Abstract
The identification of acute cardioprotective strategies against myocardial ischemia/reperfusion (I/R) injury that can be applied in the catheterization room is currently an unmet clinical need and several interventions evaluated in the past at the pre-clinical level have failed in translation. Autonomic imbalance, sustained by an abnormal afferent signalling, is a key component of I/R injury. Accordingly, there is a strong rationale for neuromodulation strategies, aimed at reducing sympathetic activity and/or increasing vagal tone, in this setting. In this review we focus on cervical vagal nerve stimulation (cVNS) and on transcutaneous auricular vagus nerve stimulation (taVNS); the latest has the potential to overcome several of the issues of invasive cVNS, including the possibility of being used in an acute setting, while retaining its beneficial effects. First, we discuss the pathophysiology of I/R injury, that is mostly a consequence of the overproduction of reactive oxygen species. Second, we describe the functional anatomy of the parasympathetic branch of the autonomic nervous system and the most relevant principles of bioelectronic medicine applied to electrical vagal modulation, with a particular focus on taVNS. Then, we provide a detailed and comprehensive summary of the most relevant pre-clinical studies of invasive and non-invasive VNS that support its strong cardioprotective effect whenever there is an acute or chronic cardiac injury and specifically in the setting of myocardial I/R injury. The potential benefit in the emerging field of post cardiac arrest syndrome (PCAS) is also mentioned. Indeed, electrical cVNS has a strong anti-adrenergic, anti-inflammatory, antioxidants, anti-apoptotic and pro-angiogenic effect; most of the involved molecular pathways were already directly confirmed to take place at the cardiac level for taVNS. Pre-clinical data clearly show that the sooner VNS is applied, the better the outcome, with the possibility of a marked infarct size reduction and almost complete left ventricular reverse remodelling when VNS is applied immediately before and during reperfusion. Finally, we describe in detail the limited but very promising clinical experience of taVNS in I/R injury available so far.
Similar content being viewed by others
Introduction
Despite substantial improvement in surgical and percutaneous revascularization techniques, myocardial infarction (MI), defined as the irreversible damage caused by severe and sustained myocardial ischemia, and the chronic heart failure (HF) which may follow, remain among the leading causes of morbidity and mortality worldwide (Moran et al. 2014). The size of the infarcted area is one of the major determinants of the prognosis of these patients; accordingly, the main goal of cardioprotective strategies is to reduce its extension.
Although restoring coronary perfusion, therapeutically known as reperfusion, is the only strategy able to prevent the progression from ischemia to infarction, it may itself contribute to worsening tissue damage due to the so called ischemia/reperfusion (I/R) injury (Heusch 2015; Hausenloy et al. 2017; Hausenloy and Yellon 2013). As such, therapeutic interventions able to protect the heart against acute I/R injury, to reduce myocardial infarct size and to finally prevent the onset of HF (F, B., et al. 2023; Elia et al. 2024) in patients presenting with acute MI are highly needed. Unfortunately, despite the promising results of several cardioprotective interventions tested in animal models so far, the clinical translation has been challenging (Lecour et al. 2021; Caccioppo et al. 2019) and no such strategy is recommended by current guidelines (Byrne et al. 2023). Specifically, stem cell-based therapies offer huge potential, but their clinical implementation is still limited by engraftment-related side effects (Pezhouman et al. 2023); accordingly, paracrine mediators, including exosomes and microvesicles, are currently under study with cardioprotective purpose (Caccioppo et al. 2019; D’Ascenzo et al. 2021; Femminò et al. 2021).
In the present review we will focus on cervical vagal nerve stimulation (cVNS) and on transcutaneous auricular vagus nerve stimulation (taVNS), being the latest one of the most promising, not cell-based, cardioprotective strategies, currently available; we will span from the pathophysiological rationale to the modalities of use and application in the context of I/R injury. The potential benefit in the setting of post cardiac arrest syndrome (PCAS) will also be mentioned.
Pathophysiology of ischemia/reperfusion injury
The main pathophysiological feature of I/R injury is the overproduction of reactive oxygen species (ROS) as a result of a number of complex mechanisms including metabolic changes (Tian et al. 2023) and mitochondrial dysfunction (Kuznetsov et al. 2019), cell death (Cell Biology of Ischemia/Reperfusion Injury - PMC n.d.), microvascular obstruction (Sezer, et al. 2018) and inflammation (Zhou et al. 2015; Maxwell and Lip 1997; Thapalia et al. 2014), autonomic imbalance and autonomic remodelling (Intachai et al. 2018; Lambert, et al. 2016). All these mechanisms are deeply related one to the other and are mutually potentiated. Specifically, the tight association between the autonomic nervous system and the immuno-inflammatory responses, better known as neuroimmune crosstalk (Tarnawski and Olofsson 2021), will be discussed in detail due do its relevance for the I/R injury. The neuroimmune crosstalk includes but is not limited to, the cholinergic anti-inflammatory pathway.
Metabolic changes
Acute myocardial ischemia is the result of an abrupt mismatch between coronary blood flow and metabolic requirements, leading to inadequate oxygen and metabolic substrates delivery. In turn, oxygen and ATP shortage represses oxidative metabolism of fatty acids (FA), carbohydrates, ketones, and amino acids, and activates anaerobic glycolysis. During reperfusion intracellular pH tends to progressively normalize thanks to oxygen wash‐in combined to ischemic metabolites wash-out, leading to the progressive switch back to oxidative metabolism and to ongoing changes in glycolysis. These metabolic changes occurring during ischemia and early reperfusion largely determine the actual infarct size that follows an ischemic episode. Accordingly, several tailored metabolic modulation strategies have been attempted against I/R injury, starting in 1962 with potassium‐insulin‐glucose administration during MI (Sodi-Pallares et al. 1962); most of them failed in translation. For instance, in experimental models, the partial inhibition of myocardial FA oxidation with drugs such as oxfenicine, ranolazine, and trimetazidine stimulates glucose oxidation and reduces lactate production during ischemia (Stanley 2004) but it has not been translated in a significant clinical benefit so far in the acute setting (Morrow 2007).
Mitochondrial dysfunction
ATP depletion leads to the malfunction of the electrogenic sodium–potassium pumps in the sarcolemma, resulting in an initial cytoplasmic accumulation of sodium. The excess sodium is then extruded through the Na/Calcium exchanger (NCX), resulting in calcium overload, and the Na/H exchanger (NHE1), resulting in acidosis, which is further worsened by the reduced energy efficiency of anaerobic metabolism. These two conditions, beyond dissipating the transmembrane potential and leading to partially depolarized ischemic cells more prone to arrhythmias, induce a profound mitochondrial dysfunction characterized by increased mitochondria permeability through the opening of mitochondrial permeability transition pores (mPTP), increased ROS production and release of cytochrome c, and further reduced ATP generation. Restoring oxygen delivery in the dysfunctional mitochondria with perfusion further worsens ROS production and increases the proteolytic activity of calpain, a calcium activated cytosolic protease whose activation promotes apoptosis. Zinc and Magnesium, as well NCX and NHE1 inhibitors have all demonstrated potential beneficial effects in pre-clinical models of I/R injury through membrane stabilization and indirect mitochondrial protection (Skene et al. 2019; Martens et al. 2022).
Cell death
The fate of the ischemic myocardium potentially follows three paths: recovery, remodelling, and death. In the context of cell death, we first distinguish necrosis (Mishra, et al. 2019), where cell death results in rupture of mitochondria and cell membrane. Mechanisms contributing to necrosis are failure of ion pumps, acidosis, calcium overload (Tani and Neely 1989), and irreversible damage to proteins, lipids, and nucleic acids as a result of excessive ROS production.
Reperfusion typically intensifies the morphological features of necrosis, with the appearance of contraction bands (Schluter et al. 1996), due to the excessive and uncoordinated contraction produced by abnormal Ca cycling between sarcoplasmatic reticulum and the cytosol, along with an early leukocyte infiltrate. The second type of death is the programmed cell death, which can be described as apoptosis (Davidson et al. 2020), necroptosis (Zhou and Yuan 2014), and pyroptosis (Audia et al. 2018), respectively, depending on the mechanism that causes it. In the first case, the release of cytochrome c from mitochondria promotes activation of caspases with DNA fragmentation; in the second, the activation of tumor necrosis factor receptors (TNFRs) or toll-like receptors (TLRs) promotes the formation of sarcolemma pores; in the third, the release of damage-associated molecular pattern molecules (DAMPs) promotes the formation of inflammasome and gasdermin-dependent pores in the sarcolemma. In the latter two cases, as in necrosis, the loss of cell membrane integrity promotes the inflammatory response.
The mechanisms of protection from cell death can in turn be categorized into three main pathways, PKC-eNOS-PKG; RISK and SAFE pathways, the activation of which may potentially be beneficial in models of I/R injury (Amoretti et al. 2002).
Inflammation and microvascular obstruction
Activation of the innate and adaptive autoimmune system plays a major role in both I/R injury and the healing pathway. The innate response is triggered by the release of DAMPs from necrotic myocardium or gone through cardiomyocyte membrane portioning, through predominantly TLR2 and TLR4, which are broadly expressed in cardiac tissue (Vilahur and Badimon 2014).
After activation, the signalling begins via TRIF (TIR-domain-containing adaptor-inducing beta interferon) and MyD88-dependent pathways resulting in IFN3 and NFκB activation, respectively, and subsequent production of pro-inflammatory cytokines (e.g. IL-1, IL-6, and Tumor necrosis factor- alpha, TNF-α) (Meijerink et al. 2015), promoting the infiltration of neutrophils into the site of cardiac injury to initiate inflammatory responses.
In addition, the release of DAMPs promotes platelet activation, endothelial damage (Silvis et al. 2020) and the occurrence of microvascular thromboembolic events, which, together with endothelial dysfunction, promote microcirculation no-reflow after reperfusion. Microthrombi also, in turn, play a critical role in leukocyte migration to the ischemic region, amplifying the inflammatory burden (Ghasemzadeh et al. 2022).
The complement system is also involved in the innate inflammatory response; in fact, C1 inhibitors and inhibitors of mannose-binding protein-associated serine protease 2 (MASP2), two regulators of complement activation, have been shown to be effective in preclinical studies.
The adaptive immune system, including T and B lymphocytes, play a central role in infarct size extension (Flohr and Breull 1975). CD4+ T lymphocytes increase myocardial I/R injury by the production of pro-inflammatory cytokines like interferon-γ (IFNγ) and TNFα (Hofmann and Frantz 2015). Otherwise, CD4+ CD25+ regulatory T cells (Tregs), a specific subset of CD4+T lymphocytes, can beneficially control myocardial I/R injury. Treg cells exert their immunomodulatory function through two different mechanisms, including cell- contact-dependent mechanisms and the production of anti-inflammatory cytokines like IL-10 and transforming growth factor (TGF)-β, involved, together with endothelial integrity preservation, in remodelling of heart muscle and atherosclerotic plaque (Candreva 2024).
Autonomic imbalance and autonomic remodelling
I/R injury is associated with a profound autonomic imbalance characterized by sympathetic overactivity and parasympathetic withdrawal, that plays a pivotal role in the progression from ischemia to infarction, as well as in the associated risk of arrhythmias and of evolution towards heart failure (Gronda, et al. 2022).
The primum movens for this imbalance is characterized by an abnormal neuronal afferent signaling sustained by chemo and mechanoreceptor stimulation within the ischemic area due to, respectively, ischemic metabolites (mostly adenosine, but also lactic acid, bradykinin, prostaglandins, and ROS) and abnormal contraction leading to increased wall stress (Dusi and Ardell 2020). Mechano-transduction, that is mostly mediated by myelinated (A delta type) fibers, occurs fast (within few seconds after acute coronary occlusion), produces phasic activity in the afferent neurons and shows a very limited memory. On the other side, chemo-transduction, that is mostly mediated by unmyelinated (C type) fibers, generates relatively slow onset responses, characterized by tonic (not phasic) activity, and displays memory capacity (Ardell and Armour 2016). The second hit, which may dramatically enhances the first one also depending on the extension of the ischemic area, is the reduced cardiac output. The subsequent baroreceptor reflex activation, acutely aimed at sustaining cardiac function at the expense of an increased metabolism, may chronically lead to deleterious effects (Ruffinazzi and Dusi 2020). Indeed, the decrease in mean and pulsatile blood pressure (BP) that may follow I/R injury leads to a decreased afferent nerve firing from stretch receptors within the arterial wall (carotid and aortic baroreceptors), a reduced stimulation of the nucleus tractus solitarius, and a reduced inhibition of cardiovascular sympathetic outflow, which is thus increased (Dusi and Ardell 2020). Notably, after a cardiac damage, even when cardiac output is preserved, the autonomic balance shifts towards a sympathetic predominance due to the offset of the baroreflex control by increased afferent pathological signaling from cardiac receptors. In both cases (reduced or preserved cardiac output), carotid baroreflex circuits are not intrinsically malfunctioning (Floras 2009). Accordingly, baroreflex activation therapy (BAT), currently achieved through an invasive device not suitable for the acute setting, is a promising therapeutic option both for HF with reduced ejection fraction (Dusi et al. 2022) and HF with preserved ejection fraction (Salah et al. 2022). The autonomic imbalance occurring during and after I/R injury is accompanied by a morpho-functional remodeling affecting all cardiovascular autonomic neurons and stations, from the intrinsic cardiac nervous system (ICNS), that is the first integrative center involved in the beat to beat regulation of cardiac functions (Giannino et al. 2024), to the intrathoracic extracardiac centers, and up to the higher centers (Ruffinazzi and Dusi 2020). Neuronal remodeling may lead to denervation, nerve sprouting, and sympathetic hyperinnervation (D’Elia et al. 2014). The dramatic pro-arrhytmic potential of this autonomic imbalance and remodeling is well charactherized at the pre-clinical (Weperen et al. 2023) and clinical level (Dusi et al. 2019; Dusi et al. 2023; Dusi, et al. 2024; Savastano, et al. 2024; Iannaccone et al. 2022). Notably, in the setting of high-frequency and prolonged sympathetic activation such as during acute myocardial ischemia, additional sympathetic co-transmitters other than norepinephrine (NE) are co-released from sympathetic post-ganglionic efferent fibers, including ATP, galanin and neuropeptide Y (NPY) (Herring 2015). NPY is of particular interest because, beyond having the direct effect of reducing ventricular electrical stability through Y1 receptors (Dusi et al. 2020; Kalla et al. 2020), also exerts a potent vasoconstrictor effect thought Y1 (Herring et al. 2019) and Y5 type (Malmström 2002) receptors on coronary micro-arteries and its levels after reperfusion were associated with microvascular dysfunction and poor prognosis both in animals (Herring et al. 2019) and in humans (Herring et al. 2019).
Overall, there is a strong rationale for neuromodulation strategies, aimed at reducing sympathetic activity and/or increasing vagal tone, in the setting of I/R injury. Of note, since this autonomic inbalance is driven and maintaned by an abnormal afferent signaling that affects both sympathetic (Dusi and Ardell 2020) and parasympathetic (Weperen and Vaseghi 2023) fibers, several studies have been conducted to elucidate and modify afferent transmission during I/R. For instance, it was recently demonstrated in a porcine model (Yoshie, et al. 2020) that cardiac afferent signaling mediated by the transient receptor potential cation subfamily V member 1 (TRPV1) channels promotes cardio-cardiac spinal reflexes after MI that result in sympathoexcitation and arrhythmogenic ventricular remodeling. Accordingly, the selective epidural administration of resiniferatoxin, an ultrapotent TRPV1 desensitizers, to DRGs provides cardioprotection against ventricular arrhythmias in pigs by inhibiting afferent neurotransmission during I/R injury (Yamaguchi et al. 2023).
The neuroimmune crosstalk
The autonomic nervous system and the immune system are tightly related one to each other. Indeed, stimulation of adrenergic receptors on immune cells controls all crucial stages of immune responses (Dusi 2020) and sympathetic overactivity was associated with the development and exacerbation of several chronic immune‐mediated diseases (Bellinger and Lorton 2014). On the other hand, the parasympathetic nervous system acts as a powerful anti‐inflammatory neural circuit, both at the central and at the peripheral level. As for most neuronal pathways, the afferent branch plays a pivotal role in this process. The activation of afferent vagal fibres, typically elicited by inflammatory cytokines and ROS, but also by other kinds of chemical or mechanical stressors, stimulates the NTS of the medulla oblongata with several consequences (Trakhtenberg and Goldberg 2011). On one side, NTS activation reflexively elicits nearby corticotrophin-releasing factor (CRF)-releasing neurons (Chen et al. 2021). Such stimulation activates the hypothalamic-pituitary-adrenal system, with release first of ACTH and finally of glucocorticoids, with potent anti-inflammatory activity. On the other side, after subcortical processing, NTS activation leads to an increase in vagal output that in turn suppress peripheral inflammation, thus completing the inflammatory reflex mechanism better known as cholinergic anti-inflammatory pathway (CAP). Notably, recent data suggest that pro- and anti-inflammatory cytokines interact with distinct populations of vagal neurons within the NTS to alert the brain of an emerging inflammatory response (Jin et al. 2024). Genetic manipulation of these neurons was proved to profoundly affect inflammatory responses, therefore opening new possibility of neuroimmunomodulation (Jin et al. 2024).
The CAP, first proposed by Tracey (Tracey 2002), is a peripheral reflex that consists in the direct inhibition of pro-inflammatory cytokine release (TNFα, IL-1β, IL-6, IL-18) from local macrophages and other immune cells induced by the neuronally released ACh. The molecular mechanism is the binding of ACh to the α7 subunit of nicotinic receptors (α7nAChR) (Tracey 2007). Therefore, cholinergic neurons can reflexively regulate inflammatory responses in real time (within seconds). The spleen is the only visceral site not directly affected by this efferent vagal reflex (Bellinger et al. 1993). Indeed, post ganglionic splenic neurons originating in the celiac ganglion are adrenergic, not cholinergic (Pereira and Leite 2016; Rosas-Ballina et al. 2011) , and the release of norepinephrine subsequently stimulates ACh- producing T-cells (Andersson and Tracey 2012); this process is named vagal-splenic pathway (Davidson et al. 2020). Figure 1 summarizes the molecular pathways underlying the nicotinic receptor mediated cholinergic anti-inflammatory effects. In addition to these nicotinic mediated effects, ACh improves mitochondrial function by reducing mPTP opening (Miao et al. 2013), inhibits programmed cell death and promotes mitochrondriogenesis (Lu et al. 2013). Overall, vagal activation on the one hand directly and indirectly reduces the inflammatory burden and on the other hand reduces ROS production, interrupting the vicious cycle of ischemia-ROS production-inflammation-ROS release.
Nicotinic receptor mediated cholinergic anti-inflammatory effects. Acetylcholine (ACh, in green in the figure), released by parasympathetic post ganglionic terminals and, in the spleen, by ACh- producing T-cells (vagal-splenic pathway, details in the text), binds to nicotine acetylcholine receptors (a7nAChR), a ligand-gated ion channel protein, on monocytes/macrophages surface. This binding plays a key role in mediating cholinergic anti-inflammation effects, by activating phosphatidylinsotisol 3-kinase (PI3K) with Nrf2 (later effector of this molecular pathway) and Janus kinase/signal transducer and activator of transcription (JAK2/STAT3) pathway, a downstream intracellular mechanism leading to the inhibition of Nuclear Factor kappa B (NF-kB) nuclear translocation, resulting in suppression of pro-inflammatory cytokines expression and production. In parallel, during immune/inflammatory processes (such as I/R injury) the innate response is triggered by the release of DAMPs (in red in the figure), predominantly through TLR2 and TLR4, broadly expressed in the cardiac tissue. After activation, MyD88-signalling pathways results in NF-kB activation, with production of pro-inflammatory cytokines. Created with Biorender.com. Alfa7nAChR: alfa-7-acetylcholine receptor; DAMPs: damage-associated molecular patterns; IKK: IkappaB kinase; JAK2: Janus kinase 2; MyD88: Myeloid differentiation primary response 88; NF-kB nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2: factor erythroid 2–related factor 2; PI3K: phosphatidyl-inositol 3-kinase; STAT3: Signal transducer and activator of transcription 3; TLR: Toll-like receptors; TRAF6: TNF receptor associated factor 6
In 2011 Calvillo et al (2011) demonstrated the cardiac effects of this pathway, underlying the crucial role played by α7nAChR in the HR- independent anti-inflammatory and anti-apoptotic effect of cVNS. Indeed, right cVNS, applied beginning 5 minutes before myocardial ischemia till 5 minutes after reperfusion (for a total duration of 40 minutes) in anesthetized rats, was associated with a decrease in infarct size and inflammatory markers independently of the heart rate (HR). At the cardiac level, the strong and direct anti-inflammatory effect of vagal activity is a key component of sympathetic-parasympathetic antagonism. Figure 1 shows the intracellular effect of α7nAChR.
Of note, the neuroimmune crosstalk is much more complex than anticipated, including also intracellular pathways and modulatory effects, such as beta-adrenergic receptor intracellular desensitization in immune cells. Indeed, Moragon et al (2020) recently demonstrated in a mice ischemia/reperfusion model (45 min ischemia/24 hours reperfusion) that metoprolol, but not atenolol or propranolol, significantly attenuated neutrophil infiltration and ameliorated I/R injury. Different intracellular β1 adrenergic receptor conformational changes were described by the in-silico analyses following metoprolol bounding as compared to the other two β-blockers and advocated as potential explanation for the different responses. These effects are expected to be synergic to those of VNS, as already demonstrated in the setting of HF (Hamann et al. 2013).
Principles of electrical vagal modulation
The target: Anatomy of the parasympathetic branch of the ANS
The efferent parasympathetic branch of the autonomic nervous system distinguishes, in the traditional two-neurons model, a preganglionic neuron, whose soma is located at the level of the dorsal motor nucleus of the vagus and the nucleus ambiguous, both within the medulla oblongata, and a postganglionic neuron (Dusi and Ardell 2020). Post ganglionic neurons are located directly on the posterior epicardial surface of the atria and superior surface of the ventricles, where they form, together with afferents neurons, local circuit neurons and a small number of catecholaminergic-releasing neurons, the intrinsic cardiac ganglionated plexi, also referred to as the intrinsic cardiac nervous system (ICNS) (Giannino et al. 2024). The axons of cardiac pre-ganglionic neurons travel along the vagus nerve (VN), together with other non-cardiac pre-ganglionic neurons directed towards upper and lower respiratory organs, gastrointestinal organs, and ovaries, and afferent neurons, with afferent fibres being much more abundant than the efferent ones (80% versus 20%). VN fibers, classified according to the diameter and the conduction velocity, range from Aα, the largest and fastest, to unmyelinated C-fibers (Mei et al. 1980), the smallest and slowest with highest activation thresholds (Schwaber and Cohen 1978; Jones et al. 1995). Cardiac vagal control in mammalians relies on B-type fibers (McAllen and Spyer 1976) (efferent fibers, with neuronal bodies mostly located within the nucleus ambiguous (Hopkins and Armour 1998) and projecting to the sinus node (Geis and Wurster 1980)) and C-type fibers (afferent and efferent, with the neuronal bodies of the efferent ones mostly located within the dorsal motor nucleus of the vagus (Cheng et al. 1999) and projecting to the the ventricles (Geis and Wurster 1980; Gourine et al. 2016)). The distribution of pre-ganglionic fibers to the post-ganglionic neurons within the epicardial fat pads is asymmetric: the right VN has a larger influence on the sinus node activity, whereas the left VN has a predominant control over the atrio-ventricular node function (Zandstra 2021). Both directly affect atrial and ventricular cardiomyocytes (Zandstra 2021). Indeed, homogenous data from the literature confirms the presence of a direct vagal effect on ventricular electrical and mechanical activity which is independent from the vagal effects on HR (Dusi and Ardell 2020). Accordingly, as opposed to the classical, old conception that cardiac vagal control only affects atrial and nodal tissues, acetylcholinesterase (AChE) staining in animals (Coote 2013) and humans (Kawano et al. 2003) clearly showed that cardiac cholinergic innervation is widespread across all cardiac chambers. Finally, cholinergic muscarinic receptors were localized in both the right and the left ventricle (Coote 2013; Fields et al. 1978). Notably, very relevant for vagal neuromodulation in pathological conditions, Vaseghi et al (2017) demonstrated in a porcine model the anatomical integrity of the cardiac parasympathetic neuronal network in border zones and in viable myocardium of infarcted hearts (as opposed to the sympathetic fibers). Despite anatomically intact, postganglionic parasympathetic neurons, studied with in vivo neuronal recordings, showed abnormal functionality both at rest and in response to stimuli, proving a complete disruption of cardiac vagal control after MI and reinforcing the strong rationale for therapeutic interventions aimed to restore a proper cardiac vagal output.
The main neurotransmitter released from the parasympathetic postganglionic terminal is acetylcholine (ACh), that exerts its action through binding to M1-M5 muscarinic metabotropic receptors (Finlay et al. 2017). Additional parasympathetic cotransmitters include NO, that facilitates ACh release and inhibits that of NE (from sympathetic neurons) at the presynaptic level, and VIP (vasoactive intestinal peptide), which may directly affect the coronary tone with vasodilatory effects, as well as the myocardium (Feliciano and Henning 1998; Henning and Sawmiller 2001). M2 receptors, a Gi-coupled type of receptors, are the most represented at the cardiac level and their activation reduces cAMP concentration and activates the G protein-coupled inwardly rectifying potassium channels (GIRK) channels (Bywater et al. 1989), being responsible for the chronotropic, dromotropic, lusitropic, inotropic, and bathmotropic negative effects of vagal stimulation (Giannino et al. 2024). GIRKs, better known as IKACh, are the main responsible for the short-term onset strong negative chronotropic effect of vagal activation. From a purely electrical point of view, the antiarrhythmic effect of efferent vagal activation at the ventricular level mostly relies on the antagonism of the pro-arrhythmic effect of excessive sympathoexcitation and the consequent intracellular calcium overload (Dusi et al. 2020).
On the other hand, M1 and M3 receptors, which are also expressed in the heart to a much lesser extent, are coupled to Gq/11 proteins and elicit the PLC pathway and therefore the production of inositol 3-phosphate (IP3) and diacylglycerol (DAG), which may act as feedback counter-regulatory actions to M2 receptor activity (Saternos et al. 2018). For instance, Tsuchida et al (Tsuchida et al. 2013) suggested that the positive inotropic action described after the direct exposure to high dosages of ACh in isolated human (Du et al. 1995) as well as animal cardiomyocytes (Gilmour and Zipes 1985; Yang et al. 1999) as opposed to the M2 receptor mediated negative inotropic effect of neuronally released ACh, may be mediated by the stimulation of M3 receptors. M3 receptors also play a cardioprotective role in models of myocardial infarction by activating several cytoprotective molecules, such as Bcl2, and cell adhesion or communication structures, such as connexin-43, in order to increase survival signals (Yang et al. 2005). Finally, the expression and the role of M4 and M5 receptor at the cardiac level is still debated, but there is no definitive prove so far (Palma 2024). Figure 2 summarizes the main G protein coupled receptors (GPCR) mediated autonomic pathways in cardiomyocytes.
Main GPCR mediated autonomic pathways in cardiomyocytes. The main neurotransmitter released by the vagus nerve is acetylcholine (in yellow in the figure), that exerts its cardiac effects mostly through M2 receptors, and, to a lesser extent, through M3 receptors. M2 receptors are a Gi-coupled type of receptors that inhibit adenylate cyclase and the production of cyclic adenosine monophosphate (cAMP), the main second messenger of β1-adrenergic receptors. Additionally, M2 receptors are also coupled with G protein-coupled inwardly rectifying potassium channels (GIRKs), better known as IKACh, that are the main responsible for the short-term onset strong negative chronotropic effect of vagal activation. Overall, M2 receptors are responsible for the chronotropic, dromotropic, lusitropic, inotropic, and bathmotropic negative effects of vagal activation. In the setting of I/R injury, they their activation antagonizes at the intracellular level the effects of catecholamines and protects the cells from intracellular calcium overload. In parallel, acetylcholine binding to M3 receptors promotes the release of diacylglycerol (DAG) and inositol-3-phosphate (IP3), which are instead involved in the negative modulation of M2 receptors, as well as in the activation of survival and cytoprotective pathways (Bcl-2; Connexin-43). β2 adrenergic receptors (a Gi-coupled type of receptors) are not shown for simplicity. Created with Biorender.com. β1AR: β1-adrenergic receptors; Bcl-2: B-cell lymphoma 2; cAMP: cyclic adenosine monophosphate; Cxn-43: connexin-43; DAG: diacylglycerol; ICaL/T: If: funny current; IP3: inositol-3-phosphate; MR: muscarinic receptor
Concerning the fibers’ spatial organization within the VN trunk at the cervical level, most pre-clinical data suggest an organotopic and function-specific distribution (Settell et al. 2020). Thompson et al (Thompson, et al. 2024), using an elegant combination of trial-and-error selective VNS and ex vivo micro-computed tomography fascicle tracing, recently showed in a porcine model a significant spatial separation of cardiac afferent and efferent fibers at the mid-cervical level. They also showed that cardiac afferent fibers are in proximity to pulmonary fibers consistent with recent findings of cardiopulmonary convergent neurons and circuits (Devarajan et al. 2022). According to most anatomical studies in humans, the auricular branch of the VN (ABVN) is the afferent cutaneous branch which innervates the antihelix, the tragus and the cavity of the concha (Peuker and Filler 2002). Interestingly, as early as 475-221 Before Christ, the healing power of the auricle was included in the Nei Jing, an ancient Chinese text. In Chinese ear acupuncture, the region affecting the heart was located precisely at the tragus level (Wirz-Ridolfi 2019). Accordingly, Frangos et al (2015) recently confirmed through functional MRI that stimulating the auricular branch of the VN in humans at the cymba conchae level activates the same brain regions as cervical VNS.
The devices: bioelectronic medicine applied to parasympathetic modulation
The expression “Bioelectronic Medicine” refers to an electrical current delivered to a nervous tissue (central or peripheral) to achieve targeted therapeutic benefits. Although conceptually the first use dates to the time of ancient Greeks and Romans, who used electric shock from torpedoes rays to relieve pain, it was not until the 19th century that bioelectronic studies began to take hold in routine clinical practice. However, in the absence of strong evidence, they were gradually abandoned in favour of drugs, until the last 30 years, when a renewed interest on electrical neuromodulation has emerged for several drug-refractory conditions (Heidland et al. 2013). In this context, vagus nerve stimulation at the cervical level (cVNS), where the nerve can be easily accessed surgically, has become increasingly popular. Due the extensive nerve distribution of the VN to various organs, cVNS has the potential on one side, to modulate a plethora of responses, on the other side, depending on its characteristics, to elicit off-target side effects. Since its first human approval by FDA in 1997 for drug-refractory epilepsy, to date studies on cVNS range from modulation of autoimmune, gastrointestinal, infectious, and cardiologic diseases (Fang 2023).
The two essential components of an electrical neuromodulation system are the generator of the electrical current and the electrode (or the electrodes) that delivers the current to the target. Based on the location of the entire system, electrical neuromodulation strategies can be classified in invasive in case of a cruel implantation or non-invasive in case of an entirely percutaneous systems. Based on the type of fibres targeted, the stimulation can be either selective or not selective. Finally, based on the possibility of integrating a biofeedback from the targeted organ/system by the stimulator, the stimulation can be at open-loop (no biofeedback) or at closed loop (with biofeedback).
In addition to the classical way of achieving VNS, namely invasive cervical VNS thought an electrode implanted in the neck around the VN, in the last decades additional non-invasive techniques have been evaluated, including cervical non-invasive VNS (nVNS) and transcutaneous auricular VNS (taVNS). Cervical nVNS has been only evaluated for neurological disorders (migraine (Fernández-Hernando et al. 2023) and acute cluster headache (Fernández-Hernando et al. 2023)) so far, with an an outstanding tolerance and safety profile and promising efficacy results. The GammaCore (gammaCore®, electroCore, Inc.) is the only FDA approved clinical device for nVNS, in which the two electrodes are embedded on top of the generator. It is not very suitable for the usage in critical patients, where the neck region might be needed for other vascular/invasive procedures. On the other side, taVNS beyond being a not expensive technique, almost free from infectious risk, is also suitable for an acute setting since stimulation is applied at the ear, far from sites that might be used for interventional purposes. TaVNS is being investigated for a wide range of clinical applications but is still not FDA-cleared for any of them.
The first two most widely used commercially available taVNS devices were the NEMOS (NEMOS, taVNS technologies) and the Parasym (Parasym Ltd). The NEMOS device has been granted the European mark (CE certification, indicating legal conformity and safety, but not necessarily clinical efficacy) for the treatment of resistant epilepsy and depression in 2010, for chronic pain in 2012 and for anxiety in 2019 (Farmer et al. 2020). The second-generation device produced by the same company as the NEMOS, named Tvns-L, has CE mark since 2021 for several indications including neurological (anxiety, autism, cognitive impairment, depression, epilepsy, migraine, Parkinson's, Prader-Willi Syndrome, sleeping disorders, stroke, tinnitus), cardiological (atrial fibrillation) and immune/inflammatory (asthma, Crohn's disease, fibromyalgia) conditions. The first generation (Parasym) and the second-generation (Nurosym) taVNS device from the Parasym Ltd company have both been granted CE mark. The main differences between these systems are in the characteristics of the stimulating electrode, that only in the Parasym device has a clip design. The Parasym device has been the most studied for cardiovascular disorders so far, with one randomized study in atrial fibrillation (Stavrakis et al. 2020), two randomized studies in HF with preserved EF (Stavrakis et al. 2022; Tran et al. 2019), one randomized study in acute decompensated HF (Dasari, et al. 2023) and one randomized study in postural tachycardia syndrome (Stavrakis et al. 2024).
An additional device, the taVNS Stimulator by Soterix Medical, is currently also available, and it’s the only one with 3 different types of electrodes available (including a clip-type).
Concerning the safety profile, the non-invasive nature of taVNS granted it an impressive safety record. A systematic review by Redgrave et al (2018) summarized all the available literature on taVNS, not limited to cardiovascular diseases, and found that local irritation (tingling/pain/redness/itching) is the most common discomfort (16.7%) that is attributable to stimulation, with headache being the second (3.3%) and nasopharyngitis (1.6%) the third. Dizziness and syncope had a prevalence of 1.4%. However, only three severe adverse events were considered taVNS-related.
The dose response issue
Unlike drugs, electrical neuromodulation requires, to obtain a single "dose" of treatment, the integration of dozens of different parameters, which depend both on the device-target interface and on the mode of energy delivery, so that the chosen "dose" of treatment can vary among hundreds of possibilities (Dusi et al. 2022). Table 1 summarizes the most relevant, that can be academically divided into electrode and current-related parameters, stimulation modality–related parameters, and safety parameters.
Regarding the interface, depending in fact on the material used, its geometry, the location of the electrode, the number of poles, and how it relates to the organ, there might be different resistances offered to the passage of current (González-González et al. 2024); on the other hand, as far as the mode of energy delivery is concerned, regardless of whether a biofeedback is integrated or not, there are many modulable parameters, such as pulse frequency, pulse width, on/off cycle duration (duty cycle), and active waveform; or again, for wave amplitude, current amplitude and titration method used to reach that amplitude (Elamin et al. 2023).
Such complexity reflects the highly integrated and extremely dynamic behavior of the therapeutic target, namely the ANS, that is still far from being fully understood.
For cVNS, a major issue, that is deeply influenced by the stimulation parameters and the electrode’s characteristics, is represented by the choice of the type of stimulation to pursue to improve efficacy and limit/avoid off target side-effects, namely a preferentially efferent stimulation rather than a combined afferent and efferent stimulation and, even better, an organ/fibres specific stimulation (otherwise known as selective stimulation). The main limit to selective cVNS is the still limited knowledge of the functional anatomical organization of the vagus nerve across species and particularly in humans (Ottaviani and Macefield 2022). Transauricular VNS is by definition a purely afferent kind of VNS, yet the stimulation protocol may have a profound impact on the central effects and the consequent modulation of efferent vagal output (Farmer et al. 2020). For instance, most clinical applications of taVNS used a 20–30 Hz frequency stimulation, but Sclocco et al (2020) reported a significantly greater effect of high-frequency (100 Hz) stimulation on the modulation of medullary vagal nuclei activity. Also, another critical issue is whether taVNS should be delivered in a respiratory gated way. Indeed, physiologically, afferent inputs to the nucleus tractus solitarius (NTS) are modulated by rhythmical oscillations in the respiratory cycle, with a major degree of receptivity during exhalation (Gilbey et al. 1984; Paton et al. 2022). Accordingly, gating taVNS to exhalation was proved to significantly potentiate its effects on vagal medullary nuclei activity, peripheral cardiovagal response and blood pressure levels (Sclocco et al. 2019). This technique is known as respiratory-gated auricular vagal afferent nerve stimulation (RAVANS) (Garcia et al. 2022).
Unfortunately, clinical trials were started long before the dose issues were solved, resulting in different and often not comparable stimulation protocols, that may contribute to understand the translational troubles encountered, for examples, for cVNS in the setting of chronic HF, where most randomized clinical trials were unable to fully confirm the promising preclinical results (Dusi and Ferrari 2021; Ferrari 2014).
Preclinical data on cervical VNS
Arrhythmias and heart failure
The beneficial effects of invasive cVNS have been studied for decades in pre-clinical models of acute and chronic myocardial ischemia, mostly focusing, in the beginning, on the acute antiarrhythmic efficacy and on the chronic anti-remodelling benefits, and only more lately directly assessing the acute effects on I/R injury.
Between 1973 and 1978, several studies on anesthetized animals (Yoon et al. 1977; Kolman et al. 1975; Kent et al. 1973; Myers et al. 1974) showed that cVNS reduces the risk of VF during acute myocardial ischemia; one of them (Myers et al. 1974) also demonstrated that the acute antifibrillatory effect of cVNS was not abolished by preventing HR decrease nor by VN decentralization, underscoring a direct ventricular efferent effect. The final demonstration came in 1991 from a conscious canine model of sudden death (Vanoli et al. 1991): cholinergic antagonism favored VF, while right cVNS, applied a few seconds after a 2-minutes circumflex coronary occlusion in the setting of a previous anterior MI and an ongoing exercise stress test, protected against VF. Almost 50% of the antifibrillatory effect was due to the significant right cVNS induced HR lowering.
Starting from the early years 2000, right cVNS began to be evaluated in models of chronic HF with reduced ejection fraction (HFrEF). In 2004, Li et al (2004) showed in a randomized study on conscious rats with a previous 14 days old large anterior myocardial infarction, that right cVNS stimulation applied for 6 weeks was associated with better left ventricular (LV) function, lower normalized biventricular weight, lower norepinephrine and BNP levels and a strikingly better survival (86% versus 50%) at 140 days compared to sham-operated animals, despite a similar infarct size. Right cVNS (10 sec on, 50 sec off) was delivered at 20 Hz, with 0.2 ms pulses and the intensity was adjusted to reduce HR by 20-30 bpm from a starting value of 360 bpm. The beneficial effects of right cVNS (set to achieve a 10% HR lowering effect) were subsequently confirmed on a canine model of coronary microembolizations induced HFrEF (Sabbah et al. 2011) and proved to be additional to those achieved with metoprolol alone (Hamann et al. 2013). In addition to the favorable LV remodeling, a significant impact of a 3-month period of cVNS on several biomarkers was also demonstrated: TNF-α and IL-6 decreased, while NOS and connexin 43 (Cx43) expression tended to normalize. cVNS still retained beneficial effects when applied to the same animal model using a different VNS stimulation protocol specifically set with a low stimulation intensity, unable to affect HR (Hamann et al. 2013). Accordingly, VNS elicited significant benefits in LV hemodynamics, C-reactive protein, norepinephrine and angiotensin II levels, HR variability and baroreflex sensitivity, compared to controls, even in a high-rate ventricular pacing induced HFrEF canine model (Zhang et al. 2009). VNS intensity was individually set before starting pacing to reduce sinus rate by approximately 20%, but HR was subsequently kept constant by pacing. These data were recently confirmed by an elegant optogenetic study (Machhada et al. 2020) showing that a purely efferent VNS with almost no impact on HR led to a significantly better cardiac function (compared with the corresponding sham-control group) both in normal and in post-MI rats, despite no changes in myocardial infarct size (30% of LV mass). VNS was achieved through a 4-week program of intermittent optogenetic activation of cholinergic neurons in the vagal dorsal motor nucleus, and, in the infarcted animals, it was started 48 hours after MI induction, therefore not leading to an infarct size reduction, that would have required an earlier application. Impressively, LV function almost normalized in post-MI rats, despite a similar infarct size (at least 30% of LV mass), an effect much more pronounced than the one observed in the landmark model by Li et al (2004) , where, as previously mentioned, cVNS was started 14 days after MI. Finally, the authors also reported an improvement in cardiac function caused by optogenetic efferent VNS in normal rats, that was related to a reduced myocardial expression of GRK2 (G protein–coupled receptor kinase 2) and of β-arrestin 2, both involved in β1-adrenergic receptor desensitization and internalization. These data suggest the apparently paradoxical potential for cardiac efferent vagal activation to improve sympathetic responses of cardiomyocytes despite its well established anti-adrenergic effects, underscoring the complexity of sympathetic-parasympathetic interaction (Dusi et al. 2020) and supporting the recent concepts that that cardiac vagal nerve activity actually increases (rather than decreasing as traditionally believed) during exercise, and that the amount of cardiac vagal tone may not only reflect the fitness level of a subject, but may also determine his/her cardiac performance (Gourine and Ackland 2019). Additionally, back in 1998, Feliciano and Henning demonstrated that 5 minutes trains of bilateral cVNS at 20 Hz applied in anesthetized healthy dogs during muscarinic and beta-adrenergic blockade, cause significant coronary artery dilation that is prevented by a VIP antagonist (Feliciano and Henning 1998). A dynamic modulation of coronary artery blood flow during exercise associated with an increase in cardiac vagal activity (directly measured) and mediated by VIP (Shanks et al. 2023) was recently confirmed in a conscious sheep model. The increased NO availability associated with cVNS in animal models (Brack et al. 2007) and proved to be dependent upon the stimulation parameters (higher during low voltage and high frequency stimulation) may also contributes to this effect (Allen et al. 2022).
Concerning the dose response issues, some pre-clinical studies were performed to shed light on this matter. Kong et al (2012) evaluated the efficacy of different protocols of right cVNS, applied for 240 minutes after coronary artery ligation in anesthetized rats, in reducing the infarct size. They found that the combination of parameters leading to the largest cardioprotective effect (lower amplitude, lower frequency, and longer duration of stimulation) was not that producing the greater HR decrease. These data suggest that a purely efferent cVNS, even if feasible, may not necessarily be the best option, since afferent fibers activation might significantly contribute to the beneficial effects of cVNS. Indeed, sensory afferent vagal fibres, whose bodies reside in the nodose ganglion, sense cytokines and metabolites released during inflammation/ischemia, transmitting the signals to upper nervous structures (such as NTS) involved in generating brain-derived neural control of inflammation (Dusi and Ardell 2020; Dusi 2020). Accordingly, back in 1973 (Schwartz et al. 1973) Schwartz et al demonstrated that selective afferent cVNS increases contralateral cardiac vagal output while reducing ipsi- and contralateral cardiac sympathetic output (Schwartz et al. 1973). In order to clarify the relative contribution of afferent versus efferent fibers activation to the acute HR responses elicited by chronic right cVNS, a wide range of stimulation parameters was recently tested in a canine conscious model (Ardell et al. 2017), including electrode configuration (anode/cathode inversion), pulse frequency (2–20Hz), intensity (0–3.5 mA) and pulse widths (130–750μs). HR responses were determined for each combination over 14 months. Based on frequency–amplitude–pulse width, the authors identified an operating point, defined as “neural fulcrum” where a null HR response was evoked during the on-phase of cVNS. They showed that chronic cVNS, when delivered within the constraints of the neural fulcrum in healthy animals, was able to maintain the circadian control of HRV. In 2023 (Hadaya et al. 2023), the same group reported for the first time the effects of right cVNS (5 Hz, 250-μs) delivered with a stimulation amplitude decided according to the neural fulcrum, in a mini-pig model of MI. Three groups were studied: control subjects, untreated MI, and MI + right cVNS, with cVNS started 48 hours after distal LAD embolization. Notably, to guarantee therapeutic levels of cVNS, a 4-week titration period was used before MI; cVNS was then stopped after titration at the time of acute MI and then resumed 48 hours later at the previously determined intensity. The authors reported a pronounced mitigation of the unfavorable functional and structural remodeling in the ischemic border zone, in the stellate ganglia and in the dorsal root ganglia (including both neurons and glial cells), that was accompanied by an improvement in cardiac function, a marked reduction in induced ventricular arrhythmias, and the preservation of normal cardiac sympathetic responses compared with untreated MI. Notably, all these favorable effects of cVNS were observed despite no changes in myocardial infarct size between treated and untreated animals, underscoring the importance of a very early parasympathetic modulation in order to also reduce the infarct size (Dusi et al. 2023). The beneficial effects of cVNS on the unfavorable structural remodeling affecting the stellate ganglia fully confirms previous findings showing that continuous low-level cVNS reduces stellate ganglion nerve activity in healthy dogs (Shen et al. 2011).
Myocardial I/R injury
The first, pioneer demonstration of the protective and only partially HR-dependent effect of right cVNS on reperfusion arrhythmias was provided by our group back in 1987 (Zuanetti et al. 1987), but remained neglected for a long time, until it was finally understood that reperfusion-related arrhythmias and myocardial I/R injury share a common pathophysiological pathway that is antagonized by cVNS. In both settings, the cardioprotective effects of VNS (Kakinuma et al. 2005; Uemura et al. 2010; Mioni et al. 2005), similarly to the ones of ischemic preconditioning (Krieg et al. 2004), are mediated by the intracellular activation of phosphatidylinositol 3-kinase and Akt pathways in cardiomyocytes. The consequence is the upregulation of the anti-apoptotic protein BCL-2 and the suppression of caspase-3, that both concur to a powerful protection from cell death (Katare et al. 2009). Notably, an increase in cardiac efferent vagal tone is also likely to mediate the beneficial effect of remote ischemic preconditioning (Crimi et al. 2013; Donato et al. 2013).
In 2013, Shinlapawittayatorn et al (2013) were the first to compare the acute cardioprotective effects of intermittent and continuous left-sided cVNS in a porcine model of I/R injury, as assessed at the end of reperfusion. Left cVNS (20 Hz, 3.5 mA, 500 μs pulse width) was applied at the onset of a 1-hour complete left anterior descending artery occlusion (LAD) and throughout the end of a 120-minute period of reperfusion, either intermittently (with recurring cycles of 21-second ON and 30-second OFF) or continuously. In both cases, cVNS significantly reduced infarct size, improved LV function, decreased VF episodes, and attenuated cardiac mitochondrial ROS production, compared with the control group, but the amount of benefit, particularly of infarct size and VF episodes reduction, was greater with intermittent cVNS (approximately 89% infarct size reduction). In a subsequent work on the same model (Shinlapawittayatorn et al. 2014) the group assessed whether left cVNS (3.5 mA, 20 Hz, with continuously recurring cycles of 21-second ON, 30-second OFF) applied late during ischemia (30 minutes after LAD occlusion) or at the onset of reperfusion differentially protected against cardiac I/R injury. They showed that left cVNS started during late ischemia, but not at reperfusion, still retained beneficial effects on electrical stability, infarct size (59% reduction), cardiac function, inflammatory biomarkers, ROS production and phosphorylated Cx43 levels, providing an important translational message. Of note, since all the assessments of efficacy were performed at the end of reperfusion, the study cannot evaluate the potential for late beneficial effects of cVNS.
In 2018 (Nuntaphum et al. 2018) the same group assessed the acute effects of intermittent left cVNS with the abovementioned characteristics, according to the integrity of the ipsilateral and contralateral VN in an identical I/R porcine model. Thirty swine were randomized into five groups: I/R injury with no cVNS and left cVNS with both vagal trunks intact (LC-VNS), with left VN transection (LtVNX, selective left efferent stimulation), with right VN transection (RtVNX) and with atropine pretreatment, respectively. LC-VNS and LtVNX produced a similar and more profound cardioprotective effect (using infarct size as primary endpoint, an 89% reduction for LC-VNS and a 84% for LtVNX was observed) compared to RtVNX (63% reduction), suggesting on one side, a predominant ipsilateral efferent fibers stimulation with this cVNS protocol, on the other side, the importance of a bilateral efferent vagal activation, to achieve the full beneficial effects of cVNS in response to such a powerful proinflammatory and prooxidative stressor as I and R. Accordingly, in an in vivo rat model of acute myocardial I/R injury (Wang et al. 2015), the cardioprotective effects of cVNS applied as perconditioning (during ischemia) in combination with limb remote ischemic perconditioning (that was also proved to elicit an increase in cardiac vagal output (Mastitskaya et al. 2012)) were superior to those achieved with either treatment alone, underscoring once again the importance of powerful vagal responses to counteract I/R injury. Notably, in the 2018 study (Nuntaphum et al. 2018) all VNS-treated groups showed a significant reduction in myocardial infarct size and the effect was reversed by atropine. The study also confirmed that cVNS improves mitochondrial function and shifts cardiac fatty acid metabolism toward beta oxidation. An impressive antiarrhythmic effect during I/R was also demonstrated, as indicated by a reduced premature ventricular complexes (PVC) burden, VT/VF incidence, T-wave peak to end and T-wave peak to end per QT interval ratio, suggesting that cVNS decreases the heterogeneity of ventricular repolarization. These effects were related to a combination of reduced myocardial infarct size and increased phosphorylation of Cx43, a key protein in gap junctions that enables intercellular communication and electrical coupling. Accordingly, a reduced Cx 43 concentration at the ventricular level during ischemia has been correlated to a higher incidence of lethal VAs (Lerner et al. 2000).
Another study conducted by Ando et al (2005) further stressed the influence of Cx43 on ventricular arrhythmias during acute myocardial ischemia, as well as the protective effects of VNS. The occurrence of arrhythmias was lower in rats subjected to cVNS, which also showed higher levels of phosphorylated Cx43 when compared to the sham group. The biomolecular pathway through which cVNS may prevent ischemia-induced reduction of phosphorylated Cx 43 is mediated by the inhibition of its degradation through the muscarinic receptor, displaying a complementary beneficial effect compared to the anti-inflammatory one mediated by nicotine receptors. All this evidence suggests that functional preservation of phosphorylated Cx43 by cVNS would play an important antiarrhythmic effect in the setting of acute ischemia.
Finally, a recent pooled review (Xu et al. 2023) including a total of 10 preclinical studies published from 2011 and 2019, assessing the acute cardioprotective effects of cVNS against myocardial I/R injury and satisfying the inclusion criteria, confirmed that cVNS was associated with a significant reduction in myocardial infarct size [Weighted mean difference (WMD): 25.24, 95% confidence interval (CI): 32.24 to 18.23, p < 0.001] when compared to the control group. A significant improvement in LVEF (WMD: 10.12, 95% CI: 4.28; 15.97, p = 0.001) and end-diastolic pressure (EDP) (WMD: 5.79, 95% CI: 9.84; -1.74, p = 0.005) was also detected. Notably, no significant influences of pre-specified covariates (i.e., stimulation type or site, VNS duration, condition, and species) on the primary estimates were identified at the meta-regression.
Post-cardiac arrest syndrome
A peculiar clinical condition that shares several pathophysiological pathways with I/R injury is the so called post-cardiac arrest syndrome (PCAS). The term refers to the severe condition that may follow a cardiac arrest (CA), whatever the causes are. The syndrome is characterized by myocardial dysfunction, microcirculatory dysfunction, global brain injury, increased vulnerability to infection, and persistent advancing pathology (Penketh and Nolan 2023). Notably, VF complicating an acute coronary occlusion represents the most common cause of cardiac arrest in the adult population in western countries (Fox et al. 2004), further stressing the potential link between PCAS and cardiac I/R injury sometimes as two sides of the same, life-threatening, coin. Accordingly, a reduced vagal tone and an increased sympathetic output were both proved to favor the occurrence of VF during acute coronary occlusion both at the pre-clinical (Schwartz et al. 1984) and at the clinical level (Ferrari et al. 2020).Notably, we were the first to demonstrate that physical inactivity, that has been consistently associated with reduced vagal tone and reflexes (Gourine and Ackland 2019), significantly increases the risk of VF occurring during a first myocardial infarction in humans (Ferrari et al. 2019). Yet, the possibility that interventional neuromodulation strategies, and specifically cVNS may exert acute beneficial effects even on PCAS was only recently unraveled.
Three studies assessed the effects of cVNS in this setting, using a model of asphyxia cardiac arrest in 2 cases (both receiving left cVNS) (Kim et al. 2022; Choudhary et al. 2022) , and one of VF-induced CA in the third one (treated with right c-VNS) (Sun et al. 2018). In the first study (Choudhary et al. 2022), anesthetized rats were assigned to either post-resuscitation cVNS for 2 h or no cVNS (control group) after 12 minutes of CA. In comparison to the control group, cVNS significantly improved 72 h survival and brain functional recovery, reduced the number of damaged neurons in the CA1 hippocampal region of the brain, as well as the levels of plasma troponin I and creatinine. In the second study (Kim et al. 2022), using anesthetized male rats, cardiopulmonary resuscitation (CPR) was performed 450 seconds after pulseless electrical activity. After the return of spontaneous circulation (ROSC), left cVNS (1 Hz, 1 mA, pulse duration 10 ms) was performed for 3 h in the CA + cVNS group. VNS ameliorated mitochondrial dysfunction in the hippocampus after ROSC and improved neurological outcomes. In the third study (Sun et al. 2018), forty rats were randomized into four groups, and all underwent CPR (n = 10 each): CPR alone (control); cVNS during CPR; α7nAChR antagonist with cVNS; α7nAChR agonist without cVNS. VF was induced and untreated for 8 min and defibrillation was attempted after 8 min of CPR. cVNS (10 Hz, voltage 2–6 V, pulse duration 0.5 ms) was initiated at the beginning of precordial chest compressions and continued for 4 h after ROSC. VNS-treated animals showed a better post-resuscitation myocardial and cerebral function and improved survival. The protective effects of cVNS were abolished by the α7nAChR antagonist and reproduced by the α7nAChR agonist, once again confirming the importance of the CAP. In addition, cVNS decreased the number of electrical shocks and the duration of CPR required for stable ROSC.
Preclinical data on taVNS in I/R injury
Like cVNS, taVNS has been mostly evaluated in preclinical models of chronic HF so far (both with reduced (Wang et al. 2014) and with preserved EF (Zhou et al. 2019)), with promising results. In a rat model of HF with preserved EF induced by high salt intake (Zhou et al. 2019), taVNS, beyond promoting a favourable reverse remodelling with reduced cardiac inflammation and fibrosis, was associated with a lower expression of pro-inflammatory and pro-fibrotic genes (TNF-α, osteopontin, interleukin (IL)-11, IL-18 and IL-23A). Chronic taVNS was delivered at 20 Hz frequency, 2 mA amplitude and 0.2 ms pulse duration, for 30 minutes daily over a 4-week period. Notably, during acute stimulation active taVNS induced a small, but significant heart rate drop. Only a few studies assessed the effectiveness of taVNS in the acute setting of myocardial I/R injury (Elamin et al. 2023).
A combined acute and chronic study was the one reported in 2014 by Wang et (2014), that assessed the long-term effects of low-level tragus stimulation applied since the day of coronary artery ligation in conscious dogs with anterior MI. In the treatment group, taVNS (20 Hz, pulse width 1 ms, with duty cycle of 5 sec on and 5 sec off) was delivered 4 hours per day bilaterally (from 7 to 9 AM and from 4 to 6 PM) to the tragus at 80% below the threshold which slowed sinus rate. After a 90-day period follow-up, taVNS significantly reduced LA and LV dimensions, improved LV systolic and diastolic function and reduced infarct size by ≈50% compared with MI group. These beneficial effects were accompanied by reduced levels of profibrotic factors (collagen I, collagen III, transforming growth factor β1, and matrix metallopeptidase 9), hs-CRP, norepinephrine and NT-proBNP starting from the 7th day to the end of follow-up. In the same canine model (Yu et al. 2016), it was subsequently demonstrated that a right-side only low-level taVNS lasting 2 hours/day per 2 months significantly reduced VAs inducibility, decreased cardiac sympathetic output, as testified by a reduced left stellate ganglion (LSG) activity, and attenuated cardiac sympathetic remodeling induced by chronic MI compared to controls, as testified by NGF downregulation and small conductance calcium-activated potassium channel type2 (SK2) protein up-regulation within the LSG.
In the neurological field, a number of pre-clinical studies indicate that taVNS delivered in acute middle cerebral artery occlusion reduces infarct size through anti-inflammatory effects, reduced excitotoxicity and increased blood-brain barrier integrity (Baig et al. 2022). The long term neuroprotective effects of taVNS in stroke may be mediated by microglial polarization, angiogenesis and neurogenesis (Baig et al. 2022), with peroxisome proliferator-activated receptor-γ (PPAR-γ) being a candidate mediator of taVNS-induced angiogenesis and neuroprotection against cerebral I/R injury (Li 2020). A recent systematic review and meta-analysis (Melo et al. 2023) including a total of 8 pre-clinical studies published between 2015 and 2022, confirmed, albeit with the limitations of a high heterogeneity and high risk of bias of the included studies, a significant reduction in neurological deficits (SMD = -1.97, 95% CI -2.57 to -1.36, I2 = 44%) and in neuronal infarct size (SMD = -1.51, 95% CI -2.42 to -0.60, I2 = 58%) following taVNS. Markers of neuroplasticity were also significantly improved, as testified by an increased microcapillary density, CD31 proliferation, and brain-derived neurotrophic factor (BDNF) protein levels and RNA expression. This data underscores the strong cytoprotective effect of taVNS in the setting of I/R injury independently on the cell type exposed to ischemia, as also suggested by the favorable pre-clinical results of cVNS in PCAS. Of note, as expected, as for cardio protection, even for neuroprotection, amelioration of mitochondrial dysfunction played a pivotal role (Kim et al. 2022).
Summary of pre-clinical data of cVNS and taVNS
Overall, pre-clinical data consistently showed a strong cardioprotective effect against acute and chronic cardiac injuries of both cVNS and taVNS (Elamin et al. 2023), even when applied at a low-level, meaning at a stimulation intensity unable to evoke acute HR changes. In the setting of myocardial I/R damage, the earlier the application, the greater the benefit (Dusi et al. 2023). No study has ever directly compared in the same model the amount of benefit of the two techniques, that do share the same final pathway represented by an improved autonomic balance (Dusi and Ardell 2020). The main unraveled mechanisms underlying the previously described beneficial effects of cVNS at the cardiac level (Fig. 3) include a direct antiadrenergic effect (both at the central and at the peripheral level, pre-synaptic and post-synaptic) that encompasses a HR-lowering effect, pre-conditioning like effects, anti-apoptotic and antioxidant effects, pro-angiogenic and anti-inflammatory effects (Dusi and Ardell 2020; Dusi et al. 2020). The majority of these effects, as well as the underlying molecular pathways, have already been confirmed as the bases for the cardioprotective actions of taVNS (Table 2); the remaining, that include the metabolic and the mitochondrial effects, still lack a direct demonstration at the cardiac level but were consistently associated with the cerebral protection provided by taVNS against neuronal I/R damage. Since both cVNS (Frangos et al. 2015; Shen et al. 2011; Komisaruk and Frangos 2022) and taVNS (Wang et al. 2014) were proved to reduce cardiac sympathetic output, a reduced released of the proarrhythmic and vasoconstrictor NPY, that, as already mentioned, is co-released by sympathetic neurons in the setting of high-level activation, could be inferred. The first, direct confirmation that cVNS can reduce in vivo NPY levels in the ventricles during bilateral stellate ganglia stimulation in healthy pigs was only recently reported (Jani et al. 2024) and associated with the activation of presynaptic sympathetic muscarinic receptors.
Potential approaches to increase cardiac vagal output before (green), during (yellow) and after (red) myocardial ischemia and reperfusion. NPY Neuropeptide Y; VIP: Vasoactive intestinal peptide. Modified from Dusi et al. 2023
As already underlined, cVNS directly delivers a mixed (afferent and efferent) stimulation, with the relative afferent and efferent contribution depending on the electrode characteristics and the stimulation protocol (Dusi and Ferrari 2021), while taVNS directly conveys a purely afferent stimulation. Additionally, cVNS affects most vagal afferent fibers (including all the cardiac ones), while taVNS only those terminating at the auricular level. Therefore, from a theoretical point of view, cVNS would be expected to be more effective than taVNS. Yet, it must be remembered that, lacking a direct efferent effect, taVNS might be a more physiological way to increase cardiac vagal output because the central integration leading to the improved autonomic balance is preserved (as for BAT). Accordingly, the entire rationale of the “neural fulcrum” (Ardell et al. 2017) concept proposed by Ardell and colleagues for cVNS relies on the concept that the best way to achieve cVNS is the one not acutely leading to a significant HR change while chronically preserving the physiological circadian HR pattern. Table 3 summarizes the main studies assessing the effects of cVNS in myocardial I/R injury and in post-cardiac arrest syndrome and the effects of taVNS in the setting of acute (within 24 hours) myocardial ischemia.
Clinical data on taVNS in myocardial I/R injury
As already mentioned, taVNS may overcome the issues related to the use of an invasive method, particularly in the acute setting, while retaining the advantages of invasive cVNS.
In humans, taVNS has already been tested in several non-cardiological conditions including neurological disorders (epilepsy, depression, migraine, tinnitus, anxiety, mild cognitive impairment, chronic stroke) (Baig et al. 2022; Zhu et al. 2022; Tan et al. 2023) and inflammatory disorders (acute COVID-19 infection (Uehara et al. 2022) and long COVID (Badran, et al. 2022)). Specifically, pilot clinical trials of taVNS show that taVNS paired with rehabilitation may improve upper limb motor and sensory function in patients with chronic stroke (Baig et al. 2022). At present, there are several registered randomized clinical trials of taVNS in stroke (Baig et al. 2023).
In the cardiovascular area, most clinical studies with taVNS were performed in the setting of atrial fibrillation and of HF with both reduced and preserved LVEF, with promising results (Elamin et al. 2023); notably, in all studies a consistent reduction in inflammatory markers was observed (Elamin et al. 2023). As already mentioned, the Parasym device has been the most studied in these settings so far (Elamin et al. 2023). Concerning the stimulation protocols, all cardiac studies used a low-level (unable to acutely affect HR) mode of taVNS delivery, mostly at a 20 Hz pulse frequency and a 0.2 msec pulse duration, and with a current amplitude mostly titrated up to a 1 mAmp below the discomfort threshold (typically in the range of 10–50 mA). Yet, the overall daily duration of the taVNS was extremely variable, from a minimum of 1 hour in outpatients with atrial fibrillation (Stavrakis et al. 2020) to a maximum of 8 hours (4 hours twice daily, between 6 AM-10 AM and 6 PM −10 PM) in hospitalized patients with acute decompensated HF (Dasari, et al. 2023).
Specific clinical experiences in the setting of acute and chronic coronary artery disease are very limited. Back in 2001, Zamotrinsky et al (2001). were the first to report, in a small, randomized study including a total of 18 patients, about a custom-made form of taVNS for the treatment of patients with severe refractory angina candidates to coronary artery bypass grafting (CABG). Patients were randomized 1:1 to taVNS or control (with no sham treatment). All patients were male, aged 48–58 years, with stable angina pectoris class IV of the Canadian and Cardiovascular Society classification, with daily anginal attacks at rest and under a low workload as assessed by bicycle exercise testing. None of them had diabetes mellitus or atrial fibrillation; LVEF range was 49–59%. After admission, the patients spent 25–30 days in the hospital awaiting surgery, during which they received a course of 10 stimulation procedures lasting 15 min, repeated for 10 consecutive days. TaVNS was delivered with an impulse current generator connected to a pair of electrodes and attached to short acupuncture needles put into the skin. TaVNS treatment abolished angina at rest, decreased HR and blood pressure, improved LVEF and LV diastolic filling and decreased the QRS as well as the QT interval (with an associated T wave morphology improvement represented by augmented T-wave amplitude in precordial leads). In the postoperative period, the percentage of patients developing HF dramatically decreased from 90% in the taVNS group to 12% in the control group. of patients treated with taVNS. Intriguingly, taVNS was reported to have acute anti-anginal effects like that of glycerol trinitrate. Of note, almost none of the patients was on beta-blockers as per procedure local management in patients with depressed LVEF, and that might have amplified the effects of taVNS. Finally, surgical specimens obtained at the time of surgery showed a higher density of cardiac microcirculatory vessels in the taVNS group compared to controls.
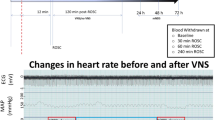
More than 15 years later, in 2017, Yu et al (2017) reported the first and, so far, only, proof of concept clinical study, of taVNS in I/R injury. Patients presenting with sopraST elevation myocardial infarction (STEMI) within 12 hours of symptom and undergoing primary percutaneous coronary intervention, were randomized 1:1 to active right taVNS (n=47) or sham-stimulation (n=49). Patients with left main or multiple coronary artery disease were excluded. Low-level taVNS was delivered at 50% below the sinus rate lowering amplitude threshold, at a pulse frequency of 20 Hz and pulse duration of 1 ms with a duty cycle of 5 sec on and 5 sec off. Active stimulation or sham control was started as soon as the patients arrived in the cardiac catheterization lab and maintained for 2 hours after reperfusion (balloon dilation); patients were subsequently monitored for the next 7 days. Most patients (75%) were male, 32% had diabetes (not better characterized), 66% had the LAD as culprit artery and the mean ischemia time (from symptoms onset to reperfusion start) was 6 hours. Reperfusion-related ventricular arrhythmias, assessed with 24 hours Holter ECG, were markedly reduced by taVNS, both the isolated PVCs, and the repetitive forms; indeed, ventricular tachycardia episodes were 18±12 in the sham compared to 2±3 in the active group (p < 0.05). Inflammatory marker levels at 24 hours after reperfusion (including IL-6, IL-1β, high mobility group-box 1 protein, TNF–α) were also significantly improved, despite no differences at the first blood sampling determination before reperfusion. The area under the curve over 72 h for the markers of myocardial damage (creatine kinase-MB and myoglobin) was smaller in the taVNS group, and that was associated with lower NT-proBNP levels, and better cardiac systolic and diastolic function as assessed by blinded 2-dimensional echocardiography assessment on days 5 to 7 after revascularization (LVEF 52± 64% versus 49± 8%, p= 0.01). The main limitation of this study is that it lacks a detailed estimate (by cardiac MRI) of the final infarct size compared to the initial area at risk to further reinforce these findings and provide a better comparison with animal data; also, the troponin levels were not provided. As expected, the feasibility, tolerability and safety profile of the device were excellent since no side-effects nor discontinuation cases were reported. All patients were conscious and were not given any sedatives during taVNS. Notably, as opposed to pre-clinical studies, the usage of taVNS on unconscious patients has never been reported so far. Local electrical capture, central integration of the inputs and peripheral effects are expected to be influenced by the level of consciousness and/or the usage of neurodepressant drugs, and that might be an issue for the taVNS implementation, for instance in the emergency setting of PCAS. On the other side, the feasibility of taVNS in an acute setting with preserved consciousness, such as acute decompensated heart failure (ADHF) was recently confirmed (Dasari, et al. 2023).
Future prospectives
The non-invasive nature of taVNS, in addition to its strong pathophysiological rationale shared with cVNS, makes it a very appealing treatment option for several conditions including I/R injury. Yet, there are still important issues slowing the clinical transition of both taVNS and cVNS, being the extensive assessment of the dose response relationship the most important one.
A better understanding of the functional anatomical organization of the vagus nerve across species is highly needed and currently pursued through ongoing clinical (cadaveric) and pre-clinical studies using advanced imaging techniques such as micro-computed tomography (Thompson et al. 2020). Despite that, several studies with hundreds of subjects would be required to identify the most suitable electrode configuration and stimulation protocol, with obvious ethical and practical concerns. Computational model strategies (Capogrosso and Lempka 2020) integrated with artificial intelligence (AI) techniques (Gallone et al. 2022) are expected to help overcoming these huge limitations of in vivo studies, and to provide an important drive in the clinical implementation of electrical neuromodulation in the near future. For instance, a multilayer computational model for neurocardiac modulation that includes sympathetic and parasympathetic branches, synaptic dynamics and conductance based integrate-and-fire neurons was recently developed. The pivotal characteristic of the model is that it recapitulates the connection between the autonomic nervous system and both pacemaker and contractile cardiomyocytes (Yang et al. 2023). The model was specifically developed to evaluate the impact of autonomic changes on arrhythmias susceptibility, but a similar model aimed to assess the effects on infarct size, albeit much more complex to realize, is now foreseeable (Liu and Ajijola 2023). Additionally, extending the now well-established health care applications of AI from the clinical (D’Ascenzo et al. 2021; Filippo et al. 2023; Saglietto et al. 2024) to the pre-clinical field (Diaconu et al. 2022) will lead to a tremendous improvement of the mechanistic understanding of the interactions between electric fields and neural circuits, that is still currently incomplete. Applied to taVNS, that includes both the central areas activated by taVNS, as well as the short and long-term impact of taVNS on the VN and on the ICNS structure and function (Giannino et al. 2024). Underpinning the complex connectivity within the ICNS, as well as its real-time changes, is particularly complex, and requires advanced techniques of in vivo neuronal recording and processing and dedicated expertise and laboratories (Mehra et al. 2022).
From a clinical standpoint, the rigorous assessment of the efficacy of taVNS in a multicentric randomized controlled study would require the evaluation of the final infarct size as percentage of the area at risk through cardiac MRI, that is still not easily available in the clinical practice in the acute and post-acute setting (Arai 2011). Universal standardization of cardiac MRI protocols/endpoints is also of the utmost importance to promote the incorporation of cardioprotective interventions into clinical practice (Ibanez et al. 2019). The implementation of novel predictive markers of autonomic dysfunction is also an area of extensive research (Mehra et al. 2022).
Finally, the development of closed loop systems, with the possibility to continuously monitor and integrate a biofeedback and adjust the stimulation parameters accordingly, albeit particularly complex to incorporate in a non-invasive system, is already under evaluation even for taVNS (Yu et al. 2022). Indeed, electrocardiography (ECG)-gated closed loop (CL)-taVNS, at present not commercially available but technically feasible using wearable ECG devices and Bluetooth based networks (Beniwal et al. 2021), might be able to set the intensity of stimulation based on hear rate, heart rate variability and the presence and the severity of arrhythmias. Even mor complex, a subcutaneous humoral signal (SHS)-gated CL-taVNS might also be developed, using as biofeedback circulating biomarkers and cytokines levels (Yu et al. 2022), assessed at a subcutaneous level.
To underscore the importance of sharing experiences, knowledge and technological tools to improve our overall understanding of autonomic nervous system functioning and pave the way for successful neuromodulation strategies, an international consortium named SPARC (Stimulating Peripheral Activity to Relieve Conditions) founded by the NIH, was instituted (Stimulating Peripheral Activity to Relieve Conditions (SPARC) n.d.). All the experimental data and mathematical models continuously supplied by the consortium members are curated, annotated and semantically linked in a single, freely accessible, data portal. The SPARC program has recently begun Phase 2, that includes a new initiative (called SPARC-V) dedicated to the vagus nerve, encompassing the REVA project, aimed at producing high-resolution maps of the human vagus nerve, and the VESPA project, aimed at assessing the effects of a common set of VNS parameters on many different organs including the heart in coordinated clinical studies.
Conclusions
The pathophysiological rational for therapeutic interventions aimed at improving vagal output in the setting of I/R injury is very strong, due to the anti-adrenergic, anti-inflammatory, antioxidants, anti-apoptotic and pro-angiogenic effect that finally leads to cardioprotection, reduced infarct size and reduced arrhythmic risk. Transcutaneous auricular VNS may overcome the limitations related to invasive cervical VNS, particularly in the acute setting, but more data are needed to better characterize the dose-effect relationship and the most suitable candidates. A better understanding of the functional and anatomical organization of the vagus nerve, combined with computational model strategies integrated with artificial intelligence tools, will provide an important drive in the clinical implementation of electrical neuromodulation in the next future.
Availability of data and materials
Not applicable.
Abbreviations
- Ach:
-
Acetylcholine
- AChRs:
-
ACh receptors
- AI:
-
Artificial Intelligence
- ANS:
-
Autonomic nervous system
- BAT:
-
Baroreflex activation therapy
- CAP:
-
Cholinergic anti-inflammatory pathway
- CL-taVNS:
-
Closed loop transcutaneous auricular vagus nerve stimulation
- CNS:
-
Central nervous system
- CPR:
-
cardiopulmonary resuscitation
- cVNS:
-
Cervical VNS
- DAMPs:
-
Damage-associated molecular pattern molecules
- HF:
-
Heart failure
- ICNS:
-
Intrinsic Cardiac Nervous System
- IL-:
-
Interleukin
- I/R:
-
Ichemia/Reperfusion
- LAD:
-
Left anterior descending artery
- LSG:
-
left stellate ganglion
- mPTP:
-
Mitochondrial permeability transition pores
- NCX:
-
Na/Calcium exchanger
- NPY:
-
Neuropeptide Y
- PCAS:
-
Post cardiac arrest syndrome
- PPAR-γ :
-
Peroxisome proliferator-activated receptor-γ
- ROS:
-
Reactive oxygen species
- ROSC:
-
Return of spontaneous circulation
- STEMI:
-
ST elevation myocardial infarction
- taVNS:
-
Transcutaneous auricular vagus nerve stimulation
- TLRs:
-
Toll-like receptors
- TNFα:
-
Tumor necrosis factor alpha
- TNFRs:
-
Tumor necrosis factor receptors
- VIP:
-
Vasoactive intestinal peptide
- VN:
-
Vagus nerve
- VNS:
-
Vagus Nerve stimulation
- α7nAChR α7:
-
Nicotinic Cholinergic Receptor
References
Allen, E., Pongpaopattanakul, P., Chauhan, R. A., Brack, K. E. & Ng, G. A. The Effects of Vagus Nerve Stimulation on Ventricular Electrophysiology and Nitric Oxide Release in the Rabbit Heart. Front. Physiol. 13, (2022).
Amoretti M, et al. Production and detection of cold antihydrogen atoms. Nature. 2002;419:456–9.
Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057–68.
Ando M, et al. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation. 2005;112:164–70.
Arai AE. MRI for Area at Risk, Myocardial Infarction, and Myocardial Salvage. J Cardiovasc Pharmacol Ther. 2011;16:313–20.
Ardell JL, Armour J. A Neurocardiology: Structure-Based Function Compr Physiol. 2016;6:1635–53.
Ardell JL, et al. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J Physiol. 2017;595:6887–903.
Audia JP, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018;113:32.
Badran, B. W. et al. A pilot randomized controlled trial of supervised, at-home, self-administered transcutaneous auricular vagus nerve stimulation (taVNS) to manage long COVID symptoms. Res. Sq. rs.3.rs-1716096 (2022) https://doi.org/10.21203/rs.3.rs-1716096/v1.
Baig SS, et al. Transcutaneous vagus nerve stimulation (tVNS) in stroke: the evidence, challenges and future directions. Auton Neurosci Basic Clin. 2022;237: 102909.
Baig SS, et al. tVNS in Stroke: A Narrative Review on the Current State and the Future. Stroke. 2023;54:2676–87.
Bellinger DL, Lorton D. Autonomic regulation of cellular immune function. Auton Neurosci Basic Clin. 2014;182:15–41.
Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204.
Beniwal, V., Mishra, T., Jain, A. & Mathur, G. Bluetooth-Based Smart Sensor Networks. in 349–355 (2021). https://doi.org/10.1007/978-981-15-6707-0_33.
Brack KE, Patel VH, Coote JH, Ng GA. Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol. 2007;583:695–704.
Byrne RA, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720–826.
Bywater RA, Campbell G, Edwards FR, Hirst GD, O’Shea JE. The effects of vagal stimulation and applied acetylcholine on the sinus venosus of the toad. J Physiol. 1989;415:35–56.
Caccioppo A, et al. Ischemia Reperfusion Injury: Mechanisms of Damage/Protection and Novel Strategies for Cardiac Recovery/Regeneration. Int J Mol Sci. 2019;20:5024.
Calvillo L, et al. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J Cardiovasc Pharmacol. 2011;58:500–7.
Candreva, A. et al. Impact of endothelial shear stress on coronary atherosclerotic plaque progression and composition: A meta-analysis and systematic review. Int. J. Cardiol. 407, (2024).
Capogrosso M, Lempka SF. A computational outlook on neurostimulation. Bioelectron Med. 2020;6:10.
Cell Biology of Ischemia/Reperfusion Injury - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3904795/.
Chen X, et al. Vagus nerve stimulation suppresses corticotropin-releasing factor-induced adrenocorticotropic hormone release in rats. Neuroreport. 2021;32:792–6.
Cheng Z, Powley TL, Schwaber JS, Doyle FJ. Projections of the dorsal motor nucleus of the vagus to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol. 1999;410:320–41.
Choudhary RC, et al. Threshold adjusted vagus nerve stimulation after asphyxial cardiac arrest results in neuroprotection and improved survival. Bioelectron Med. 2022;8:10.
Clemente-Moragón A, et al. Metoprolol exerts a non-class effect against ischaemia–reperfusion injury by abrogating exacerbated inflammation. Eur Heart J. 2020;41:4425–40.
Coote JH. Myths and realities of the cardiac vagus. J Physiol. 2013;591:4073–85.
Crimi G, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc Interv. 2013;6:1055–63.
D’Ascenzo F, et al. Extracellular vesicles from patients with Acute Coronary Syndrome impact on ischemia-reperfusion injury. Pharmacol Res. 2021;170: 105715.
D’Ascenzo F, et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): a modelling study of pooled datasets. Lancet Lond Engl. 2021;397:199–207.
D’Elia E, et al. Novel approaches to the post-myocardial infarction/heart failure neural remodeling. Heart Fail Rev. 2014;19:611–9.
Dasari, T. et al. Noninvasive low-level tragus stimulation attenuates inflammation and oxidative stress in acute heart failure. Res. Sq. rs.3.rs-3323086 (2023) https://doi.org/10.21203/rs.3.rs-3323086/v1.
Davidson SM, et al. Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J Cell Mol Med. 2020;24:3795–806.
De Ferrari GM. Vagal stimulation in heart failure. J Cardiovasc Transl Res. 2014;7:310–20.
De Ferrari GM, et al. Physical Inactivity Is a Risk Factor for Primary Ventricular Fibrillation. J Am Coll Cardiol. 2019;73:2117–8.
De Ferrari GM, et al. Risk factors for primary ventricular fibrillation during a first myocardial infarction: Clinical findings from PREDESTINATION (PRimary vEntricular fibrillation and suDden dEath during firST myocardIal iNfArcTION). Int J Cardiol. 2020;302:164–70.
De Filippo O, et al. Machine learning-based prediction of in-hospital death for patients with takotsubo syndrome: The InterTAK-ML model. Eur J Heart Fail. 2023;25:2299–311.
de Melo P, et al. Understanding the Neuroplastic Effects of Auricular Vagus Nerve Stimulation in Animal Models of Stroke: A Systematic Review and Meta-Analysis. Neurorehabil Neural Repair. 2023;37:15459683231177596.
Devarajan A, et al. Convergent cardiorespiratory neurons represent a significant portion of cardiac and respiratory neurons in the vagal ganglia. Front Cardiovasc Med. 2022;9: 959815.
Diaconu A, et al. Expending the power of artificial intelligence in preclinical research: an overview. IOP Conf Ser Mater Sci Eng. 2022;1254: 012036.
Donato M, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol. 2013;98:425–34.
Du XY, Schoemaker RG, Bos E, Saxena PR. Characterization of the positive and negative inotropic effects of acetylcholine in the human myocardium. Eur J Pharmacol. 1995;284:119–27.
Dusi V, De Ferrari GM. Vagal stimulation in heart failure. Herz. 2021;46:541–9.
Dusi V, Zhu C, Ajijola OA. Neuromodulation Approaches for Cardiac Arrhythmias: Recent Advances. Curr Cardiol Rep. 2019;21:32.
Dusi V, De FGM, Mann DL. Cardiac Sympathetic-Parasympathetic Interaction. JACC Basic Transl Sci. 2020;5:811–4.
Dusi V, De Ferrari GM, Schwartz PJ. There are 100 ways by which the sympathetic nervous system can trigger life-threatening arrhythmias. Eur Heart J. 2020;41:2180–2.
Dusi V, Angelini F, De Ferrari GM. Vagus Nerve Stimulation for Myocardial Ischemia. JACC Basic Transl Sci. 2023;8:1119–22.
Dusi, V. Nociception, Sympathetic Nervous System, and Inflammation: When the Heart and the Skin Speak the Same Language. in Brain and Heart Dynamics (eds. Govoni, S., Politi, P. & Vanoli, E.) 117–145 (Springer International Publishing, Cham, 2020). https://doi.org/10.1007/978-3-030-28008-6_63.
Dusi, V. & Ardell, J. L. Brain-Heart Afferent-Efferent Traffic. in Brain and Heart Dynamics (eds. Govoni, S., Politi, P. & Vanoli, E.) 3–24 (Springer International Publishing, Cham, 2020). https://doi.org/10.1007/978-3-030-28008-6_2.
Dusi, V., Angelini, F., Zile, M. R. & De Ferrari, G. M. Neuromodulation devices for heart failure. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 24, E12–E27 (2022).
Dusi, V., Angelini, F., Gravinese, C., Frea, S. & De Ferrari, G. M. Electrical storm management in structural heart disease. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 25, C242–C248 (2023).
Dusi, V. et al. Continuous stellate ganglion block for ventricular arrhythmias: case series, systematic review, and differences from thoracic epidural anaesthesia. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 26, euae074 (2024).
Elamin ABA, Forsat K, Senok SS, Goswami N. Vagus Nerve Stimulation and Its Cardioprotective Abilities: A Systematic Review. J Clin Med. 2023;12:1717.
Elia E, et al. Gender differences in the development of heart failure after acute coronary syndrome: Insight from the CORALYS registry. Int J Cardiol. 2024;397: 131622.
F, B. et al. Impact of Complete Revascularization on Development of Heart Failure in Patients With Acute Coronary Syndrome and Multivessel Disease: A Subanalysis of the CORALYS Registry. J. Am. Heart Assoc. 12, (2023).
Fang, Y.-T. et al. Neuroimmunomodulation of vagus nerve stimulation and the therapeutic implications. Front. Aging Neurosci. 15, (2023).
Farmer AD, et al. International Consensus Based Review and Recommendations for Minimum Reporting Standards in Research on Transcutaneous Vagus Nerve Stimulation (Version 2020). Front Hum Neurosci. 2020;14: 568051.
Feliciano L, Henning RJ. Vagal nerve stimulation during muscarinic and beta-adrenergic blockade causes significant coronary artery dilation. J Auton Nerv Syst. 1998;68:78–88.
Feliciano L, Henning RJ. Vagal nerve stimulation releases vasoactive intestinal peptide which significantly increases coronary artery blood flow. Cardiovasc Res. 1998;40:45–55.
Femminò S, et al. Percutaneous Coronary Intervention (PCI) Reprograms Circulating Extracellular Vesicles from ACS Patients Impairing Their Cardio-Protective Properties. Int J Mol Sci. 2021;22:10270.
Fernández-Hernando D, Justribó Manion C, Pareja JA, García-Esteo FJ, Mesa-Jiménez JA. Effects of Non-Invasive Neuromodulation of the Vagus Nerve for the Management of Cluster Headache: A Systematic Review. J Clin Med. 2023;12:6315.
Fernández-Hernando D, Fernández-de-Las-Peñas C, Pareja-Grande JA, García-Esteo FJ, Mesa-Jiménez JA. Management of auricular transcutaneous neuromodulation and electro-acupuncture of the vagus nerve for chronic migraine: a systematic review. Front Neurosci. 2023;17:1151892.
Fields, J. Z., Roeske, W. R., Morkin, E. & Yamamura, H. I. Cardiac muscarinic cholinergic receptors. Biochemical identification and characterization. J. Biol. Chem. 253, 3251–3258 (1978).
Finlay M, Harmer SC, Tinker A. The control of cardiac ventricular excitability by autonomic pathways. Pharmacol Ther. 2017;174:97–111.
Flohr H, Breull W. Effect of etafenone on total and regional myocardial blood flow. Arzneimittelforschung. 1975;25:1400–3.
Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–85.
Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–7.
Frangos E, Ellrich J, Komisaruk BR. Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimulat. 2015;8:624–36.
Gallone G, Bruno F, D’Ascenzo F, Ferrari DE, G. M. What will we ask to artificial intelligence for cardiovascular medicine in the next decade? Minerva Cardiol Angiol. 2022;70:92–101.
Garcia RG, et al. Optimization of respiratory-gated auricular vagus afferent nerve stimulation for the modulation of blood pressure in hypertension. Front Neurosci. 2022;16:1038339.
Geis GS, Wurster RD. Cardiac responses during stimulation of the dorsal motor nucleus and nucleus ambiguus in the cat. Circ Res. 1980;46:606–11.
Ghasemzadeh M, Ahmadi J, Hosseini E. Platelet-leukocyte crosstalk in COVID-19: How might the reciprocal links between thrombotic events and inflammatory state affect treatment strategies and disease prognosis? Thromb Res. 2022;213:179–94.
Giannino G, et al. The Intrinsic Cardiac Nervous System: From Pathophysiology to Therapeutic Implications. Biology. 2024;13:105.
Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J Physiol. 1984;356:65–78.
Gilmour RF, Zipes DP. Positive inotropic effect of acetylcholine in canine cardiac Purkinje fibers. Am J Physiol. 1985;249:H735-740.
González-González MA, et al. Bioelectronic Medicine: a multidisciplinary roadmap from biophysics to precision therapies. Front Integr Neurosci. 2024;18:1321872.
Gourine AV, Ackland GL. Cardiac Vagus and Exercise. Physiology. 2019;34:71–80.
Gourine AV, Machhada A, Trapp S, Spyer KM. Cardiac vagal preganglionic neurones: An update. Auton Neurosci Basic Clin. 2016;199:24–8.
Gronda, E. et al. Sympathetic activation in heart failure. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 24, E4–E11 (2022).
Hadaya J, et al. Vagal Nerve Stimulation Reduces Ventricular Arrhythmias and Mitigates Adverse Neural Cardiac Remodeling Post-Myocardial Infarction. JACC Basic Transl Sci. 2023;8:1100–18.
Hamann JJ, et al. Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure. Eur J Heart Fail. 2013;15:1319–26.
Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100.
Hausenloy DJ, et al. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J. 2017;38:935–41.
Heidland A, et al. Neuromuscular electrostimulation techniques: historical aspects and current possibilities in treatment of pain and muscle waisting. Clin Nephrol. 2013;79(Suppl 1):S12-23.
Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49:27–37.
Herring N. Autonomic control of the heart: going beyond the classical neurotransmitters. Exp Physiol. 2015;100:354–8.
Herring N, et al. Neuropeptide-Y causes coronary microvascular constriction and is associated with reduced ejection fraction following ST-elevation myocardial infarction. Eur Heart J. 2019;40:1920–9.
Heusch G. Treatment of Myocardial Ischemia/Reperfusion Injury by Ischemic and Pharmacological Postconditioning. Compr Physiol. 2015;5:1123–45.
Hoang JD, et al. Proarrhythmic Effects of Sympathetic Activation Are Mitigated by Vagal Nerve Stimulation in Infarcted Hearts. JACC Clin Electrophysiol. 2022;8:513–25.
Hofmann U, Frantz S. Role of Lymphocytes in Myocardial Injury, Healing, and Remodeling After Myocardial Infarction. Circ Res. 2015;116:354–67.
Hopkins DA, Armour JA. Brainstem cells of origin of physiologically identified cardiopulmonary nerves in the rhesus monkey (Macaca mulatta). J Auton Nerv Syst. 1998;68:21–32.
Hua K, et al. Cardiovascular effects of auricular stimulation -a systematic review and meta-analysis of randomized controlled clinical trials. Front Neurosci. 2023;17:1227858.
Iannaccone M, et al. Impact of Successful Chronic Coronary Total Occlusion Recanalization on Recurrence of Ventricular Arrhythmias in Implantable Cardioverter-Defibrillator Recipients for Ischemic Cardiomyopathy (VACTO PCI Study). Cardiovasc Revasc Med. 2022;43:104–11.
Ibanez B, et al. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;74:238–56.
Intachai, K., C. Chattipakorn, S., Chattipakorn, N. and Shinlapawittayatorn, K. Revisiting the Cardioprotective Effects of Acetylcholine Receptor Activation against Myocardial Ischemia/Reperfusion Injury Int J Mol Sci. 2018;19:2466.
Jani N, et al. PO-01-173 VAGAL NERVE STIMULATION REDUCES INTERSTITIAL VENTRICULAR NEUROPEPTIDE Y RELEASE VIA ACTIVATION OF PRESYNAPTIC SYMPATHETIC MUSCARINIC RECEPTORS. Heart Rhythm. 2024;21:S172.
Jin, H., Li, M., Jeong, E., Castro-Martinez, F. & Zuker, C. S. A body–brain circuit that regulates body inflammatory responses. Nature 1–3 (2024) https://doi.org/10.1038/s41586-024-07469-y.
Jones JF, Wang Y, Jordan D. Heart rate responses to selective stimulation of cardiac vagal C fibres in anaesthetized cats, rats and rabbits. J Physiol. 1995;489(Pt 1):203–14.
Kakinuma Y, et al. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-1alpha. FEBS Lett. 2005;579:2111–8.
Kalla M, et al. The cardiac sympathetic co-transmitter neuropeptide Y is pro-arrhythmic following ST-elevation myocardial infarction despite beta-blockade. Eur Heart J. 2020;41:2168–79.
Katare RG, et al. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg. 2009;137:223–31.
Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–9.
Kent, K. M., Smith, E. R., Redwood, D. R. & Epstein, S. E. Electrical stability of acutely ischemic myocardium. Influences of heart rate and vagal stimulation. Circulation 47, 291–298 (1973).
Kim S, et al. Vagus Nerve Stimulation Improves Mitochondrial Dysfunction in Post-cardiac Arrest Syndrome in the Asphyxial Cardiac Arrest Model in Rats. Front Neurosci. 2022;16: 762007.
Kolman BS, Verrier RL, Lown B. The effect of vagus nerve stimulation upon vulnerability of the canine ventricle: role of sympathetic-parasympathetic interactions. Circulation. 1975;52:578–85.
Komisaruk BR, Frangos E. Vagus nerve afferent stimulation: Projection into the brain, reflexive physiological, perceptual, and behavioral responses, and clinical relevance. Auton Neurosci. 2022;237: 102908.
Kong S-S, et al. Optimizing the Parameters of Vagus Nerve Stimulation by Uniform Design in Rats with Acute Myocardial Infarction. PLOS ONE. 2012;7: e42799.
Krieg T, et al. Acetylcholine and bradykinin trigger preconditioning in the heart through a pathway that includes Akt and NOS. Am J Physiol Heart Circ Physiol. 2004;287:H2606-2611.
Kuznetsov AV, et al. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants. 2019;8:454.
Lambert, E. A. et al. Sympathetic nervous response to ischemia-reperfusion injury in humans is altered with remote ischemic preconditioning. Am. J. Physiol.-Heart Circ. Physiol. 311, H364–H370 (2016).
Lecour S, et al. IMproving Preclinical Assessment of Cardioprotective Therapies (IMPACT) criteria: guidelines of the EU-CARDIOPROTECTION COST Action. Basic Res Cardiol. 2021;116:52.
Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation. 2000;101:547–52.
Li M, et al. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–4.
Li, J. et al. PPAR-γ Mediates Ta-VNS-Induced Angiogenesis and Subsequent Functional Recovery after Experimental Stroke in Rats. BioMed Res. Int. 2020, (2020).
Liu MB, Ajijola OA. The promise of cardiac neuromodulation: can computational modelling bridge the gap? J Physiol. 2023;601:3693–4.
Lu X, et al. Vagal nerve stimulation protects cardiac injury by attenuating mitochondrial dysfunction in a murine burn injury model. J Cell Mol Med. 2013;17:664–71.
Machhada A, et al. Optogenetic Stimulation of Vagal Efferent Activity Preserves Left Ventricular Function in Experimental Heart Failure. JACC Basic Transl Sci. 2020;5:799–810.
Malmström, R. E. Pharmacology of neuropeptide Y receptor antagonists. Focus on cardiovascular functions. Eur. J. Pharmacol. 447, 11–30 (2002).
Martens P, et al. Prognostic relevance of magnesium alterations in patients with a myocardial infarction and left ventricular dysfunction: insights from the EPHESUS trial. Eur Heart J Acute Cardiovasc Care. 2022;11:148–59.
Mastitskaya S, et al. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res. 2012;95:487–94.
Maxwell SRJ, Lip GYH. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58:95–117.
McAllen RM, Spyer KM. The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. J Physiol. 1976;258:187–204.
Mehra R, et al. Research Opportunities in Autonomic Neural Mechanisms of Cardiopulmonary Regulation: A Report From the National Heart, Lung, and Blood Institute and the National Institutes of Health Office of the Director Workshop. JACC Basic Transl Sci. 2022;7:265–93.
Mei N, Condamin M, Boyer A. The composition of the vagus nerve of the cat. Cell Tissue Res. 1980;209:423–31.
Meijerink J, et al. Inhibition of COX -2-mediated eicosanoid production plays a major role in the anti-inflammatory effects of the endocannabinoid N - docosahexaenoylethanolamine ( DHEA ) in macrophages. Br J Pharmacol. 2015;172:24–37.
Miao Y, et al. Acetylcholine Attenuates Hypoxia/ Reoxygenation-Induced Mitochondrial and Cytosolic ROS Formation in H9c2 Cells via M2 Acetylcholine Receptor. Cell Physiol Biochem. 2013;31:189–98.
Mioni C, et al. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–8.
Mishra, P. K. et al. Guidelines for evaluating myocardial cell death. Am. J. Physiol.-Heart Circ. Physiol. 317, H891–H922 (2019).
Moran AE, et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–501.
Morrow DA. Effects of Ranolazine on Recurrent Cardiovascular Events in Patients With Non–ST-Elevation Acute Coronary SyndromesThe MERLIN-TIMI 36 Randomized Trial. JAMA. 2007;297:1775.
Myers RW, et al. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation. 1974;49:943–7.
Nuntaphum W, et al. Vagus nerve stimulation exerts cardioprotection against myocardial ischemia/reperfusion injury predominantly through its efferent vagal fibers. Basic Res Cardiol. 2018;113:22.
Ottaviani MM, Macefield VG. Structure and Functions of the Vagus Nerve in Mammals. Compr Physiol. 2022;12:3989–4037.
Palma J-A. Muscarinic control of cardiovascular function in humans: a review of current clinical evidence. Clin Auton Res Off J Clin Auton Res Soc. 2024;34:31–44.
Paton JFR, et al. Advancing respiratory–cardiovascular physiology with the working heart–brainstem preparation over 25 years. J Physiol. 2022;600:2049–75.
Penketh J, Nolan JP. Post-Cardiac Arrest Syndrome. J Neurosurg Anesthesiol. 2023;35:260–4.
Pereira MR, Leite PEC. The Involvement of Parasympathetic and Sympathetic Nerve in the Inflammatory Reflex. J Cell Physiol. 2016;231:1862–9.
Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat N Y N. 2002;15:35–7.
Pezhouman A, et al. Cardiac regeneration – Past advancements, current challenges, and future directions. J Mol Cell Cardiol. 2023;182:75–85.
Redgrave J, et al. Safety and tolerability of Transcutaneous Vagus Nerve stimulation in humans; a systematic review. Brain Stimulat. 2018;11:1225–38.
Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101.
Ruffinazzi, M. & Dusi, V. Central Nervous System Management of Autonomic Cardiovascular Control. in Brain and Heart Dynamics (eds. Govoni, S., Politi, P. & Vanoli, E.) 1–27 (Springer International Publishing, Cham, 2020). https://doi.org/10.1007/978-3-319-90305-7_65-1.
Sabbah HN, et al. Vagus Nerve Stimulation in Experimental Heart Failure. Heart Fail Rev. 2011;16:171–8.
Saglietto A, et al. Convolutional neural network (CNN)-enabled electrocardiogram (ECG) analysis: a comparison between standard twelve-lead and single-lead setups. Front Cardiovasc Med. 2024;11:1327179.
Salah HM, Levin AP, Fudim M. Device Therapy for Heart Failure with Preserved Ejection Fraction. Cardiol Clin. 2022;40:507–15.
Saternos HC, et al. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics. 2018;50:1–9.
Savastano, S. et al. Electrical storm treatment by percutaneous stellate ganglion block: the STAR study. Eur. Heart J. ehae021 (2024) https://doi.org/10.1093/eurheartj/ehae021.
Schluter, K. D., Jakob, G., Ruiz-Meana, M., Garcia-Dorado, D. & Piper, H. M. Protection of reoxygenated cardiomyocytes against osmotic fragility by nitric oxide donors. Am. J. Physiol.-Heart Circ. Physiol. 271, H428–H434 (1996).
Schwaber JS, Cohen DH. Electrophysiological and electron microscopic analysis of the vagus nerve of the pigeon, with particular reference to the cardiac innervation. Brain Res. 1978;147:65–78.
Schwartz PJ, Pagani M, Lombardi F, Malliani A, Brown AM. A cardiocardiac sympathovagal reflex in the cat. Circ Res. 1973;32:215–20.
Schwartz, P. J., Billman, G. E. & Stone, H. L. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction. An experimental preparation for sudden cardiac death. Circulation 69, 790–800 (1984).
Sclocco R, et al. The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: A multimodal ultrahigh-field (7T) fMRI study. Brain Stimulat. 2019;12:911–21.
Sclocco R, et al. Stimulus frequency modulates brainstem response to respiratory-gated transcutaneous auricular vagus nerve stimulation. Brain Stimulat. 2020;13:970–8.
Settell ML, et al. Functional vagotopy in the cervical vagus nerve of the domestic pig: implications for the study of vagus nerve stimulation. J Neural Eng. 2020;17: 026022.
Sezer, M. et al. Coronary Microvascular Injury in Reperfused Acute Myocardial Infarction: A View From an Integrative Perspective. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 7, e009949 (2018).
Shanks J, Pachen M, Chang JW-H, George B, Ramchandra R. Cardiac Vagal Nerve Activity Increases During Exercise to Enhance Coronary Blood Flow. Circ Res. 2023;133:559–71.
Shen MJ, et al. Continuous Low-Level Vagus Nerve Stimulation Reduces Stellate Ganglion Nerve Activity and Paroxysmal Atrial Tachyarrhythmias in Ambulatory Canines. Circulation. 2011;123:2204–12.
Shinlapawittayatorn K, et al. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm. 2013;10:1700–7.
Shinlapawittayatorn K, et al. Vagus nerve stimulation initiated late during ischemia, but not reperfusion, exerts cardioprotection via amelioration of cardiac mitochondrial dysfunction. Heart Rhythm. 2014;11:2278–87.
Silvis MJM, et al. Damage-Associated Molecular Patterns in Myocardial Infarction and Heart Transplantation: The Road to Translational Success. Front Immunol. 2020;11: 599511.
Skene K, et al. Acute dietary zinc deficiency in rats exacerbates myocardial ischaemia–reperfusion injury through depletion of glutathione. Br J Nutr. 2019;121:961–73.
Sodi-Pallares D, et al. Effects of an intravenous infusion of a potassium-glucose-insulin solution on the electrocardiographic signs of myocardial infarction. Am J Cardiol. 1962;9:166–81.
Stanley WC. Myocardial Energy Metabolism During Ischemia and the Mechanisms of Metabolic Therapies. J Cardiovasc Pharmacol Ther. 2004;9:S31–45.
Stavrakis S, et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin Electrophysiol. 2020;6:282–91.
Stavrakis S, et al. Neuromodulation of Inflammation to Treat Heart Failure With Preserved Ejection Fraction: A Pilot Randomized Clinical Trial. J Am Heart Assoc. 2022;11: e023582.
Stavrakis S, et al. Noninvasive Vagus Nerve Stimulation in Postural Tachycardia Syndrome. JACC Clin Electrophysiol. 2024;10(2):346–55. https://doi.org/10.1016/j.jacep.2023.10.015.
Stimulating Peripheral Activity to Relieve Conditions (SPARC). https://commonfund.nih.gov/sparc.
Sun P, et al. Improved Outcomes of Cardiopulmonary Resuscitation in Rats Treated With Vagus Nerve Stimulation and Its Potential Mechanism. Shock Augusta Ga. 2018;49:698–703.
Tan C, et al. The efficacy and safety of transcutaneous auricular vagus nerve stimulation in the treatment of depressive disorder: A systematic review and meta-analysis of randomized controlled trials. J Affect Disord. 2023;337:37–49.
Tani, M. & Neely, J. R. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ. Res. 65, 1045–1056 (1989).
Tarnawski L, Olofsson PS. Inflammation neuroscience: neuro-immune crosstalk and interfaces. Clin Transl Immunol. 2021;10: e1352.
Thapalia BA, Zhou Z, Lin X. Autophagy, a process within reperfusion injury: an update. Int J Clin Exp Pathol. 2014;7:8322–41.
Thompson N, et al. MicroCT optimisation for imaging fascicular anatomy in peripheral nerves. J Neurosci Methods. 2020;338: 108652.
Thompson, N. et al. Anatomical and functional organization of cardiac fibers in the porcine cervical vagus nerve allows spatially selective efferent neuromodulation. BioRxiv Prepr. Serv. Biol. 2024.01.09.574861 (2024) https://doi.org/10.1101/2024.01.09.574861.
Tian H, Zhao X, Zhang Y, Xia Z. Abnormalities of glucose and lipid metabolism in myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2023;163: 114827.
Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9.
Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96.
Trakhtenberg EF, Goldberg JL. Immunology. Neuroimmune communication. Science. 2011;334:47–8.
Tran N, et al. Autonomic Neuromodulation Acutely Ameliorates Left Ventricular Strain in Humans. J Cardiovasc Transl Res. 2019;12:221–30.
Tsuchida K, Mizukawa Y, Urushidani T, Tachibana S, Naito Y. An inotropic action caused by muscarinic receptor subtype 3 in canine cardiac purkinje fibers. ISRN Pharmacol. 2013;2013: 207671.
Uehara L, et al. Transcutaneous auricular vagus nerve stimulation effects on inflammatory markers and clinical evolution of patients with COVID-19: a pilot randomized clinical trial. Expert Rev Med Devices. 2022;19:915–20.
Uemura K, Zheng C, Li M, Kawada T, Sugimachi M. Early short-term vagal nerve stimulation attenuates cardiac remodeling after reperfused myocardial infarction. J Card Fail. 2010;16:689–99.
van Weperen VYH, Vaseghi M. Cardiac vagal afferent neurotransmission in health and disease: review and knowledge gaps. Front Neurosci. 2023;17:1192188.
van Weperen VYH, Ripplinger CM, Vaseghi M. Autonomic control of ventricular function in health and disease: current state of the art. Clin Auton Res Off J Clin Auton Res Soc. 2023;33:491–517.
Vanoli E, et al. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–81.
Vaseghi, M. et al. Parasympathetic dysfunction and antiarrhythmic effect of vagal nerve stimulation following myocardial infarction. JCI Insight 2, e86715, 86715 (2017).
Vilahur, G. & Badimon, L. Ischemia/reperfusion activates myocardial innate immune response: the key role of the toll-like receptor. Front. Physiol. 5, (2014).
Wang Z, et al. Chronic intermittent low-level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail. 2014;7:1014–21.
Wang Q, et al. Combined Vagal Stimulation and Limb Remote Ischemic Perconditioning Enhances Cardioprotection via an Anti-inflammatory Pathway. Inflammation. 2015;38:1748–60.
Wirz-Ridolfi A. The History of Ear Acupuncture and Ear Cartography: Why Precise Mapping of Auricular Points Is Important. Med Acupunct. 2019;31:145–56.
Xu, Y.-P., Lu, X.-Y., Song, Z.-Q., Lin, H. & Chen, Y.-H. The protective effect of vagus nerve stimulation against myocardial ischemia/reperfusion injury: pooled review from preclinical studies. Front. Pharmacol. 14, (2023).
Yamaguchi T, et al. Thoracic Dorsal Root Ganglion Application of Resiniferatoxin Reduces Myocardial Ischemia-Induced Ventricular Arrhythmias. Biomedicines. 2023;11:2720.
Yang JM, Chung KT, Yang SN. Muscarinic activation causes biphasic inotropic response and decreases cellular Na+ activity in canine cardiac Purkinje fibers. J Biomed Sci. 1999;6:176–82.
Yang B, et al. Choline produces cytoprotective effects against ischemic myocardial injuries: evidence for the role of cardiac m3 subtype muscarinic acetylcholine receptors. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2005;16:163–74.
Yang P-C, et al. A multiscale predictive digital twin for neurocardiac modulation. J Physiol. 2023;601:3789–812.
Yoon MS, Han J, Tse WW, Rogers R. Effects of vagal stimulation, atropine, and propranolol on fibrillation threshold of normal and ischemic ventricles. Am Heart J. 1977;93:60–5.
Yoshie, K. et al. Cardiac TRPV1 afferent signaling promotes arrhythmogenic ventricular remodeling after myocardial infarction. JCI Insight 5, e124477, 124477 (2020).
Yu L, et al. Chronic Intermittent Low-Level Stimulation of Tragus Reduces Cardiac Autonomic Remodeling and Ventricular Arrhythmia Inducibility in a Post-Infarction Canine Model. JACC Clin Electrophysiol. 2016;2:330–9.
Yu L, et al. Low-Level Tragus Stimulation for the Treatment of Ischemia and Reperfusion Injury in Patients With ST-Segment Elevation Myocardial Infarction: A Proof-of-Concept Study. JACC Cardiovasc Interv. 2017;10:1511–20.
Yu Y, Ling J, Yu L, Liu P, Jiang M. Closed-Loop Transcutaneous Auricular Vagal Nerve Stimulation: Current Situation and Future Possibilities. Front Hum Neurosci. 2022;15: 785620.
Zamotrinsky AV, Kondratiev B, de Jong JW. Vagal neurostimulation in patients with coronary artery disease. Auton Neurosci. 2001;88:109–16.
Zandstra, T. E. et al. Asymmetry and Heterogeneity: Part and Parcel in Cardiac Autonomic Innervation and Function. Front. Physiol. 12, (2021).
Zhang Y, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–9.
Zhou W, Yuan J. SnapShot: Necroptosis. Cell. 2014;158:464-464.e1.
Zhou T, Chuang C-C, Zuo L. Molecular Characterization of Reactive Oxygen Species in Myocardial Ischemia-Reperfusion Injury. BioMed Res Int. 2015;2015:1–9.
Zhou L, et al. Low-level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp Physiol. 2019;104:28–38.
Zhu, S., Zhang, X., Zhou, M., Kendrick, K. M. & Zhao, W. Therapeutic applications of transcutaneous auricular vagus nerve stimulation with potential for application in neurodevelopmental or other pediatric disorders. Front. Endocrinol. 13, (2022).
Zuanetti G, De Ferrari GM, Priori SG, Schwartz PJ. Protective effect of vagal stimulation on reperfusion arrhythmias in cats. Circ Res. 1987;61:429–35.
Acknowledgements
None.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
No funding acknowledged.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and writing of the manuscript. All authors approved of the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No human subjects.
Consent for publication
Not applicable.
Competing interests
No competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giannino, G., Nocera, L., Andolfatto, M. et al. Vagal nerve stimulation in myocardial ischemia/reperfusion injury: from bench to bedside. Bioelectron Med 10, 22 (2024). https://doi.org/10.1186/s42234-024-00153-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42234-024-00153-6