Abstract
High-dose chemotherapy followed by autologous stem cell transplantation (HD-ASCT) as well as the introduction of novel agents (NA) significantly improved survival for patients with multiple myeloma (MM). A total of 150 unselected newly diagnosed MM patients treated at our institution from 1998 to 2017 were retrospectively analyzed. Median age at diagnosis was 69 years (range 33–93 years) with a median follow-up of 48.6 months. The median overall survival (OS) for the entire cohort was 60.7 months (range 0.3–280.1). Patients who received frontline HD-ASCT (p < 0.01) or NA-based first-line treatment (p = 0.043) had a significantly better OS. According to the revised Myeloma Comorbidity Index (R-MCI), patients were defined as fit (36.5%), intermediate-fit (44.5%), or frail (19%) with a significant difference in OS between these categories (p < 0.01). Multivariate analysis revealed R-MCI as an independent prognostic factor for OS (p < 0.01). Presence of subclinical amyloid deposits (A+) was detected in 18 out of 66 patients (27.3%) and significantly correlated with a serum free light chain (sFLC) ratio ≥ 100 (p = 0.01) and bone marrow plasma cell infiltration > 60% (p = 0.04). Furthermore, patients with A+ had significantly worse OS compared with their counterparts (p = 0.048). Our results corroborate the efficacy of both early HD-ASCT and the use of new agents as initial therapy of MM patients in “real-world” daily clinical practice. The R-MCI is an easily applicable tool to stratify MM patients and may support treatment decisions. The prognostic value of subclinical amyloid deposition should be validated within prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is a heterogeneous disease characterized by proliferation of a plasma cell clone and distinct clinical and pathological features. It accounts for approximately 13% of all hematological malignancies and mainly affects the elderly population with a median age at diagnosis of about 65 years [1,2,3]. Introduction of novel agents (NA)–based treatment options improved outcome of patients both eligible and not eligible for high-dose chemotherapy-autologous stem cell transplantation (HD-ASCT) [4,5,6]. However, this improvement might be less pronounced in real life due to strict exclusion criteria used in clinical trials as well as national reimbursement policies of novel agents, and data from unselected MM patients in the era of NA are limited.
It is well known that comorbidities have substantial impact on prognosis in different hematological malignancies [7,8,9,10,11]. The elderly population is highly heterogeneous and risk stratification of myeloma patients for treatment selection is mainly based on disease- or host-specific factors such as the International Staging System (ISS), age, and comorbidities. Recently, Engelhardt et al. developed the revised Myeloma Comorbidity Index (R-MCI) which is a frailty score with simple clinical applicability [12]. Their study demonstrated that the R-MCI allows for the definition of largely different risk groups and represents a valid risk assessment tool in distinct treatment and age groups. However, data on R-MCI and patient outcome in MM patients are limited.
Immunoglobulin light chain (AL) amyloidosis is a rare and serious disease caused by misfolded proteins deriving from monoclonal light chains in patients with an underlying plasma cell disorder [13, 14]. The most commonly applied method for the detection of amyloid deposits is a subcutaneous abdominal fat pad aspirate (SAFA). Following Congo red staining, amyloid fibrils exhibit a characteristic apple-green appearance in fluorescent light microscopy [14,15,16]. Nonetheless, there is still little evidence on coincident AL amyloidosis in patients with MM. Therefore, more information about the frequency of amyloid deposits and its clinical impact are needed.
This retrospective study was performed to review the outcome and prognostic factors in an unselected cohort of MM patients treated at our hemato-oncology day unit over a period of 20 years. In addition, we aimed to describe the prevalence of amyloid deposits as well as its clinical impact in our MM patient cohort.
Methods
Patient cohort
Data collection and analysis were performed anonymously according to current data safety law and Institutional Review Board regulations. All patients with newly diagnosed multiple myeloma treated at the hemato-oncology day unit of the “Franz Tappeiner” hospital in Merano from January 1, 1998, to December 31, 2017, were included in this analysis. The “Franz Tappeiner” hospital is a public regional tertiary care hospital which covers a large area of western South Tyrol. Based on the national regulations, all patients have access to a unique healthcare system which provides universal coverage to all citizens and residents. Therefore, this study represents an unselected heterogeneous population irrespective of age, performance status, and comorbidities. All patients were followed up until June 30, 2018. Clinical data were extracted from electronic clinical records (OncoNet, http://edp-progetti.it, Bolzano, Italy). Correct diagnosis of MM was made according to the 2001–2008 WHO classification and was reviewed by a treating hematologist. Retrieved disease parameters included stages according to the ISS, cytogenetic aberrations, plasma cell infiltration of bone marrow, serum lactate dehydrogenase (LDH), serum creatinine, serum calcium, hemoglobin, and, beginning in 2008, serum free light chain ratios. The comorbidity index R-MCI was determined using a web-based calculator (www.myelomacomorbidityindex.org). High-risk MM based on FISH was defined by the presence of abnormalities such as t(4; 14), t(14; 16), t(14; 20), del(17p), and/or gain of 1q21 [17]. All other cases were considered standard risk. SAFA has been retrieved on a regular basis since 2011, unless the patient did not consent to the procedure. In cases diagnosed before 2011, SAFA has only been obtained by clinical decision upon unclear deterioration in kidney function. Subclinical amyloid deposits were detected by Congo red staining of SAFA.
Treatment was given according to the current recommendations of the National Myeloma Working Group, local approval status, and drug reimbursement policies of novel agents. Treatment characteristics included eligibility for ASCT, first-line treatment with NA-based therapy or conventional chemotherapy, and treatment at relapse. NA included the proteasome inhibitor bortezomib and the immunomodulatory agents (IMiDs) thalidomide and lenalidomide. For treatment with HD-ASCT, patients were referred to the Central Regional Hospital of South Tyrol in Bolzano.
Statistical methods
Statistical analysis was performed using R, version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria) and RStudio, version 1.1.456 (RStudio, Inc., Boston, MA, USA), using the packages “survival” (version 2.38), “survminer” (version 0.4.3) and “gplots” (version 3.0.1). The primary end point was OS, defined as the time between diagnosis and death by any cause or end of follow-up. Survival curves were calculated by the Kaplan-Maier method and compared by the log-rank test. Multivariate analysis was performed using a Cox proportional hazards regression model for OS. Correlations between subclinical amyloid deposits and clinicopathological variables were assessed with the chi-square test or Fisher’s exact test as appropriate. For all analyses, a p value < 0.05 was considered statistically significant. Due to the exploratory, hypothesis-generating design of the study, no correction for multiple testing was applied [18].
Results
Patients’ characteristics
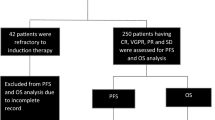
Baseline characteristics of the patients included in the study are summarized in Table 1. A total of 150 patients were diagnosed with MM at our hemato-oncology day unit between January 1, 1998, and December 31, 2017. Patients were regularly followed up with a median follow-up time of 48.6 months. The median age at diagnosis of MM was 69 years (range 33–93 years), while the median age for patients treated with and without HD-ASCT was 57 years (range 33–70) and 73 years (range 41–93), respectively. The male/female ratio was 1.08 (78/150 male, 72/150 female). At the time of analysis, 51/150 (34%) patients were still alive. Briefly, 116/150 patients (77.3%) had a positive serum M-spike, 25 (16.7%) patients had a light chain MM, whereas 6 (4.0%) were non-secretory MM, and 3 (2.0%) patients were classified having a solitary plasmacytoma, respectively. 62/150 (41.3%) patients were staged according to ISS stage I, 32 (21.3%) patients as stage II, and 56 (37.3%) patients as stage III. Cytogenetic studies by fluorescence in situ hybridization (FISH) analysis from bone marrow were performed in 86/150 (57.3%) patients, with 38/86 (44.2%) and 48/86 (55.8%) patients displaying high- and standard-risk aberrations, respectively. Where laboratory parameters were available, elevated levels (> upper limit of normal; ULN) of serum calcium concentration, LDH, and creatinine were found in 7.0%, 25.3%, and 30.7% of patients, respectively. Anemia at diagnosis of MM (as defined by Hb < 12 mg/dL) was present in 60.4% of patients, whereas bone marrow plasma cell infiltration > 60% was observed in 25.4% of patients. In total, a serum free light chain (sFLC) ratio ≥ 100 at diagnosis was detectable in 68.2% of patients. Of the 150 patients included in this retrospective study, data on amyloid deposits were evaluable in 66 (44.0%) patients. Amyloid deposits (A+) were detected by SAFA in 18/66 (27.3%) cases. Of these, all patients at time of diagnosis had MM without clinical signs of AL amyloidosis as previously defined [19], whereas patients with MM and classical symptoms of AL at diagnosis were excluded from the study (n = 6). In 48/66 (72.7%) patients, no amyloid deposits could be detected.
Frequency of comorbidities and R-MCI scoring
In our patient cohort, 92.7% (139/150) of patients had at least one comorbid condition. The most prevalent comorbidities are listed in Table 2. Cardiovascular comorbid conditions were the most frequent, present in 107/150 (71.3%) patients, among which arterial hypertension was the most common (58/150 patients; 38.7%). Kidney disease was observed in 25 (16.7%) patients, while chronic lung disease was present in 7 (4.7%) patients. Of endocrine disorders, diabetes mellitus was diagnosed in 15 (10.0%) patients, while hypothyroidism was identified in 14 (9.3%) patients. Peripheral neuropathy was present in 19 (12.7%) patients. Second primary malignancies were observed in 8 (5.3%) patients of the total group, whereas 14 (9.3%) patients had pre-existing neoplasms other than MM. Most frequent pre-existing neoplasms were prostate (n = 6), breast (n = 3), and colorectal (n = 2) cancers, as well as cervical, lung, and bladder cancers in one patient, each. Second primary malignancies comprised two melanomas and one patient with neoplasms in the liver, hypopharynx, cervix, urinary bladder, thyroid, and kidney, each. No hematological malignancies have been observed. R-MCI calculation was feasible in 137/150 (91.3%) patients, while it could not be evaluated in 13 patients due to missing data. According to the R-MCI, 50/137 patients (36.5%) were defined as fit, 61 patients (44.5%) as intermediate-fit, and 26 patients (19%) as frail. Treatment modalities of different R-MCI subgroups are summarized in the Supplementary Table 1. Briefly, frail patients were more likely to receive no treatment as 6 out of 7 patients that received best supportive care were classified as frail according to R-MCI. In addition, only 1 out of 26 frail patients underwent HD-ASCT.
Treatment characteristics and survival analysis
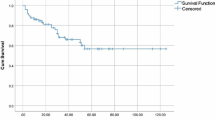
Among the total cohort, 76/150 (50.7%) of patients received NA-based first-line treatment, whereas 63/150 (42.0%) of patients were treated with conventional chemotherapy and 11/150 (7.3%) patients had never been treated or followed by best supportive care. In total, 46/150 (30.7%) patients received HD-ASCT. The 100-day treatment-related mortality of the transplanted patients was 1/46 (2.2%). In accordance with the respective national approval dates of novel compounds, maintenance therapy after ASCT was not performed. In total, 88/150 (58.7%) of patients received second-line treatment with lenalidomide being the most commonly used drug in this setting. Bisphosphonates were given in 114/150 (76%) patients. The median OS for the entire cohort was 60.7 months (range 0.3–280.1; 95% CI 51.7–81.1 months). Univariate survival analysis is summarized in Table 3. Patient age > 70 years, elevated serum creatinine, calcium and LDH levels > ULN, presence of anemia, advanced ISS stage (I vs II vs III), bone marrow plasma cell infiltration > 60%, and an involved-to-uninvolved sFLC ratio ≥ 100 had a negative impact on OS. Of note, median OS for A+ patients was significantly inferior compared with patients without amyloid deposits detected by SAFA (p = 0.048; Fig. 1). Patients with standard-risk cytogenetic abnormalities showed a numerical trend towards better OS as compared with patients with high-risk cytogenetics (p = 0.26). Finally, progressively increasing R-MCI score (fit, intermediate-fit, frail) was associated with worse OS (p < 0.01; Fig. 2). The median OS in these subgroups was 114.7 months, 59.0 months, and 13.3 months, respectively. The median OS reached 70.9 months for patients who received any treatment and was 15.1 months for patients that had never been treated or were followed by best supportive care (data not shown). Overall, patients who received a NA-based first-line therapy had a median OS of 73.1 months (95% CI 49.3–114.2), compared with 58.4 months (95% CI 42.5–76.6) for patients who did not receive NA-based therapy (p = 0.043). As expected, median OS was significantly better for MM patients who have undergone HD-ASCT compared with that for patients who were not eligible for HD-ASCT (114.2 months (95% CI 76.8–153.1) vs. 48.6 months (95% CI 38.0–58.4); p < 0.01). Multivariate analysis was performed using age, serum creatinine and calcium, hemoglobin, ISS, HD-ASCT, the use of NA-based first-line therapy, BM plasma cell infiltration, and R-MCI as covariates, with available data in 116 patients (Table 4). The Cox regression model revealed R-MCI as an independent prognostic factor for OS in our cohort (p < 0.01).
Patient cohort with available data from amyloid deposits
Patients in whom SAFA was performed had significantly better OS than those where the procedure was not performed (p < 0.001; Supplementary Fig. 1). Of note, the majority of patients in whom SAFA was obtained was diagnosed after 2011 and these patients were more likely treated with NA-based therapy (52/66 (78.8%) vs. 24/84 (28.6%), p < 0.001, chi-square test) or were eligible for HD-ASCT (28/66 (42.4%) vs. 18/84 (21.4%), p = 0.01, chi-square test) as compared with patients who were not tested for subclinical amyloid deposits.
Subsequently, a detailed analysis for the subgroup of patients for whom data of amyloid deposits were available was performed. The baseline characteristics of the patients from this subgroup are summarized in Supplementary Table 2. At the time of diagnosis, 17/18 (94.4%) A+ patients had elevated sFLC (with 68% λ), with a median level of 870 mg/L (range 10–3316 mg/L) in A+ patients compared with a median level of 191.5 mg/L (range 9–8578 mg/L) in A− patients, respectively. No correlation was found between the presence of amyloid deposits and age (≤ vs. > 70 years), sex, ISS, and cytogenetic risk stratification as well as normal vs. ULN of hemoglobin, creatinine, calcium, or LDH- and NA-based therapy (data not shown, chi-square/Fisher’s exact test). However, presence of amyloid deposits significantly correlated with a sFLC ratio ≥ 100 (p < 0.01, Fisher’s exact test), percentage of bone marrow plasma cell infiltration (p = 0.04, chi-square test), and eligibility for HD-ASCT (p = 0.012, Fisher’s exact test). During follow-up, 12/18 (66.7%) patients with amyloid deposits developed clinical findings of systemic amyloidosis. In contrast, progression to systemic amyloidosis in patients being negative for AL amyloidosis at the time of diagnosis was observed only in 3/48 (6.3%) cases.
Discussion
In the past decade, major advances have been made in the treatment of MM with the introduction of new active drugs in the transplant as well as non-transplant setting with an improved median survival of 8–10 years [20]. Most published data, however, are derived from patients who were treated within clinical trials but these cohorts represent only a minority of the heterogeneous real-world patient population due to rigorous patient selection criteria. In this regard, the study by Costa et al. [21] reported a significant lower median age (61 years) of subjects enrolled in clinical trials compared with unselected patients with a median age of 69 years diagnosed with MM during the same period. Moreover, trial subjects with untreated MM had less advanced stage than unselected patients and comorbid conditions are often a criterion of exclusion. In addition, access to novel agent–based therapies may be limited by approval status and level of drug reimbursement, which vary through different countries.
The present study examined the clinicopathological features and treatment outcomes of 150 MM patients treated at our central regional hospital in South Tyrol within the period from 1998 to 2017. In addition, we evaluated the impact of subclinical amyloid deposits and comorbidities on OS. Based on this retrospective analysis, the prognosis of patients with MM has improved significantly due to the introduction of NA-based treatment modalities. Bortezomib has been used at our hospital since late 2004 and improved outcomes were linked closely to the increased use of new therapeutic agents. These results are well in line with those from other population-based cohort studies of real-world experience, which demonstrated an improvement in survival not only in transplant-eligible MM patients but also for the older patient population not eligible for HD-ASCT [4, 22,23,24,25,26,27,28,29]. In a large observational, cross-sectional patient chart review between 2014 and 2016 across five European countries including Italy, the most commonly used first-line novel agent was bortezomib [30,31,32]. Bortezomib-based regimens were also the most widely used first-line types of treatment during our observation period, reflecting the local availability of this new drug since 2004. However, 38% of patients were treated with conventional first-line chemotherapy, with the majority of them starting therapy before approval of novel agents.
High-dose melphalan with ASCT has led to improved response rates and prolonged progression-free survival in several clinical trials [33,34,35,36]. Although currently the standard approach in transplant-eligible patients with newly diagnosed MM, the benefit in terms of OS as well as its role in the era of novel agents is less clear. Two recent meta-analyses did not show a significant OS benefit in patients treated with HD-ASCT compared with standard-dose therapy [37, 38]. However, there was a significant heterogeneity present across these enrolled studies. In addition, the lack of OS benefit in some trials may be associated with a crossover or the use of HD-ASCT at relapse [37]. The median OS for patients treated with and without HD-ASCT observed in our study is comparable with that published in other real-world studies and further corroborates the efficacy of HD-ASCT in MM patients treated in daily clinical practice. Treatment-related mortality of the transplanted patients was low.
With the introduction of novel agents, MM has changed to a chronic disease with increased life expectancy. In this context, prolonged exposure to alkylating and immunomodulatory agents has raised concerns over the long-term risk of hematologic or solid secondary primary malignancies (SPM). However, data are inconsistent and recent studies reported that the overall risk of SPM in MM is low and multifactorial, and NA-based therapies may largely outweigh the negative side effects [39,40,41]. Consistent with recent findings, we also observed that solid tumors are more common than hematologic malignancies in patients with MM, both prior and subsequent to the diagnosis of MM [40, 42,43,44].
Among newly diagnosed patients with MM, a considerable number of patients have comorbidities related or unrelated to the disease [45, 46]. Kleber et al. originally developed the Myeloma Comorbidity Index (MCI; also called Freiburg Comorbidity Index), which is a simple and valid comorbidity index in MM patients including the risk factors renal impairment, moderate to severe lung disease, and performance status [47, 48]. Only recently, the score was revised (R-MCI) by adding the factors age, frailty, and cytogenetic aberrations [12]. The R-MCI allows the definition of fit (R-MCI score ≤ 3), intermediate-fit (R-MCI scores 4–6), and frail (R-MCI score > 6) patients showing strong clinical relevance for OS and PFS. Of note, the R-MCI remained a significant-risk tool in different treatments (patients treated with vs. without HD-ASCT or NA-based therapy) and age groups (≤ 65 years vs. > 65 years) [12]. The score is simply applicable within a web-based application.
In our retrospective study, hypertension was the most common comorbidity followed by moderate to severe renal dysfunction and neuropathy. According to the proposed R-MCI score, the majority of our patients was scored as intermediate-fit, followed by fit and frail patients, with similar distribution as described in the original study by Engelhardt et al. [12]. A higher R-MCI score was associated with a significantly shortened OS in our patients. Moreover, multivariate analysis identified R-MCI score as an independent prognostic factor for OS.
Notably, we also examined 66 patients for evidence of amyloid deposits in subcutaneous fat pads. Data on coexistent amyloid deposits in MM patients are limited and heterogeneous. Depending on the analyzed tissue specimen, amyloid deposits can be detected in 1–40% of patients with MM [49,50,51,52,53]. The study by Desikan et al. investigated the incidence of AL amyloid in patients with MM within a prospective phase II trial [49]. By SAFA, amyloid was detectable in 25 (31%) out of 81 patients, whereas bone marrow biopsy was positive for amyloid in 8 (10%) patients. In total, overall incidence of AL amyloidosis was 38% in transplant patients as opposed to earlier observations reporting MM-associated AL amyloidosis in only 3–15% [54,55,56]. Interestingly, no lambda predominance in patients with AL amyloidosis was detected, and median overall and event-free survival were similar in both subgroups. A similar incidence of AL in patients with MM has been demonstrated by Vela-Ojeda et al. [50]. Multiple myeloma-associated amyloidosis was found in 68 (34%) out of 201 patients by fat-pad biopsy needle aspiration and Congo red staining. In contrast to the Desikan et al. study, they could demonstrate that the presence of amyloid deposits in patients with MM is an independent adverse prognostic factor regardless of the presence or absence of amyloid symptoms at the time of diagnosis. In a retrospective study on 166 MM patients, Petruzziello et al. could find amyloid deposits in the bone marrow in 40% of patients studied at diagnosis or with advanced disease [51]. In contrast, frequency of amyloid deposition in the bone marrow of newly diagnosed patients with MM and SMM was only 2% reported by the study of Siragusa et al. [52].
Our findings are in line with the data by Vela-Ojeda et al., as patients without amyloid deposits at time of diagnosis of MM had a significantly better OS compared with patients with amyloid deposits. Similarly, the majority of our patients positive for amyloid deposits developed clinical findings of amyloidosis during the follow-up. This indicates that the presence of subclinical amyloidosis may confer a poor prognosis. Particularly, presence of subclinical amyloidopathy may affect therapeutic decisions regarding the choice of first-line therapy or intensity of the conditioning regimen.
Major limitations of our study include the retrospective study design within a single institution, relative small sample size for subgroup analyses, and heterogeneity of patients as well as treatment protocols. However, our results reflect a “real-life” cohort of MM patients for the population in South Tyrol and demonstrated that both HD-ASCT and the use of new agents as a part of first-line treatment seem to impact the survival of MM patients outside clinical trials. The R-MCI is a practical and efficient tool for assessing patients’ physical conditions and prognosis as well as possible treatment-associated risks. Subclinical amyloid deposition in MM may be associated with worse outcome. Population-based studies add important knowledge on what happens to MM patients in real life.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR (2003) Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78:21–33. https://doi.org/10.4065/78.1.21
Palumbo A, Anderson K (2011) Multiple myeloma. N Engl J Med 364:1046–1060. https://doi.org/10.1056/NEJMra1011442
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, Lust J, McCurdy A, Russell SJ, Zeldenrust SR, Kyle RA, Rajkumar SV (2014) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28:1122–1128. https://doi.org/10.1038/leu.2013.313
Pulte D, Gondos A, Brenner H (2011) Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist 16:1600–1603. https://doi.org/10.1634/theoncologist.2011-0229
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, Siniscalchi A, Magarotto V, Pour L, Carella A, Malfitano A, Petrò D, Evangelista A, Spada S, Pescosta N, Omedè P, Campbell P, Liberati AM, Offidani M, Ria R, Pulini S, Patriarca F, Hajek R, Spencer A, Boccadoro M, Palumbo A (2015) Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol 16:1617–1629. https://doi.org/10.1016/S1470-2045(15)00389-7
Deschler B, Binek K, Ihorst G et al (2010) Prognostic factor and quality of life analysis in 160 patients aged > or =60 years with hematologic neoplasias treated with allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 16:967–975. https://doi.org/10.1016/j.bbmt.2010.02.004
Newberry KJ, Naqvi K, Nguyen KT, Cardenas-Turanzas M, Florencia Tanaka M, Pierce S, Verstovsek S (2014) Comorbidities predict worse prognosis in patients with primary myelofibrosis. Cancer 120:2996–3002. https://doi.org/10.1002/cncr.28857
Antic D, Jelicic J, Trajkovic G, Balint MT, Bila J, Markovic O, Petkovic I, Nikolic V, Andjelic B, Djurasinovic V, Sretenovic A, Smiljanic M, Vukovic V, Mihaljevic B (2018) Is it possible to improve prognostic value of NCCN-IPI in patients with diffuse large B cell lymphoma? The prognostic significance of comorbidities. Ann Hematol 97:267–276. https://doi.org/10.1007/s00277-017-3170-z
Kleber M, Ihorst G, Deschler B, Jakob C, Liebisch P, Koch B, Sezer O, Engelhardt M (2009) Detection of renal impairment as one specific comorbidity factor in multiple myeloma: multicenter study in 198 consecutive patients. Eur J Haematol 83:519–527. https://doi.org/10.1111/j.1600-0609.2009.01318.x
Wasterlid T, Mohammadi M, Smedby KE et al (2019) Impact of comorbidity on disease characteristics, treatment intent and outcome in diffuse large B-cell lymphoma: a Swedish lymphoma register study. J Intern Med 285:455–468. https://doi.org/10.1111/joim.12849
Engelhardt M, Domm A-S, Dold SM, Ihorst G, Reinhardt H, Zober A, Hieke S, Baayen C, Müller SJ, Einsele H, Sonneveld P, Landgren O, Schumacher M, Wäsch R (2017) A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 102:910–921. https://doi.org/10.3324/haematol.2016.162693
Bhat A, Selmi C, Naguwa SM, Cheema GS, Gershwin ME (2010) Currents concepts on the immunopathology of amyloidosis. Clin Rev Allergy Immunol 38:97–106. https://doi.org/10.1007/s12016-009-8163-9
Falk RH, Comenzo RL, Skinner M (1997) The systemic amyloidoses. N Engl J Med 337:898–909. https://doi.org/10.1056/NEJM199709253371306
Puchtler H, Sweat F (1965) Congo red as a stain for fluorescence microscopy of amyloid. J Histochem Cytochem 13:693–694. https://doi.org/10.1177/13.8.693
Glenner GG, Ein D, Eanes ED, Bladen HA, Terry W, Page DL (1971) Creation of “amyloid” fibrils from Bence Jones proteins in vitro. Science 174:712–714
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle RA, Caers J, Hillengass J, San Miguel J, van de Donk N, Einsele H, Bladé J, Durie BG, Goldschmidt H, Mateos MV, Palumbo A, Orlowski R (2016) Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood 127:2955–2962. https://doi.org/10.1182/blood-2016-01-631200
Bender R, Lange S (2001) Adjusting for multiple testing - when and how? J Clin Epidemiol 54:343–349. https://doi.org/10.1016/S0895-4356(00)00314-0
Gertz MA (2018) Immunoglobulin light chain amyloidosis: 2018 update on diagnosis, prognosis, and treatment. Am J Hematol 93:1169–1180. https://doi.org/10.1002/ajh.25149
Gay F, Engelhardt M, Terpos E, Wäsch R, Giaccone L, Auner HW, Caers J, Gramatzki M, van de Donk N, Oliva S, Zamagni E, Garderet L, Straka C, Hajek R, Ludwig H, Einsele H, Dimopoulos M, Boccadoro M, Kröger N, Cavo M, Goldschmidt H, Bruno B, Sonneveld P (2018) From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica 103:197–211. https://doi.org/10.3324/haematol.2017.174573
Costa LJ, Hari PN, Kumar SK (2016) Differences between unselected patients and participants in multiple myeloma clinical trials in US: a threat to external validity. Leuk Lymphoma 57:2827–2832. https://doi.org/10.3109/10428194.2016.1170828
Hock BD, Mulholland KS, Ganly P et al (2018) Impact of increased access to novel agents on the survival of multiple myeloma patients treated at a single New Zealand centre. Intern Med J. https://doi.org/10.1111/imj.14155
Thoennissen GB, Gorlich D, Bacher U et al (2017) Autologous stem cell transplantation in multiple myeloma in the era of novel drug induction: a retrospective single-center analysis. Acta Haematol 137:163–172. https://doi.org/10.1159/000463534
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood 111:2516–2520. https://doi.org/10.1182/blood-2007-10-116129
Pozzi S, Marcheselli L, Bari A, Liardo EV, Marcheselli R, Luminari S, Quaresima M, Cirilli C, Ferri P, Federico M, Sacchi S (2013) Survival of multiple myeloma patients in the era of novel therapies confirms the improvement in patients younger than 75 years: a population-based analysis. Br J Haematol 163:40–46. https://doi.org/10.1111/bjh.12465
Tangen J-M, Tjonnfjord GE, Gulbrandsen N et al (2018) Improved outcome in patients following autologous stem cell transplantation for multiple myeloma in south eastern Norway 2001-2010: a retrospective, population based analysis. BMC Cancer 18:801. https://doi.org/10.1186/s12885-018-4722-x
Warren JL, Harlan LC, Stevens J, Little RF, Abel GA (2013) Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States. J Clin Oncol 31:1984–1989. https://doi.org/10.1200/JCO.2012.46.3323
Mey UJM, Leitner C, Driessen C, Cathomas R, Klingbiel D, Hitz F (2016) Improved survival of older patients with multiple myeloma in the era of novel agents. Hematol Oncol 34:217–223. https://doi.org/10.1002/hon.2205
Remes K, Anttila P, Silvennoinen R et al (2018) Real-world treatment outcomes in multiple myeloma: multicenter registry results from Finland 2009-2013. PLoS One 13:e0208507. https://doi.org/10.1371/journal.pone.0208507
Yong K, Delforge M, Driessen C, Fink L, Flinois A, Gonzalez-McQuire S, Safaei R, Karlin L, Mateos MV, Raab MS, Schoen P, Cavo M (2016) Multiple myeloma: patient outcomes in real-world practice. Br J Haematol 175:252–264. https://doi.org/10.1111/bjh.14213
Raab MS, Cavo M, Delforge M, Driessen C, Fink L, Flinois A, Gonzalez-McQuire S, Safaei R, Karlin L, Mateos MV, Schoen P, Yong K (2016) Multiple myeloma: practice patterns across Europe. Br J Haematol 175:66–76. https://doi.org/10.1111/bjh.14193
Raab MS, Fink L, Schoen P et al (2018) Evolution of multiple myeloma treatment practices in Europe from 2014 to 2016. Br J Haematol. https://doi.org/10.1111/bjh.15680
Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, Macro M, Pertuiset E, Dreyfus F, Mariette X, Boccacio C, Brouet JC (1998) High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood 92:3131–3136
Blade J, Rosinol L, Sureda A et al (2005) High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood 106:3755–3759. https://doi.org/10.1182/blood-2005-03-1301
Fermand J-P, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, Arnulf B, Royer B, Mariette X, Pertuiset E, Belanger C, Janvier M, Chevret S, Brouet JC, Ravaud P, Group Myelome-Autogreffe (2005) High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol 23:9227–9233. https://doi.org/10.1200/JCO.2005.03.0551
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ, Medical Research Council Adult Leukaemia Working Party (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348:1875–1883. https://doi.org/10.1056/NEJMoa022340
Dhakal B, Szabo A, Chhabra S, Hamadani M, D’Souza A, Usmani SZ, Sieracki R, Gyawali B, Jackson JL, Asimakopoulos F, Hari PN (2018) Autologous transplantation for newly diagnosed multiple myeloma in the era of novel agent induction: a systematic review and meta-analysis. JAMA Oncol 4:343–350. https://doi.org/10.1001/jamaoncol.2017.4600
Su B, Zhu X, Jiang Y et al (2018) A meta-analysis of autologous transplantation for newly diagnosed multiple myeloma in the era of novel agents. Leuk Lymphoma:1–8. https://doi.org/10.1080/10428194.2018.1543874
Musto P, Anderson KC, Attal M et al (2017) Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol 28:228–245. https://doi.org/10.1093/annonc/mdw606
Sahebi F, Iacobelli S, Sbianchi G, Koster L, Blaise D, Reményi P, Russell NH, Ljungman P, Kobbe G, Apperley J, Trneny M, Krejci M, Wiktor-Jedrzejczak W, Sanchez JF, Schaap N, Isaksson C, Lenhoff S, Browne P, Scheid C, Wilson KMO, Yakoub-Agha I, Muñiz SG, Schönland S, Morris C, Garderet L, Kröger N (2018) Incidence of second primary malignancies after autologous transplantation for multiple myeloma in the era of novel agents. Biol Blood Marrow Transplant 24:930–936. https://doi.org/10.1016/j.bbmt.2018.01.006
Razavi P, Rand KA, Cozen W, Chanan-Khan A, Usmani S, Ailawadhi S (2013) Patterns of second primary malignancy risk in multiple myeloma patients before and after the introduction of novel therapeutics. Blood Cancer J 3:e121. https://doi.org/10.1038/bcj.2013.19
Jonsdottir G, Lund SH, Bjorkholm M et al (2017) The impact of prior malignancies on second malignancies and survival in MM patients: a population-based study. Blood Adv 1:2392–2398. https://doi.org/10.1182/bloodadvances.2017007930
Hasskarl J, Ihorst G, De Pasquale D et al (2011) Association of multiple myeloma with different neoplasms: systematic analysis in consecutive patients with myeloma. Leuk Lymphoma 52:247–259. https://doi.org/10.3109/10428194.2010.529207
Jones JR, Cairns DA, Gregory WM, Collett C, Pawlyn C, Sigsworth R, Striha A, Henderson R, Kaiser MF, Jenner M, Cook G, Russell NH, Williams C, Pratt G, Kishore B, Lindsay J, Drayson MT, Davies FE, Boyd KD, Owen RG, Jackson GH, Morgan GJ (2016) Second malignancies in the context of lenalidomide treatment: an analysis of 2732 myeloma patients enrolled to the Myeloma XI trial. Blood Cancer J 6:e506. https://doi.org/10.1038/bcj.2016.114
Knudsen LM, Hjorth M, Hippe E (2000) Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol 65:175–181
Kistler KD, Kalman J, Sahni G et al (2017) Incidence and risk of cardiac events in patients with previously treated multiple myeloma versus matched patients without multiple myeloma: an observational, retrospective, cohort study. Clin Lymphoma Myeloma Leuk 17:89–96.e3. https://doi.org/10.1016/j.clml.2016.11.009
Kleber M, Ihorst G, Terhorst M, Koch B, Deschler B, Wäsch R, Engelhardt M (2011) Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM-comorbidity score. Blood Cancer J 1:e35. https://doi.org/10.1038/bcj.2011.34
Kleber M, Ihorst G, Gross B et al (2013) Validation of the Freiburg Comorbidity Index in 466 multiple myeloma patients and combination with the international staging system are highly predictive for outcome. Clin Lymphoma Myeloma Leuk 13:541–551. https://doi.org/10.1016/j.clml.2013.03.013
Desikan KR, Dhodapkar MV, Hough A, Waldron T, Jagannath S, Siegel D, Barlogie B, Tricot G (1997) Incidence and impact of light chain associated (AL) amyloidosis on the prognosis of patients with multiple myeloma treated with autologous transplantation. Leuk Lymphoma 27:315–319. https://doi.org/10.3109/10428199709059685
Vela-Ojeda J, Garcia-Ruiz Esparza MA, Padilla-Gonzalez Y et al (2009) Multiple myeloma-associated amyloidosis is an independent high-risk prognostic factor. Ann Hematol 88:59–66. https://doi.org/10.1007/s00277-008-0554-0
Petruzziello F, Zeppa P, Catalano L, Cozzolino I, Gargiulo G, Musto P, D’Auria F, Liso V, Rizzi R, Caruso N, Califano C, Piro E, Musso M, Bonanno V, Pia Falcone A, Tafuto S, di Raimondo F, de Laurentiis M, Pane F, Palombini L, Rotoli B (2010) Amyloid in bone marrow smears of patients affected by multiple myeloma. Ann Hematol 89:469–474. https://doi.org/10.1007/s00277-009-0857-9
Siragusa S, Morice W, Gertz MA, Kyle RA, Greipp PR, Lust JA, Witzig TE, Lacy MQ, Zeldenrust SR, Rajkumar SV, Russell SJ, Hayman SR, Buadi F, Kumar SK, Dingli D, Dispenzieri A (2011) Asymptomatic immunoglobulin light chain amyloidosis (AL) at the time of diagnostic bone marrow biopsy in newly diagnosed patients with multiple myeloma and smoldering myeloma. A series of 144 cases and a review of the literature. Ann Hematol 90:101–106. https://doi.org/10.1007/s00277-010-1028-8
Chakraborty R, Gertz MA, Dispenzieri A, Gonsalves WI, Zeldenrust SR, Russell SJ, Go RS, Kapoor P, Rajkumar VS, Hayman SR, Hwa YL, Lacy MQ, Kyle RA, Leung N, Kumar SK (2017) Natural history of amyloidosis isolated to fat and bone marrow aspirate. Br J Haematol 179:170–172. https://doi.org/10.1111/bjh.14205
Ivanyi B (1990) Frequency of light chain deposition nephropathy relative to renal amyloidosis and Bence Jones cast nephropathy in a necropsy study of patients with myeloma. Arch Pathol Lab Med 114:986–987
Kapadia SB (1980) Multiple myeloma: a clinicopathologic study of 62 consecutively autopsied cases. Medicine (Baltimore) 59:380–392
Kyle RA (1975) Multiple myeloma: review of 869 cases. Mayo Clin Proc 50:29–40
Funding
This study was funded by the budget of the Südtiroler Sanitätsbetrieb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study that involved human participants were in accordance with the ethical standards of the institutional committee, national research committee, and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The Ethics Committee of the “Südtiroler Sanitätsbetrieb” approved this study by the protocol number 81-2018.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Mair, M., Straka, C., Buratti, T. et al. Clinical outcomes and prognostic factors in patients with multiple myeloma in South Tyrol: a retrospective single-center analysis. Ann Hematol 99, 1031–1040 (2020). https://doi.org/10.1007/s00277-020-03969-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-03969-9