Abstract
Background
Multiple myeloma (MM) is characterized by infiltration of neoplastic plasma cells in the bone marrow. Although many novel agents have been developed in the last decade, MM remains a non-curable disease. The association between bone marrow fibrosis (BMF) and MM survival is unknown, and the considerable changes in patient survival during the last few decades necessitates new studies to examine survival and associated factors in patients with MM.
Results
A total of 72 patients with MM, 39 (54.17%) males and 33 (45.83%) females, were included in this retrospective study. Fifteen patients did not have BMF, 55 had BMF (grades 1–4); there were no significant differences between these groups in terms of any of the parameters examined. The 5-year overall survival (OS) rate was 56.5 ± 7.4%. Mean OS was 81.54 ± 7.01 months, mean progression-free survival (PFS) after first-line treatment was 14.07 ± 2.54 months, and mean PFS after autologous stem cell transplantation (ASCT) was 25.92 ± 3.66 months. Survival times or mortality risk were not found to be associated with BMF in any of the analyses (HR 1.208, [95% CI 0.408–3.578], p = 0.733). Mortality risk was increased by 8.163-fold in patients with hypercalcemia (HR 8.163, 95% CI 2.413–27.617, p = 0.001), while it was decreased by 0.243-fold in patients with favourable response to first-line treatment (HR 0.243, 95% CI 0.078–0.756, p = 0.015). Younger patients (< 60 years) had a 1.981-fold greater risk of progression after first-line treatment (HR 1.981, 95% CI 1.111–3.532, p = 0.021), while those with hypercalcemia had a 3.160-fold greater risk of progression after ASCT (HR 3.160, 95% CI 1.103–9.052, p = 0.032). Low haemoglobin levels were also associated with increased mortality risk (p = 0.024).
Conclusion
Although hypercalcemia, unfavourable treatment response, young age and a low haemoglobin level were found to be indicators of poor prognosis in patients with MM, no relationship was found between BMF and survival.
Similar content being viewed by others
Background
Multiple myeloma (MM) is a monoclonal plasma cell disease characterized by lytic bone lesions, anaemia, hypercalcemia and renal failure (Rajkumar 2011). MM accounts for 1% of all malignancies and approximately 10% of haematological malignancies and is the second most frequently diagnosed haematologic malignancy (Rajkumar 2019; Siegel et al. 2015). It has been reported that the age of onset of MM is about 66 years and it is mostly seen in males (Kyle et al. 2003).
Prolonging the time to disease progression in patients with newly diagnosed MM is currently the primary treatment goal (Fonseca et al. 2017). The treatment steps of MM consist of important stages such as initial (first-line) treatment, stem cell transplantation if appropriate, consolidation / maintenance treatment, and treatment for relapse (Rajkumar and Kyle 2005). Treatment paradigms and outcomes for patients with MM have changed dramatically, with more effective and less toxic therapeutic agents recently introduced (Kumar et al. 2014). One of the most important parameters that change positively in this process is the survival time of patients with MM (Pulte et al. 2015).
Although the average survival time in MM is approximately 5–7 years, large differences in survival may occur depending on various factors (Rajkumar and Kumar 2016). The stage of the disease, cytogenetic abnormalities and response to treatment are some of the factors that affect survival. In patients with MM, increased bone marrow fibrosis (BMF) has also been reported as one of the factors that reduce the survival time (Subramanian et al. 2007). Patients with MM with BMF (especially those with extensive BMF) have a worse prognosis even when treated with immunomodulatory agents and proteasome inhibitors (Paul et al. 2020). Understanding the changes in the bone marrow microenvironment and the prognostic implications of these changes is of great importance to further improve the efficacy of myeloma treatment and outcomes for patients with MM. However, few studies have evaluated the relationship between BMF and disease prognosis and survival in patients with MM (Rajkumar 2016; S Vincent Rajkumar et al. 2014a, b).
Herein, our aims were to report the incidence and grade of BMF among patients with MM and to investigate whether presence or degree of BMF was associated with survival duration. In addition, we sought to describe the demographic, clinical and laboratory characteristics of patients with MM and to determine factors independently associated with survival times and survival rates.
Methods
Study plan and data collection
Our study was carried out with a pre-specified plan to include all eligible patients who were followed up with a diagnosis of MM in University of Health Sciences Prof. Dr. Cemil Taşçıoğlu City Hospital and Ege University Medical Faculty Hospital. The study was approved by the Ethics Committee of the Okmeydani Training and Research Hospital (No: 1389, Date: 06/08/2019). Informed consent was obtained from all individual participants included in the study.
Within the scope of the study, the medical records of patients who were diagnosed with MM between 2008 and 2019 in two institutions were evaluated retrospectively. Data of patients aged over 18 years who met the diagnostic criteria for MM and were evaluated for BMF in bone marrow biopsies, were included in the study. Patients diagnosed as having advanced heart failure, decompensated liver cirrhosis and advanced lung disease were not included in the study group.
Data on demographic characteristics, myeloma type, myeloma stage, treatment protocols, date of diagnosis, genetic mutation status, presence of hypercalcemia, anaemia or lytic lesion at diagnosis, serum lactate dehydrogenase (LDH) and creatinine levels, concomitant diseases, presence of concomitant plasmacytoma, autologous transplant status were recorded from the patients’ medical records retrospectively.
Outcome measures
The primary outcomes of this study were to assess the incidence and grade of BMF and to determine whether BMF was associated with survival in patients with MM. Secondary outcomes included the assessment of demographic, clinical and laboratory characteristics of patients with MM, and to determine factors independently associated with survival times and survival rates.
Diagnosis and staging of multiple myeloma
The Updated International Myeloma Study Group MM diagnostic criteria were used to diagnose MM. Accordingly, a bone marrow clonal plasma cell ratio of ≥ 10% or the presence of biopsy-proven plasmacytoma together with one or more myeloma-defining events is required for the diagnosis of MM in bone marrow evaluation. Myeloma defining events consist of hypercalcemia, renal failure, anaemia, lytic bone lesions (CRAB findings) and three specific biomarkers [clonal bone marrow plasma cells ≥ 60%, serum free light chain ratio ≥ 100, multiple focal lesions on magnetic resonance imaging (MRI). In the absence of myeloma-defining events, at least one of the SLiM criteria is required (S: bone marrow clonal plasma cell ratio ≥ 60%, Li: affected/unaffected serum free light chain ratio ≥ 100, M: Presence of multiple focal lesions of 5 mm or larger on whole-body MRI) (Rajkumar 2016; S. V. Rajkumar et al. 2014a, b).
The Durie-Salmon staging system was used for the staging of MM. In this staging system developed by Durie and Salmon in 1975, disease stage is determined by evaluating factors such as haemoglobin, calcium and lytic bone lesions (Durie et al. 2015).
Treatments, response and prognosis
The chemotherapy regimens used in the majority of patients with MM were Vincristine-Adriamycin-Dexamethasone (VAD), Bortezomib-Cyclophosphamide-Dexamethasone (VCD), Bortezomib-Melphalan-Dexamethasone (VMP), Bortezomib-Dexamethasone (BD), Revlimid-Dexamethasone (RD), Lenalidomide-Cyclophosphamide-Dexamethasone (LCD) and Velcade-Lenalidomide-Dexamethasone (VRD).
Responses to treatments and allogeneic stem cell transplantation in patients with MM were grouped as progressive disease (PD), stable disease (SD), partial response (PR), very good partial response (VGPR) and complete response (CR), as defined by the International Myeloma Working Group (IMWG).
Prognosis was assessed as duration (months) of overall survival (OS), progression-free survival (PFS), and the percentage of patients who were alive at 5 years (5-year OS) and at the end of the study.
Evaluation of bone marrow fibrosis
The presence of fibrosis in the bone marrow was evaluated in four groups (Grade 0: No reticulin fibres visible, Grade 1: Occasional fine individual fibres and fine mesh foci, Grade 2: Fine fibre network throughout most of the section; no coarse fibres, Grade 3: Messy fibre network with scattered thick coarse fibres but no mature collagen, Grade 4: Wide, thick fibre network with areas of collagenisation) (Bain et al. 2019). During the analyses, two groups were formed among the patients in terms of BMF, Grade 0 and Grades 1–2-3–4, and comparisons were performed.
Statistical analysis
All analyses were performed using the SPSS v21 statistical software package (SPSS Inc., Chicago, IL, USA). Prior to the analysis we assessed the distribution of key variables for normality. According to the normality of the distribution, continuous data are given as mean ± standard deviation or median (minimum–maximum). Categorical data is summarized with frequency and relative percentage. Between-group comparisons of the continuous variables were performed with the independent samples t-test or the Mann–Whitney U test depending on normality of distribution. Between-group comparisons of categorical variables were performed with appropriate chi-square tests or the Fisher’s exact test. Survival times were calculated using the Kaplan–Meier method. Comparisons of survival times between groups were performed using the log-rank test. Cox regression analysis (forward conditional method) was utilized to identify factors independently associated with prognosis. Statistical significance value was accepted as p < 0.05.
Results
Demographic and clinical characteristics
A total of 72 patients with MM were included in our study, 39 (54.17%) were male and 33 (45.83%) were female. The mean age of the patients was 62.43 ± 13.41 (range 34–87) years. The most common comorbidity in the patients was hypertension, the most common type of MM was immunoglobulin (Ig)-G kappa and the most common stage was stage-III (A/B). Genetic mutation evaluation was performed in 38.89% (n = 28) of the patients and the most common genetic mutation was found to be del 17p (10.71%). ASCT had been performed in 49.3% (n = 35) of the patients. Mortality rate was found to be 33.33% (n = 24) (Table 1).
Bone marrow fibrosis
Any-grade BMF was present in 55 (78.57%) patients, while 15 (21.43%) patients did not have fibrosis. When reported according to grades, Grade 1 fibrosis was identified in 31 (44.29%), Grade 2 was identified in 15 (21.43%), Grade 3 was identified in 8 (11.43%), and Grade 4 was identified in 1 (1.43%) patient(s). The comparison of patients without BMF (Grade 0) and those with any degree of fibrosis (Grades 1–2–3–4) did not reveal any significant differences between the groups, including disease staging, clinical characteristics, treatment(s), treatment response(s), progression after therapy, and survival rates (1–5 years and study duration).
Overall survival
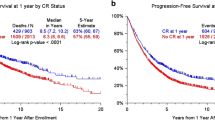
Five-year OS rate was determined to be 56.5 ± 7.4% and mean OS was 81.54 ± 7.01 (95.% CI: 67.80–95.29) months. No significant associations were found between OS duration and age, sex, comorbidity, MM type, lytic lesion, plasmacytoma and BMF grade (Table 2). The OS of patients in stage III (A/B) (62.21 ± 7.53 [95% CI 47.46–76.96]) was lower than in other stages (117.77 ± 6.95 [95% CI 104.15–131.39], p = 0.003). The OS of patients with hypercalcemia (32.94 ± 7.90 [95% CI 17.46–48.43], p = 0.023) and high creatinine level (39.07 ± 7.18 [95% CI 25.00–53.13], p = 0.012) was found to be significantly lower than those without. The 5-year OS rates of patients with VGPR or CR to first-line therapy (78.5 ± 8.8, p = 0.003) and those with ASCT (73.6 ± 8.8, p = 0.007) were significantly higher than others. BMF was not associated with OS (HR 1.208, [95% CI 0.408–3.578], p = 0.733) (Table 2, Fig. 1).
Cox regression analysis was performed and hypercalcemia and low haemoglobin level were found as poor prognostic factors, while having VGPR or CR after first-line treatment were found as good prognostic factors. Patients with hypercalcemia had a mortality risk 8.163 times higher than other patients (HR 8.163, 95% CI 2.413–27.617, p = 0.001). Patients whose response to first-line treatment was VGPR or CR had a 0.243-fold lower mortality risk than other patients (HR 0.243, 95% CI 0.078–0.756, p = 0.015). In addition, a low haemoglobin level was found to be associated with an increased risk of mortality (p = 0.024). BMF was not found to be independently associated with mortality (Table 3, Figs. 2, 3).
Progression-free survival
The mean PFS in the study group after first-line treatment was 14.07 ± 2.54 months. No association was found between post-treatment PFS and sex, presence of comorbidity, presence of lytic lesion, presence of plasmacytoma, hypercalcemia, high creatinine and BMF level. The duration of PFS was significantly shorter in those younger than 60 years (7.34 ± 2.13 [95% CI 3.17–11.52], p = 0.011), those who had heavy chain type (9.89 ± 1.99 [95% CI 6.00–13.78], p = 0.007) and those in stage III (A/B) (8.82 ± 2.55 [95% CI 3.82–13.82], p = 0.024). BMF was not associated with PFS (Table 4, Fig. 4).
In Cox regression analysis, younger patients (< 60 years) were found to have a 1.981-fold greater risk of progression after first-line treatment than older patients (HR 1.981, 95 CI%: 1.111–3.532, p = 0.021) (Table 5, Fig. 5).
When PFS values after ASCT were assessed, we found that mean PFS was 25.92 ± 3.66 months after ASCT in patients with MM. In the presence of hypercalcemia, the mean PFS was found to be significantly shorter after ASCT (p = 0.021) (Table 6, Fig. 6). Patients with hypercalcemia had a 3.160-fold greater risk of progression after ASCT compared to those without hypercalcemia (HR 3.160, 95% CI 1.103–9.052, p = 0.032) (Table 7, Fig. 7).
Discussion
New therapeutic agents and treatment modalities in MM have achieved significantly longer OS in the last two decades (Landgren and Rajkumar 2016). Despite these advances, MM is not a curable disease and it is assumed that the disease will relapse in every patient. The OS in patients with MM was reported as 33 months before the 2000s, whereas today the average survival is 5–7 years (Paul et al. 2020; Rajkumar and Kumar 2016).
In our study, any-grade BMF was found to be present in 78.57% of our patients. The presence or absence of BMF and its grade were not associated with any of the parameters examined. The mean OS in patients with MM was 81.54 ± 7.01 months, similar to the range reported in the literature. In addition, the mean PFS was 14.07 ± 2.54 months after first-line therapy and 25.92 ± 3.66 months after ASCT. Survival duration and survival rates were not found to be associated with BMF presence or degree. Cox regression with mortality as the dependent variable revealed that mortality risk was significantly increased in the presence of hypercalcemia (8.163-fold) and significantly decreased in patients with VGPR or CR to first-line therapy (0.243-fold). Low haemoglobin was also independently associated with higher risk of mortality. PFS was independently associated with age, with patients younger than 60 years of age demonstrating shorter time until progression. When patients with ASCT were examined, hypercalcemia was again found to be associated with shorter PFS and higher likelihood of progression (3.160-fold).
Estimates of survival in patients with MM may vary depending on the source of the data and can be affected by the age of the patients, along with many other factors (Rajkumar 2020). In previous studies, younger patients were reported to have better OS (Durie et al. 2015; Kaya et al. 2012; Yusuf et al. 2016) and it was reported that a 1-year increase in age increased the risk of mortality 1.03 times (Paul et al. 2020). In this study, we found that OS did not differ according to age groups, while PFS was shorter in younger patients. The characteristics of the patients in the study group in terms of comorbidity and performance may have caused this result.
As expected survival in MM is affected by the response to treatment (Rajkumar 2020) and CR to treatment was reported to be an independent predictive factor for increased OS and PFS times (Babarović et al. 2012; Gay et al. 2011). Similarly, in our study, mortality was found to be lower in patients with at least a VGPR response to treatment. This relationship we found between treatment response and survival may be a guide for new treatment strategies. Obtaining a deep response with strong treatments in the early step will contribute to OS.
In patients with myeloma bone disease, together with the lack of bone formation, excessive calcium release occurs as a result of excessive bone resorption, leading to hypercalcemia (Walker et al. 2014) and hypercalcemia is one of the defining symptoms of MM (Rajkumar 2016). The prevalence of hypercalcemia has been reported as 9–19.5% in different studies (Yusuf et al. 2016; Zagouri et al. 2017) and has been associated with increased mortality risk and shorter survival time (Bao et al. 2020; Kastritis et al. 2011). In our study group, hypercalcemia was detected in 13.24% of patients with MM. Hypercalcemia was found to increase the risk of mortality (p = 0.023) and progression after ASCT (p = 0.032).
Anaemia, which is associated with poor quality of life, decreased performance, hypoxia and ischaemic complications in patients with MM, has been reported to be one of the poor prognostic factors (Caro et al. 2001; Mittelman 2003). These effects become even more important given that most patients with MM are older (Mittelman 2003). We found that low haemoglobin levels increased the risk of mortality, consistent with the literature.
It has been reported that the bone marrow microenvironment, consisting of extracellular matrix proteins, cytokines/chemokines, bone marrow stromal cells, mesenchymal stem cells, osteoblasts and osteoclasts, inflammatory cells, megakaryocytes and microvessels, plays an important role in the survival, clonal evolution of myeloma cells, and the development of drug resistance (Paul et al. 2020). Reticulin is a normal component of the bone marrow microenvironment and can be increased in many malignant and non-malignant diseases. BMF occurs with the deposition of reticulin or collagen in the bone marrow stromal environment (Paul et al. 2020). Although the frequency of BMF has been reported as 30%–38% in previous studies (Hallgrimsdottir et al. 2013; Singhal et al. 2004), in our patient group, the rate was quite high (78.57%) compared with other studies. The reason for this is that patients who were evaluated for BMF were included in the study. In addition, the staging systems and the treatment regimens used in the studies were not similar, which may have caused the differences between the results. In the study of Paul et al., it was reported that patients with BMF had significantly shorter OS and PFS. In their univariate analysis, it was reported that BMF was significantly associated with both survival times in patients with MM, but this effect was not preserved in the multivariate analysis (Paul et al. 2020). On the other hand, there are also studies reporting that no relationship was found between BMF and OS and PFS in patients with MM. In our study group, no association was found between the level of BMF and OS and PFS of patients with MM.
Limitations
The most important limitation of the study is its retrospective design. Data from patients with MM who were not examined for BMF could have yielded different results. However, this factor can only be controlled in a prospective study. Another limitation of our study is that we did not use a more up-to-date staging system than the Durie-Salmon system for staging MM. The reason for this was the inclusion of patients from the past. The Durie-Salmon staging system was used because this is the only classification system that can be used for both old and new patients. Despite these limitations, our study is valuable in that it includes detailed analyses of patient data from two major centres over a 12-year period and also shares the results of BMF evaluated by limited studies.
Conclusions
As a result of the analyses, BMF was found to be common among patients with MM (78.57%), but its presence or degree was not associated with survival. The mean OS in patients with MM was 81.54 ± 7.01 months, the mean PFS was 14.07 ± 2.54 months after first-line therapy and 25.92 ± 3.66 months after ASCT. It was determined that hypercalcemia and low haemoglobin levels increased the risk of mortality and favourable treatment response decreased the risk of mortality. Being younger (< 60 years) was to be associated with increased risk of progression after first-line therapy. Hypercalcemia presence increased the risk of progression after ASCT. Patients with MM should be closely monitored in terms of hypercalcemia, low haemoglobin and poor treatment response, which adversely affect survival. Prospective studies that determine the effect of BMF on survival and prognosis in patients with MM will be beneficial.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- MM:
-
Multiple myeloma
- BMF:
-
Bone marrow fibrosis
- LDH:
-
Lactate dehydrogenase
- CRAB:
-
Consist of hypercalcemia, renal failure, anaemia, lytic bone lesions
- MRI:
-
Magnetic resonance imaging
- VAD:
-
Vincristine-Adriamycin-Dexamethasone
- VMP:
-
Bortezomib-Melphalan-Dexamethasone
- BD:
-
Bortezomib-Dexamethasone
- RD:
-
Revlimid-Dexamethasone
- LCD:
-
Lenalidomide-Cyclophosphamide-Dexamethasone
- VRD:
-
Velcade-Lenalidomide-Dexamethasone
- PD:
-
Progressive disease
- SD:
-
Stable disease
- PR:
-
Partial response
- VGPR:
-
Very good partial response
- CR:
-
Complete response
- IMWG:
-
International Myeloma Working Group
References
Babarović E, Valković T, Štifter S, Budisavljević I, Seili-Bekafigo I, Duletić-Načinović A, Lučin K, Jonjić N (2012) Assessment of bone marrow fibrosis and angiogenesis in monitoring patients with multiple myeloma. Am J Clin Pathol 137(6):870–878
Bain BJ, Clark DM, Wilkins BS (2019) Bone marrow pathology. Wiley, Oxford
Bao L, Wang Y, Lu M, Chu B, Shi L, Gao S, Fang L, Xiang Q (2020) Hypercalcemia caused by humoral effects and bone damage indicate poor outcomes in newly diagnosed multiple myeloma patients. Cancer Med 9(23):8962–8969
Caro JJ, Salas M, Ward A, Goss G (2001) Anemia as an independent prognostic factor for survival in patients with cancer: a systematic, quantitative review. Cancer 91(12):2214–2221
Durie B, Hoering A, Rajkumar SV, Abidi MH, Epstein J, Kahanic SP, Thakuri MC, Reu FJ, Reynolds CM, Sexton R (2015) Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT): results of the randomized phase III trial SWOG S0777. Blood 126(23):25
Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L, Richardson P (2017) Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia 31(9):1915–1921
Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R, Genuardi M, Romano A, Liberati AM, Siniscalchi A (2011) Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood 117(11):3025–3031
Hallgrimsdottir T, Porwit A, Björkholm M, Rossmann E, Steingrimsdottir H, Lund SH, Kristinsson SY (2013) Bone marrow fibrosis in patients with multiple myeloma: a new prognostic factor for survival? Blood 122(21):1946
Kastritis E, Katodritou E, Pouli A, Hatzimichael E, Delimpasi S, Michalis E, Zomas A, Kartasis Z, Parcharidou A, Gika D (2011) Frequency and prognostic significance of hypercalcemia in patients with multiple myeloma: an analysis of the database of the Greek Myeloma Study Group. Blood 118(21):5083
Kaya H, Peressini B, Jawed I, Martincic D, Elaimy AL, Lamoreaux WT, Fairbanks RK, Weeks KA, Lee CM (2012) Impact of age, race and decade of treatment on overall survival in a critical population analysis of 40,000 multiple myeloma patients. Int J Hematol 95(1):64–70
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N (2014) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28(5):1122–1128
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR (2003) Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78(1):21–33
Landgren O, Rajkumar SV (2016) New developments in diagnosis, prognosis, and assessment of response in multiple myeloma. Clin Cancer Res 22(22):5428–5433
Mittelman M (2003) The implications of anemia in multiple myeloma. Clin Lymphoma 4:S23–S29
Paul B, Zhao Y, Loitsch G, Feinberg D, Mathews P, Barak I, Dupuis M, Li Z, Rein L, Wang E (2020) The impact of bone marrow fibrosis and JAK2 expression on clinical outcomes in patients with newly diagnosed multiple myeloma treated with immunomodulatory agents and/or proteasome inhibitors. Cancer Med 9(16):5869–5880
Pulte D, Jansen L, Castro FA, Emrich K, Katalinic A, Holleczek B, Brenner H, Group GCSW (2015) Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br J Haematol 171(2):189–196
Rajkumar SV (2011) Treatment of multiple myeloma. Nat Rev Clin Oncol 8(8):479–491
Rajkumar SV (2016) Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 91(7):719–734
Rajkumar SV (2019) Multiple myeloma: Every year a new standard? Hematol Oncol 37:62–65
Rajkumar SV (2020) Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol 95(5):548–567
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, Kumar S, Hillengass J, Kastritis E, Richardson P (2014a) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15(12):e538-548
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF (2014b) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15(12):e538-548
Rajkumar SV, Kumar S (2016) Multiple myeloma: diagnosis and treatment. Mayo Clin Proc 91(1):101–119
Rajkumar SV, Kyle RA (2005) Multiple myeloma: diagnosis and treatment. Mayo Clin Proc 80(10):1371–1382
Siegel RL, Miller KD, Jemal A (2015) (2015) Cancer statistics. CA Cancer J Clin 65(1):5–29
Singhal N, Singh T, Singh ZN, Shome D, Gaiha M (2004) Histomorphology of multiple myeloma on bone marrow biopsy. Indian J Pathol Microbiol 47(3):359–363
Subramanian R, Basu D, Dutta TK (2007) Significance of bone marrow fibrosis in multiple myeloma. Pathology 39(5):512–515
Walker RE, Lawson MA, Buckle CH, Snowden JA, Chantry AD (2014) Myeloma bone disease: pathogenesis, current treatments and future targets. Br Med Bull 111(1):117–138
Yusuf AA, Natwick T, Werther W, Felici D, Mahue M, Bridges KR, Peng Y (2016) A retrospective analysis to examine factors associated with mortality in Medicare beneficiaries newly diagnosed with multiple myeloma. Curr Med Res Opin 32(12):1989–1996
Zagouri F, Kastritis E, Zomas A, Terpos E, Katodritou E, Symeonidis A, Delimpasi S, Pouli A, Vassilakopoulos TP, Michalis E (2017) Hypercalcemia remains an adverse prognostic factor for newly diagnosed multiple myeloma patients in the era of novel antimyeloma therapies. Eur J Haematol 99(5):409–414
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
EED contributed to design, data collection, data analyses, results, discussion, literature review and writing—editing; AA contributed to data collection, data analyses; ND contributed to data collection, data analyses, results; DA contributed to data collection, data analyses, results; IM contributed to data collection, discussion and writing—editing; SA contributed to data collection, data analyses, results; FKK contributed to data analyses; RE contributed to design, data collection, discussion and literature review; GS contributed to design and data analyses. All authors read and approved the final manuscript for submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Okmeydani Training and Research Hospital (No: 1389, Date: 06/08/2019). Informed consent was obtained from all individual participants included in this study.
Consent for publication
Not applicable.
Competing interests
No competing interest within authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dogan, E.E., Arslan, A., Demirel, N. et al. Survival in patients with multiple myeloma: evaluation of possible associations with bone marrow fibrosis and investigation of factors independently associated with survival. Bull Natl Res Cent 46, 242 (2022). https://doi.org/10.1186/s42269-022-00926-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00926-6