paraoxonase 1 gene is a risk factor for coronary artery disease in Saudi subjects. Mol Cell Biochem 380:121–128. https://doi.org/10.1007/s11010-013-1665-z
Liu T, Zhang X, Zhang J, Liang Z, Cai W, Huang M, Yan C, Zhu Z, Han Y (2014) Association between PON1 rs662 polymorphism and coronary artery disease. Eur J Clin Nutr 68(9):1029–1035. https://doi.org/10.1038/ejcn.2014.105
Kotur-Stevuljevic J, Spasic S, Stefanovic A, Zeljkovic A, Bogavac-Stanojevic N, Kalimanovska-Ostric D et al (2006) Paraoxonase-1 (PON1) activity, but not PON1(Q192R) phenotype, is a predictor of coronary artery disease in a middle-aged Serbian population. Clin Chem Lab Med 44(10):1206–1213. https://doi.org/10.1515/CCLM.2006.216
Deshpande CS, Singhal RS, Mukherjee MS (2013) Association of paraoxonase1 gene Q192R polymorphism and apolipoprotein B in Asian Indian Women with coronary artery disease risk. Genet Test Mol Biomark 17(2):140–146. https://doi.org/10.1089/gtmb.2012.0193
Siller-Lopez F, Garzon-Castano S, Ramos-Marquez ME, Hernandez-Canaveral I (2017) Association of paraoxonase-1 Q192R (rs662) single nucleotide variation with cardiovascular risk in coffee harvesters of central Colombia. J Toxicol. https://doi.org/10.1155/2017/6913106
Ombres D, Pannitteri G, Montali A, Cande-loro A, Seccareccia F, Campagna F et al (1998) The Gln-Arg192 polymorphism of human paraoxonase gene is not associated with coronary artery disease in Italian patients. Arterioscler Thromb Vasc Biol 18(10):1611–1616. https://doi.org/10.1161/01.atv.18.10.1611
Saadat M (2018) Evaluation of association between Q192R and L55M genetic polymorphisms of PON1 and serum paraoxonase-1 activity in healthy individuals, a metaanalysis. Rom J Diabetes Nutr Metab Dis 25:171–180. https://doi.org/10.2478/rjdnmd-2018-0020
Eom SY, Kim YS, Lee CJ, Lee CH, Kim YD, Kim H (2011) Effects of intronic and exonic polymorphisms of paraoxonase 1 (PON1) gene on serum PON1 activity in a Korean population. J Korean Med Sci 26:720–725. https://doi.org/10.3346/jkms.2011.26.6.720
Camps J, Iftimie S, García-Heredia A, Castro A, Joven J (2017) Paraoxonases and infectious diseases. Clin Biochem 50:80411. https://doi.org/10.1016/j.clinbiochem.2017.04.016
Seres I, Bajnok L, Harangi M, Sztanek F, Koncsos P, Paragh G (2010) Alteration of PON1 activity in adult and childhood obesity and its relation to adipokine levels. Adv Exp Med Biol 660:129–142. https://doi.org/10.1007/978-1-60761-350-3_12
Costa LG, Cole TB, Jarvik GP, Furlong CE (2003) Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Ann Rev Med 54:371–392. https://doi.org/10.1146/annurev.med.54.101601.152421
Tsimikas S (2006) Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr Atheroscler Rep 8:55–61. https://doi.org/10.1007/s11883-006-0065-1
Toshima S, Hasegawa A, Kurabayashi M, Itabe H, Takano T, Sugano J, Shimamura K, Kimura J, Michishita I, Suzuki T, Nagai R (2000) Circulating oxidized low-density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol 20:2243–2247. https://doi.org/10.1161/01.ATV.20.10.2243
Jiang XL, Li M, Zhou JG, Yang QB, Du LJ, Du J (2011) Plasma paraoxonase-1, oxidized low-density lipoprotein and lipid peroxidation levels in gout patients. Cell Biochem Biophys 61:461–466. https://doi.org/10.1007/s12013-011-9221-5
Howell KW, Meng X, Fullerton DA, Jin C, Reece TB, Cleveland JC Jr (2011) Toll-like receptor 4 mediates oxidized LDL-induced macrophage differentiation to foam cells. J Surg Res 171:e27-31. https://doi.org/10.1016/j.jss.2011.06.033
Holvoet P (1999) Endothelial dysfunction, oxidation of low-density lipoprotein, and cardiovascular disease. Ther Apher 3:287–293. https://doi.org/10.1046/j.1526-0968.1999.00169.x
Weinbrenner T, Cladellas M, Isabel Covas M, Fitó M, Tomás M, Sentí M, Bruguera J, Marrugat J (2003) High oxidative stress in patients with stable coronary heart disease. Atherosclerosis 168:99–106. https://doi.org/10.1016/s0021-9150(03)00053-4
Yurdagul A Jr, Green J, Albert P, McInnis MI, Mazar AP (2014) Orr AW2. α5β1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler Thromb Vasc Biol 34:1362–1373. https://doi.org/10.1161/ATVBAHA.114.303863
Legein B, Temmerman L, Biessen EAL, Lutgens E (2013) Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci 70:3847–3869. https://doi.org/10.1007/s00018-013-1289-1
Tajbakhsh A, Rezaee M, Rivandi M, Forouzanfar F, Afzaljavan F, Pasdar A (2017) Paraoxonase 1 (PON1) and stroke; the dilemma of genetic variation. Clin Biochem 50:1298–1305. https://doi.org/10.1016/j.clinbiochem.2017.08.001
Mucientes A, Fernández-Gutierrez B, Herranz E, Rodriguez-Rodriguez L, Varade J, Urcelay E, Lamas JR (2019) Functional implications of single nucleotide polymorphisms rs662 and rs854860 on the antioxidative activity of paraoxonase1 (PON1) in patients with rheumatoid arthritis. Clin Rheumatol 38(5):1329–1337. https://doi.org/10.1007/s10067-018-4394-6
Wysocka A, Cybulski M, Wysokiński AP, Berbeć H, Stążka J, Zapolski T (2019) Paraoxonase 1 activity, polymorphism and atherosclerosis risk factors in patients undergoing coronary artery surgery. J Clin Med 8(4):441. https://doi.org/10.3390/jcm8040441
Mackness MI, Arrol S, Mackness B, Durrington PN (1997) Alloenzymes of paraoxonase and effectiveness of high-density lipoproteins in protecting low-density lipoprotein against lipid peroxidation. Lancet 349(9055):851–852. https://doi.org/10.1016/S0140-6736(05)61755-2
Mackness M, Mackness B (2015) Human paraoxonase-1 (PON1): gene structure and expression, promiscuous activities, and multiple physiological roles. Gene 67:12–21. https://doi.org/10.1016/j.gene.2015.04.088
Roest M, van Himbergen TM, Barendrecht AB, Peeters PHM, van der Schouw YT, Voorbij HAM (2007) Genetic and environmental determinants of the PON-1 phenotype. Eur J Clin Investig 37:187–196. https://doi.org/10.1111/j.1365-2362.2007.01769
Mackness B, Davies GK, Turkie W, Lee E, Roberts DH, Hill E, Roberts C, Durrington PN, Mackness MI (2001) Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol 21:1451–1457. https://doi.org/10.1161/hq0901.094247
Sun T, Hu J, Yin Z, Xu Z, Zhang L, Fan L, Zhuo Y, Wang C (2017) Low serum paraoxonase1 activity levels predict coronary artery disease severity. Oncotarget. 8(12):19443–19454. https://doi.org/10.18632/oncotarget.14305
Acknowledgements
The authors would like to extend their gratitude to Dr. Pramod and Ms. Mamta, who helped us in the sampling and examination process in this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial and intellectual contribution to the work and approved it for publication. RK and CK contributed to the conceptualization. RK and VS were involved in formal analysis and literature search. RK was involved in data acquisition and methodology. VS contributed to the visualization, software and manuscript preparation. CK and HI contributed to the supervision and project administration. NT, VS and SS contributed to the manuscript editing and review. NT and SS contributed to the manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted after gaining approval from the institutional ethical committee (VMMC & Safdarjung Hospital, New Delhi) vide Reference No. IEC/VMMC/SJH/Thesis/October/2018-01 dated 29.10.2018. Written informed consent was taken from the study participants.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, R., Saini, V., Kaur, C. et al. Association between PON1 rs662 gene polymorphism and serum paraoxonase1 level in coronary artery disease patients in Northern India. Egypt J Med Hum Genet 22, 74 (2021). https://doi.org/10.1186/s43042-021-00196-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-021-00196-3