Abstract
Objectives
To describe the feature of expression of syndecan-4 in serum, synovial fluid (SF) and synovium in rheumatoid arthritis (RA) patients, and to analyze the correlation of syndecan-4 with disease activity and serological characteristic of RA.
Methods
Syndecan-4 in sera of 60 RA patients, 20 osteoarthritis (OA) patients, 20 healthy controls, and in SF of 25 RA patients and 25 OA patients were tested by enzyme linked immunosorbant assay. The expressions of syndecan-4 in synovium of RA and OA patients were detected by immunohistochemistry. The expression of syndecan-4 on synovial fibroblasts from RA and OA patients were detected by immunofluorescence. The correlation between serum syndecan-4 concentration and disease activity were analyzed in RA patients.
Results
The serum syndedcan-4 concentration was significantly higher in RA patients than in OA patients and healthy controls, and was higher in rheumatoid factor (RF)-positive RA patients than in RF-negative ones. Syndecan-4 concentration in SF of RA patients was comparable with OA patients. Syndecan-4 expression in synovial tissue was similar between RA and OA patients. The syndecan-4 concentration was significantly lower in SF than in serum of RA and OA patients. Syndecan-4 concentration in both serum and SF was positively correlated with disease activity of RA patients.
Conclusion
The serum syndecan-4 concentration was higher in RA patients than in OA patients, and significantly higher in RF-positive RA patients than in RF-negative ones. Syndecan-4 concentration in both serum and SF was positively correlated with disease activity of RA patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized as articular synovitis, progressive cartilage destruction and bone erosion. Although the aetiology of RA remains unclear, the aberrant B-cells activation and cytokine-mediated pathway are central to the pathogenesis of RA.
Syndecan-4, one of the members of transmembrane heparan sulphate proteoglycans (HSPGs), is the receptor of various cytokines and chemokines that can regulate a series of signal transductions [1]. It could be expressed in several cells under inflammation and involved in regulation of inflammation, angiogenesis, focal adhesion and cell migration [2]. Syndecan-4 ectodomains are constitutively shed and replaced under physiological conditions to maintain balance. However, in response to certain stimuli, such as extracellular cytokines, metalloprotease (MMP) and oxidative stress, syndecan-4 shedding increases dramatically, and the soluble syndecan-4 can promote inflammation and the synthesis of new syndecan-4 [3].
Syndecan-4 could up-regulate the levels of MMP with thrombospondin motifs (ADAMTS)‐5 to promote the inflammation and cartilage degeneration [4]. It has been shown previously that the loss of syndecan-4 protects mice from cartilage damage in animal models of osteoarthritis (OA) [5] and RA [6]. Syndecan-4 deficient mice were resistant to the induction of collagen-induced arthritis (CIA) due to loss of the migration ability of B cells [7]. The tumour necrosis factor transgenic (hTNFtg) mouse model, an established animal model of RA, demonstrated that syndecan-4 was prominently involved in synovial fibroblasts activation and its attachment to the cartilage in the early stage of arthritis [6]. Intra-articular injection of anti-syndecan-4 antibodies into wild-type mice prevented cartilage damage [5]. Syndecan-4 blocking antibody reduced interleukin-1 receptor 1 (IL1R1) surface presentation, leading to reduced RA symptoms in the hTNFtg RA mouse model [8].
Syndecan-4 can be highly expressed by the fibroblast-like synoviocyte (FLS) of RA, as well as interact with receptor protein tyrosine phosphatase sigma (RPTPσ), a highly-expressed phosphatase in arthritic FLS which can regulate the RA FLS migration [9]. Godmann et al. reported that syndecan-4 was highly expressed in RA synovium, and messenger RNA (mRNA) level was also upregulated in RA‐FLS [8]. Syndecan-4 modulates the response of RA‐FLS to the inflammatory cytokine IL-1 [8]. A most recent study demonstrated that highly expressed syndecan-4 led to the initiation of inflammation and decreased apoptosis in RA‐FLS, thereby illustrating the important role of syndecan-4 in the pathogenesis of RA [10].
Despite evidence implicating the relevant role of syndecan-4 in the pathogenesis of RA, the syndecan-4 expression in serum and synovial fluid (SF) of RA patients has not been reported. The correlation of syndencan-4 with clinical features of RA, including disease activity and autoantibody, also remains unclear. In the present study, we aimed to describe the expression of syndecan-4 in serum and SF of RA patients, and reveal the correlation between syndecan-4 and disease activity in RA.
Materials and methods
Patients and samples
The syndecan-4 concentrations in sera were tested in 60 RA patients, and 20 OA patients and 20 healthy controls by enzyme linked immunosorbant assay (ELISA). The OA patients and healthy controls were matched with the RA patients by age and gender. The correlation of syndecan-4 concentration in sera and disease activity of RA patients were analyzed.
SF samples were collected from 25 RA patients and 25 OA patients. The correlation of syndecan-4 concentration in sera and disease activity of RA patients were also analyzed.
Syndecan-4 expression in synovial tissues of 5 RA patients and 5 OA patients were tested by immunohistochemistry. All the synovial tissues were derived from patients undergoing knee replacement. These RA patients and OA patients were also matched by age and gender.
All the above RA patients fulfilled the 2010 American College of Rheumatology/The European League Against Rheumatism (ACR/EULAR) classification criteria. All the enrolled OA patients fulfilled the OA classification criteria [11, 12]. Patients with comorbidities that may increase serum syndecan-4 levels., including pneumonia, malignancies, heart failure, dilated cardiomyopathy, atopic dermatitis, haemodialysis, inflammatory arthritis other than RA, connective tissue diseases and infections were excluded.
None of the patients have received biological disease modifying anti-rheumatic drugs or target therapy. Disease activity of RA patients were evaluated by disease activity score based on 28 joint count-C reactive protein (DAS28-CRP), DAS28-erythrocyte sedimentation rate (DAS28-ESR), simplified disease activity index (SDAI) and clinical disease activity index (CDAI).
This trial was approved by the Peking University Ethics Committee.
All the participants gave written informed consent.
Syndecan-4 concentrations in sera and synovial fluid
Blood and SF were collected into tubes free of anticoagulants, centrifuged 3000 rpm for 10 min to remove cellular debris and stored at − 80 °C until use. Syndecan-4 concentrations in sera and SF were measured by the human syndecan-4 ELISA kit (R&D systems, the USA), following the manufacturer’s protocols. Goat anti human syndecan-4 capture antibody was diluted to a terminal concentration of 0.8 ug/mL using PBS, coated onto the wells of a polystyrene microtiter plate and incubated at room temperature overnight. Test sera without dilution were added to the microwells and incubated at room temperature for 2 h. After washed for 3 times, a detective antibody with a terminal concentration of 100 ng/mL was then added and incubated at room temperature for 2 h. Streptavidin-HRP was then added after washed for 3 times. After incubation for 20 min, substrate solution was added to the microwells. The reaction was terminated by the addition of stop solution and absorbance was measured at 450 nm and 540 nm. Finally, sample concentration of syndecan-4 was determined by extrapolation in a standard curve prepared from seven geometric syndecan-4 standards.
Immunohistochemistry
The protocol of previous study was followed [13] with minor modifications by anti-syndecan-4 antibodies. Briefly, 10 mm thick serial cryostat sections of synovia were dried for 1 h and stored at liquid nitrogen (− 196 ℃) until required. Prior to immunohistochemical analysis, slides were left to equilibrate to room temperature for 30 min, fixed in acetone (4 ℃) for 10 min, air dried, and then sections were rehydrated in phosphate buffered saline (PBS) for 5 min. All primary antibodies were used at 5 mg/ml since initial experiments showed that this concentration gave the optimum specific staining over the range of 2.5–10 mg/ml. Antibody binding was detected using DAB staining kit (Vector Labs, Burlingame, California, USA). Sections were counterstained with Mayer Haematoxylin and mounted. In control experiments, isotype matched control mouse Ig or rabbit Ig were added instead of primary antibodies and stained with the DAB kit as above.
Synovial fibroblast culture
Synovial tissues were obtained from RA and OA patients during joint replacement surgery of knees. According to Kellgren Lawrence grade (K–L grade) of osteoarthritis, all these ten patients were graded as 4. Tissues were minced and treated with 4 mg/ml type I collagenase (Gibco, USA) in serum free Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) at 37 °C for 1 h. Primary cultured synovial cell lines were established and maintained in DMEM including 10% heat-inactivated fetal bovine serum (FBS; Gibco, USA), 1% penicillin–streptomycin (100 U/ml; Gibco, USA), and 1% L-glutamine (2 mM; Gibco, USA), in a humidified incubator at 37 °C in the presence of 5% CO2. Harvested cells were continuously cultured to obtain synovial fibroblasts, and cells of passages 3–6 were used in the next experiments.
Immunofluorescence
Cultured synovial fibroblasts of passages 3–6 were used for immunofluorescence. Cells were treated with rabbit anti-human syndecan-4, 4 °C overnight. Washed with PBS and incubated with goat anti-rabbit IgG–Alexa Fluor ®488 (Abcam, UK), room temperature for 1 h. The biotinylated secondary antibody was detected by streptavidin-Alexa 594 (1:1000 Molecular Probes Code S-11227; Molecular Probes, Eugene, Oregon, USA).
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (IBM Company, the USA) and all charts were performed by GraphPad Prism 5.0 (CA Company, the USA). The results of immunohistochemistry and immunofluorescence were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics Company, the USA). Quantitative variables were expressed as mean ± SD or median (min–max) for normal distribution or non normal distribution data, respectively. Statistical analysis was performed by the χ2 test (frequencies), nonparametric Mann–Whitney U test (non normal distribution data) and Student’s t test (normal distribution data), as appropriate. Correlation analysis was performed by Spearman correlation analysis. P value less than 0.05 was considered significant.
Results
Demographic and clinical features of RA patients
Among the 60 RA patients, there were 12 (20%) males and 48 (80%) females with mean age of 54.8 ± 11.6 years and median disease duration of 6.3 (0.5–34) years. 37 (61.7%) were rheumatoid factor (RF) positive and 53 (88.3%) were anti-cyclic citrullinated protein (anti-CCP) positive. The baseline demographic and clinic features of 60 RA patients, 20 OA patients and 20 healthy controls were demonstrated in Table 1. Generally, the age and gender were comparable among the three groups.
The expression of syndecan-4 in serum, synovial fluid and synovial tissue
The median syndecan-4 concentration in sera of 60 RA patients, 20 OA patients and 20 healthy controls were 509.3 (345.2–769.8) pg/mL, 363.6 ± 137.6 pg/mL and 204.4 ± 59.1 pg/mL, respectively. The serum syndecan-4 concentration was significantly higher in RA than in OA patients and healthy controls (p < 0.001, p < 0.001, respectively). It was also higher in OA patients than in healthy controls (p < 0.001). (Fig. 1).
Syndecan-4 concentration was significantly higher in sera than in SF of RA patients [509.3 (345.2–769.8) pg/mL vs 94.8 (23.5–280.3) pg/mL, p < 0.001] (Additional file 1: Figure S1).
Syndecan-4 concentrations was also significantly higher in sera than in SF of OA patients [363.6 ± 137.6 pg/mL vs 51.0 (27.2–163.6) pg/mL, p < 0.001] (Additional file 1: Figure S1).
Syndecan-4 concentration in SF was slightly higher in RA patients compared to OA patients, however without statistical difference (p = 0.497).
Syndecan-4 was detected by immunohistochemistry in the lining and sublining layers of all the 5 RA and 5 OA patents (Fig. 2). The mean area, mean diater, mean density and mean integrated optical density (IOD) were comparable between RA and OA patients (Additional file 1: Table S1).
Immunofluorescence demonstrated that Syndecan-4 was positively stained in cultured synovial fibroblasts from both RA and OA patients. The IOD was similar between RA and OA patients (10,668 ± 7570 vs. 8106 ± 12,760, p = 0.255). (Additional file 1: Figure S2).
The comparison of serum syndecan-4 concentration between sera-positive and sera-negative RA patients
Serum syndecan-4 concentration was significantly higher in RF positive patients than in RF negative patients [586.2 (390.2–1124.8) pg/mL vs 434.3 (312.0–537.5) pg/mL, (p = 0.009)]. But it was comparable between anti-CCP antibody positive and negative patients [517.4 (346.2–781.0) pg/mL vs. 500.7 (373.8–537.5) pg/mL, p = 0.542] (Additional file 1: Figure S3).
Then we defined patients with at least one of the above two antibodies positive as “sera positive”, and there were 53 sera positive and 7 sera negative patients. The result was the same as that when stratified by anti-CCP antibody.
The correlation between Syndecan-4 concentration and disease activity of RA patients
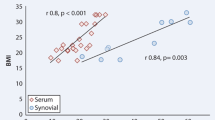
Among the 60 RA patients, the syndecan-4 concentration in sera was positively correlated with TJC, SJC, PGA, EGA, and the disease activity scores, including DAS28-ESR, DAS28-CRP, SDAI and CDAI (Table 2). The correlation coefficents (r value) were mild to moderate (Table 2). Based on any of these disease activity scores, the patients in high disease activity (HDA) had the highest serum syndecan-4 concentration, compared with those in remission, low disease activity (LDA) or moderate disease activity (MDA). (Table 3, Fig. 3) Plotted correlation graphs showed the exact Corresponding Relation of sera Syndecan-4 concentration and disease activity. (Additional file 1: Figure S4).
The syndecan-4 concentration in SF from the 25 RA patients,was positively correlated with PGA, EGA, CRP, DAS28-ESR, DAS28-CRP and SDAI (Additional file 1: Table S2). All of the correlation coefficents (r value) were higher than 0.5.
Discussion
In the current study, we demonstrated higher serum syndecan-4 concentration in RA patients than OA patients and healthy controls. We also confirmed the correlation between syndecan-4 with RA phenotypes, including disease activity and RF. Our findings provided further evidence for the involvement of syndecan-4 in the pathogenesis of RA.
The role of syndecan-4 in articular cartilage degeneration has been proved by substantial evidences [4,5,6, 14], but its involvement in inflammation of arthritis has not been widely recognized. We found serum syndecan-4 was positively related with disease activity, TJC and SJC of RA, suggesting syndecan-4 may be involved in RA-associated inflammation. Syndecan-4 can be up-regulated by TNFα, IL-1β and lipopolysaccharide (LPS) through a functional nuclear factor (NF)-κB site in its promoter [3]. Considering the promotion effect of IL-17 on osteoclastogenesis by inducing the expression of the receptor activator of NF-κB,Th17 might have potential role in inducing syndecan-4 expression [15]. The ectodomain of syndecan-4 could shed from the membrane under oxidative stress and inflammation [16, 17]. Sheded heparan sulfate-substituted syndecan-4 ectodomains in turn increased ICAM-1, VCAM1, TNFα and IL-1β expression and NF-κB activation which all participate in the progression of RA, suggesting positive feedback regulation of inflammatory pathways [18]. TNF alpha inhibitors may control syndecan-4 [19]. It needs further investigation.
The exact pathways and mechanisms by which syndecan-4 contribute to inflammation and joint destruction in RA has emerged. Syndecan-4 was required for the action of tenascin-C which was identified as a major target of autoantibodies in RA and might regulate the pannus invasion into cartilage [20]. Inhibition of syndecan-4 suppressed tenascin-C activity and over-expression of syndecan-4 enhanced the effects of tenascin-C. Tenascin-C and syndecan-4 might work together to control fibroblast morphology and regulate matrix contraction [21]. Dimerisation of syndecan-4 is critically involved in IL-1 signal transduction and IL-1R1 surface presentation via caveolin-dependent mechanisms both in vitro and an animal model of human RA [8]. A most recent published study demonstrated syndecan-4 silencing significantly suppressed the levels of reactive oxygen species (ROS), nitric oxide (NO) and inflammation and promoted the apoptosis of RA‐FLSs. The apoptosis of RA‐fibroblast-like synoviocytes (FLSs) regulated by syndecan-4 was mainly through the intrinsic pathway in which the P53, Caspase‐3, B‐cell leukemia/lymphoma 2 (Bcl2), and Bcl2‐associated X protein (Bax) were involved. It provided evidence that Syndecan-4 mediated the inflammation and apoptosis of RA‐FLSs by mediating the ROS and NO release [10].
We found the concentration of syndecan-4 was significantly lower in SF than in serum both in RA patients and in OA patients. The underlying mechanism remains unclear. The Syndecan-4 in SF and serum may be originated from different sources. Syndecan-4 is expressed ubiquitously by a variety of cells, including mesenchymal stem cells, epithelial cells, endothelial cells, fibroblast, and ect [22]. The cartilage degradation is an important source of Syndecan-4 in SF. Unfortunately, we did not investigate the cartilage inflammation and cartilage degradation of these patients. Although the SF syndecan-4 concentration was low, it was positively correlated with RA composite disease activity, as well as TJC, SJC and CRP. The correlation coefficents (r value) of syndecan-4 in SF with disease activity was even higher than that of syndecan-4 in sera with disease activity.
We also found that the serum syndecan-4 level was significantly higher in RF positive than in RF negative RA patients. This might suggest the involvement of syndecan-4 in the humoral immunity. Endo T et al. found that syndecan-4 deficient mice had reduced numbers and migration function of B cells, causing deficient germinal center (GC) formation in draining lymph nodes, suggesting that syndecan-4 may contributed to the development of CIA by promoting GC formation [7].
Syndecan-4 is one of the receptors of a proliferation inducing ligand (APRIL), which has been proved to be a critical signal for plasmablasts survival and an important molecular in the regulation of maturation and differentiation of B cells [23]. It role of APRIL on the pathogenesis of RA had been revealed [24]. Our previous study also demonstrated that the serum APRIL was higher in RA than in OA patients, higher in sero-positive RA patients than in sero-negative ones, and more infiltration of APRIL positive cells in RA synovium than in OA patients [25]. The underlying specific mechanisms require further investigation.
We found two outliers in Fig. 3, indicating patients with remarkably high levels of syndecan-4 in the remission or low disease activity groups. This may be related with cartilage degradation. Syndecan-4 is involved in the degradation of cartilage, possibly, by upregulating the expression of MMP-3 and the activity of ADAMTS5 [14].
Interestingly, we found the serum syndecan-4 concentration was higher in OA patients than healthy controls. OA is degenerative joint disease characterized by progressive loss of cartilage matrix and cartilage clefts. Studies have demonstrated the involvement of syndecan-4 in the pathogenesis of OA [5, 14, 26]. We found syndecan-4 expression in synovium was similar in RA and OA, suggesting striking similarities in the pathogenesis of these two diseases. Whereas, Godmann et al. observed dramatically higher expression of syndecan-4 in synovium of RA than OA [8]. This may be due to different degrees of joint inflammation or bone/cartilage destruction among patients.
There were several limitations in our study. First, Syndecan-4 is involved in the degradation of cartilage, but we did not investigate the cartilage inflammation and cartilage degradation. Further research must be conducted. Second, limited samples in the anti-CCP antibody negative group. This may explain why the concentration of Syndecan-4 in the anti-CCP negative group was not significantly different from the concentration in anti-CCP-positive RA patients..
Conclusions
The serum syndecan-4 concentration is higher in RA patients than in OA patients and healthy controls, and significantly higher in RF-positive RA patients than in RF-negative ones. Serum and SF syndecan-4 concentrations were both positively correlated with the disease activity of RA patients. Although the role of syndecan-4 in RA remains elusive, it should be given more attention and prioritised.
Availability of data and materials
We will provide all the data and materials if needed.
References
Kaneider NC, Feistritzer C, Gritti D, Mosheimer BA, Ricevuti G, Patsch JR, et al. Expression and function of syndecan-4 in human platelets. Thromb Haemost. 2005;93(6):1120–7.
Leonova EI, Galzitskaya OV. Role of syndecans in lipid metabolism and human diseases. Adv Exp Med Biol. 2015;855:241–58.
Strand ME, Herum KM, Rana ZA, Skrbic B, Askevold ET, Dahl CP, et al. Innate immune signaling induces expression and shedding of the heparan sulfate proteoglycan syndecan-4 in cardiac fibroblasts and myocytes, affecting inflammation in the pressure-overloaded heart. FEBS J. 2013;280(10):2228–47.
Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-α and IL-1β promote a disintegrin- like and metalloprotease with thrombospondin type I motif-5- mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286(46):39738–49.
Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15(9):1072–6.
Korb-Pap A, Stratis A, Mühlenberg K, Niederreiter B, Hayer S, Echtermeyer F, et al. Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Ann Rheum Dis. 2012;71(6):1004–11.
Endo T, Ito K, Morimoto J, Kanayama M, Ota D, Ikesue M, et al. Syndecan 4 regulation of the development of autoimmune arthritis in mice by modulating B cell migration and germinal center formation. Arthritis Rheumatol. 2015;67(9):2512–22.
Godmann L, Bollmann M, Korb-Pap A, König U, Sherwood J, Beckmann D, et al. Antibody-mediated inhibition of syndecan-4 dimerisation reduces interleukin (IL)-1 receptor trafficking and signalling. Ann Rheum Dis. 2020;79(4):481–9.
Doody KM, Stanford SM, Sacchetti C, Svensson MN, Coles CH, Mitakidis N, et al. Targeting phosphatase-dependent proteoglycan switch for rheumatoid arthritis therapy. Sci Transl Med. 2015. https://doi.org/10.1126/scitranslmed.aaa4616.
Cai P, Lu Z, Jiang T, Wang Z, Yang Y, Zheng L, et al. Syndecan-4 involves in the pathogenesis of rheumatoid arthritis by regulating the inflammatory response and apoptosis of fibroblast-like synoviocytes. J Cell Physiol. 2020;235(2):1746–58.
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum. 1986;29(8):1039–49.
Altman R, Alarcón G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American college of rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33(11):1601–10.
Roskams T, De Vos R, David G, Van Damme B, Desmet V. Heparan sulphate proteoglycan expression in human primary liver tumours. J Pathol. 1998;185(3):290–7.
Zhou K, He S, Yu H, Pei F, Zhou Z. Inhibition of syndecan-4 reduces cartilage degradation in murine models of osteoarthritis through the downregulation of HIF-2α by miR-96-5p. Lab Invest. 2021;101(8):1060–70.
Murdaca G, Colombo BM, Puppo F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern Emerg Med. 2011;6(6):487–95.
Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, et al. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284(6):3537–45.
Kliment CR, Oury TD. Extracellular superoxide dismutase protects cardiovascular syndecan-1 from oxidative shedding. Free Radic Biol Med. 2011;50(9):1075–80.
Strand ME, Aronsen JM, Braathen B, Sjaastad I, Kvaløy H, Tønnessen T, et al. Shedding of syndecan-4 promotes immune cell recruitment and mitigates cardiac dysfunction after lipopolysaccharide challenge in mice. J Mol Cell Cardiol. 2015;88:133–44.
Murdaca G, Colombo BM, Cagnati P, Gulli R, Spanò F, Puppo F. Update upon efficacy and safety of TNF-α inhibitors. Expert Opin Drug Saf. 2012;11(1):1–5.
Schwenzer A, Jiang X, Mikuls TR, Payne JB, Sayles HR, Quirke AM. Identification of an immunodominant peptide from citrullinated tenascin-C as a major target for autoantibodies in rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1876–83.
Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell. 2004;15(12):5670–7.
Xian X, Gopal S, Couchman JR. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010;339(1):31–46.
Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):235–46.
Nagatani K, Itoh K, Nakajima K, Kuroki H, Katsuragawa Y, Mochizuki M, et al. Rheumatoid arthritis fibroblast-like synoviocytes express BCMA and are stimulated by APRIL. Arthritis Rheum. 2007;56(11):3554–63.
Zhao J, Guo J, Wang L, Zhou W, Zhang Z. The role of a proliferation inducing ligand (APRIL) in the pathogenesis of Rheumatoid Arthritis. Scand J Rheumatol. 2014;43(6):462–9.
Chanalaris A, Clarke H, Guimond SE, Vincent TL, Turnbull JE, Troeberg L. Heparan sulfate proteoglycan synthesis is dysregulated in human osteoarthritic cartilage. Am J Pathol. 2019;189(3):632–47.
Acknowledgements
We acknowledged Yuhang Liu, Xiaojun Meng for the physical examination and disease activity evaluation of subjects, Li Wang for the sera and SF samples collection. The authors thank all the participating patients in this study.
Funding
The study was supported by National Natural Science Foundation of China (No. 81971524, 81901642).
Author information
Authors and Affiliations
Contributions
JZ and XY performed the laboratory test and analyzed the related data, JZ collected and analyzed the clinical data and wrote the paper. ZZ was the co-designer of the research and took the responsibility of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Consent to participate
This trial was approved by the Peking University Ethics Committee.
Consent for publication
The submission was agreed by all the authors and none of the authors has any potential financial conflict of interest related to this manuscript.
Informed consent
All the participants gave written informed consent.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. The comparison of syndencan-4 concentrations in serum and SF samples from RA patients and OA patients. RA: rheumatoid arthritis; OA: osteoarthriits; SF: synovial fluid. Figure S2. Immunofluorescence: Syndecan-4 can be expressed in the cultured synovial fibroblast of RA and OA patients. The fluorescence intensity was similar between RA and OA patients.RA: rheumatoid arthritis; OA: osteoarthritis. Figure S3. The comparison of serum syndecan-4 between sera-positive and sera-negative RA patients. RA: rheumatoid arthritis. RF: rheumatoid factor; anti-CCP: anti-cyclic citrullinated peptide. Figure S4. plotted correlation graphs of serum syndecn-4 concentration with disease activity in 60 RA patients. DAS: disease activity; SDAI: simplified disease activity index; CDAI: clinical disease activity index. Table S1. The comparison of mean area, mean diameter, mean density and mean integrated optical density (IOD) of synovia immunohistochemistry in RA and OA patients. Table S2. The correlation of syndecn-4 concentration in SF with disease activity in 25 RA patients having SF samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, J., Ye, X. & Zhang, Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol 62, 21 (2022). https://doi.org/10.1186/s42358-022-00254-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-022-00254-3