Abstract
Background
Obstructive sleep apnea (OSA) is a unique potent predictor for stroke compared to other predictors. By aiding in the adherence of leukocytes and platelets, soluble P-selectin (sP-selectin) contributes to the development of ischemic stroke. The objective of this study was to investigate the independent impact of OSA on platelet activation and development of silent brain infarction. Twenty-four OSA patients and 24 controls were studied in a case–control study, who underwent one-night polysomnography, magnetic resonance imaging for evaluation of silent brain infarctions (SBI), measurement of serum (sP-selectin) levels for assessment of increased platelet activation and C-reactive protein (CRP) serum levels.
Results
Out of 24 patients, 5 (20.8%) had mild OSA and 8 (33.3%) had moderate and 11 (45.8%) had severe OSA. Serum levels of sP-selectin were statistically significantly higher in moderate and severe groups (p < 0.001). Eleven (57.9%) patients in moderate and severe OSA had SBI. Fifty percent of patients with moderate OSA had elevated serum sP-selectin and 25.0% of them had SBI and 81.8% of severe OSA patients had elevated serum sP-selectin and 81.8% of them had SBI. Patients with mild OSA and controls had no SBI and normal serum sP-selectin level. CRP was statistically significantly higher in moderate and severe OSA groups (16.6% and 45.8%) than the mild and control groups (4.1% and 0%) (p < 0.001).
Conclusion
Moderate and severe obstructive sleep apnea were associated independently with elevated serum sP-selectin reflecting increased platelet function, elevated inflammatory marker CRP and an increased risk of silent brain infarctions.
Similar content being viewed by others
Background
Because of the widespread prevalence of obstructive sleep apnea (OSA), it has become a common health problem, defined by recurrent attacks of hypoxia during sleep [1]. Severe OSA has been related to atherosclerosis, hypertension, ischemic heart, and cerebrovascular diseases [1,2,3]. Several inflammatory cascades are triggered by OSA that may enhance platelet activation and lead to silent brain infarction (SBI), even without other known risk factors like diabetes and coronary heart disease [4].
E-, L-, and P-selectins are three forms of cell surface proteins on endothelial cells, leukocytes, and platelets. By mediating leukocyte recruitment, are supposed to enhance the processes of inflammation. Soluble P-selectin (sP-selectin) is one of the most significant proteins participating in activation of platelets [5, 6]. sP-selectin plays a role in leukocyte adhesion and recruitment, and so facilitating development of atherosclerosis [7].
C-reactive protein (CRP) is an inflammatory predictor that participates in development of atherosclerosis, by stimulating coronary arteries smooth muscle cells and endothelial cells and by promoting vascular and intracellular cell adhesion molecules expression [8].
By increasing the development of hypertension and endothelial dysfunction, OSA may be linked to an increased incidence of silent brain infarction (SBI). However, earlier research looking into the relationship between OSA and SBI have produced incongruent outcomes [9,10,11]. This study was conducted to investigate the independent impact of OSA on platelet activation and development of silent brain infarction.
Methods
This case control study was conducted in the Neurology Department, and Diagnostic Radiology Department of Suez Canal University Hospital, Ismailia, Egypt, between February 2018 to January 2019. Twenty-four patients aged ≥ 18 years of both sexes attended to the Otolaryngology and Chest outpatient clinics of Suez Canal University Hospital diagnosed clinically as OSA by professional consultants according to their complains of daytime sleepiness, or their bed partner reports of loud snoring, gasping, choking, snorting, or interruptions in breathing while sleeping or had narrow or “crowded” airway, large neck circumference or craniofacial abnormalities as retrognathia. Nonsmokers, without evidence of systemic infection for minimum 15 days prior to the study, and not using any drugs, nonobese with body mass index (BMI) < 25 [12] were included. Patients with BMI > 25, with any disease as diabetes mellitus, hypertension, dyslipidemia, atrial fibrillation, ischemic heart disease, vasculitis, old stroke, and smokers were excluded. Twenty-four age, sex and BMI-matched controls were obtained from the healthy subjects.
The Suez Canal University faculty of medicine’s ethics committee approved the study after all participants signed informed consent forms.
A thorough medical history and examination, complete blood picture, vasculitis panel, serum uric acid, serum creatinine, liver enzymes, prothrombin time and serum albumin, lipid profile, Hb A1C, fasting and 2 h postprandial blood sugar and erythrocyte sedimentation rate (ESR) were performed for each participant in the study.
C-reactive protein (CRP) was estimated using the latex agglutination test by (CRP Latex test kit, Immuno-Diagnostics, USA, Ref-310-100-31) which checks macroscopically for apparent agglutination directly after taking off the slide from the rotator, normal level was < 6 mg/l.
sP-selectin analysis was used to assess increased platelet activation, blood samples were collected after doing polysomnography (PSG) at 6:00 a.m. and before centrifugation let to clot for 15 min on ice and until analysis the serum was stored at − 40 °C. The sP-selectin enzyme-linked immunosorbent assay kit (Sino Gene Clon Biotech Co., Ltd, China, catalog No.: SG-10508) was utilized to evaluate the sP-selectin human serum levels. Following manufacturer’s instructions, ELISA assay was conducted on diluted samples. The kit's assay range was 1–140 ng/ml.
The translated Arabic form of Epworth Sleepiness Scale (ESS) was used to evaluate OSA clinically [13]. The total score ranged from 0–24. Excessive daytime sleepiness (EDS) was considered at a score ≥ 10 [14]. Full night diagnostic PSG study was carried out for all participants started sleep at 10:00 p.m. till 6:00 a.m. A sleep technician carried out the PSG study using E-Series EEG/PSG system © Compumedics Limited 2004, Australia which included video recording of electroencephalography with two frontal, two central and two occipital derivations, electrooculography with right and left eye derivations, nasal-oral airflow with thermal device, nasal pressure, snoring sensor, respiratory effort with abdominal and thoracic belts, pulse oximetry for oxygen saturation, EMG for submental, right, and left tibialis anterior muscles.
Clinical neurophysiology specialist performed sleep staging and event scoring manually according to the American Academy of Sleep Medicine [15]. An apnea episode was defined as a 90% reduction in airflow for at least 10 s and Hypopnea occurs when the airflow is reduced by 30% or more for at least 10 s, and oxygen saturation drops by 3% or more or associated with arousal [15].
The apnea/hypopnea index (AHI) was the apneas and hypopneas number per hour of sleep and OSA was categorized as mild, moderate, and severe as AHI levels of ≥ 5 to 15, ≥ 15 to 30 and ≥ 30, respectively [16].
A 1.5-Tesla magnetic resonance imaging unit (Achieva; Philips Medical Systems, the Netherlands) was utilized to scan the entire brain for silent infarctions, resulting in 20 axial images. There was an interslice gap of 2 mm between the slices and the thickness of the slices was 5 mm fluid-attenuated inversion recovery (FLAIR), T1-weighted images (T1WI), T2-weighted images (T2WI), diffusion-weighted, and axial gradient echo (GRE) images were acquired [17]. Images were presented on a workstation monitor and assessed by a professional neuroradiologist who was unaware of the patients’ identities or diagnosis.
According to the criteria of Yoshida and colleagues [18], SBI was defined as lesions with a broadest diameter ≥ 3 mm showing T2WI and FLAIR hyperintensity, and T1WI hypointensity, without clinical manifestations that match to the MRI abnormalities and with lack of clinical stroke medical history. The lesions > 3 mm in locations with a high frequency of large perivascular spaces, as the lower part of the basal ganglia were not considered as infarcts. Infarcts were classified as cortical, subcortical, basal ganglia, brainstem, and cerebellar [19].
The IBM SPSS 20.0 software package was applied to analyze the data. (Armonk, New York: IBM Corp.) Variables were analyzed for normality of distribution utilizing the Shapiro–Wilk test; categorical variables were analyzed utilizing the Chi-square test (Monte Carlo). Student’s t-test utilized for comparison between two groups for normally distributed quantitative variables while ANOVA was utilized for the four studied groups, respectively, for quantitative variables that were not normally distributed. Mann–Whitney test utilized for comparison between two groups and Kruskal–Wallis test between different groups. At 5% level, the significance of the findings was assessed.
Results
Forty-eight participants, 24 OSA patients, and 24 controls were involved in this study. The patients’ ages ranged from 19 and 53 years (mean = 36.0 ± 10.26) and the controls’ ages ranged from 18 and 50 years (mean = 35.0 ± 11.01). Fourteen subjects (58.3%) were males and 10 (41.7%) were females in patients, while in controls, 13 subjects (54.2%) were males, and 11 subjects (54.8%) were females. The mean BMI was 21.25 ± 2.27 for patients, 20.88 ± 2.25 for controls. Between the two groups, differences in age, gender, and BMI were statistically non-significant (p value = 0.746, 0.771 and 0.568), respectively. The range of ESS in patients was from 11–22 with a mean (14.33 ± 2.85) and it was statistically significantly higher than the controls which ranged from 0–8 with a mean (3.08 ± 2.62) (p value = < 0.001) (Table 1).
The different sleep parameters including sleep structure, sleep stages and sleep abnormalities among patients and controls are shown in Table 2. The patients were classified into mild OSA (20.8%), moderate OSA (33.3%), and severe OSA (45.8%) based on the AHI (Table 3).
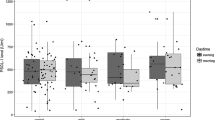
Thirteen (54.2%) patients had elevated sP-selectin serum levels, 4 patients (16.7%) had moderate OSA, and 9 patients (37.5%) had severe OSA, while all patients with mild OSA and all controls had normal sP-selectin serum level. The serum levels of sP-selectin in controls (range 1.0–135.0) (mean = 62.50 ± 45.76) and in mild OSA patients (range 25–138) (mean 78.60 ± 51.18) were within the normal range but they were higher in moderate OSA patients (range 48–201) (mean = 133.25 ± 53.78) and much higher in severe OSA patients (range 96–450) (mean = 261.27 ± 106.77) and statistically this difference was significant (p value = < 0.001) (Table 4).
Sixteen (66.7%) patients had elevated CRP, 1 (4.1%) patient had mild OSA, 4 (16.6%) patients had moderate OSA, and 11 (45.8%) patients had severe OSA while all controls had normal CRP, and statistically this difference was significant (p value = < 0.001) (Table 4).
Eleven (45.8%) patients had silent brain infarction (SBI); 2 (8.3%) patients with moderate OSA and 9 (37.5%) patients with severe OSA while all patients with mild OSA and all controls had no SBI, and statistically this difference was significant (p value = < 0.001). Nine (81.8%) SBI were lacunar SBI, 10 (90.9%) SBI were unilateral, and their size was 3–15 mm, 8 (72.7%) SBI were only one, and the subcortical white matter is the most common site of SBI 7 (63.6%) (Table 5).
The serum level of sP-selectin had a significant positive correlation with ESS, total sleep time (TST), AHI, arousal index, O2 desaturation index, CRP, and number of SBI, where r = 0.662, 0.662, 0.554, 0.819, 0.641, 0.565, 0.907 and 0.811 and p = 0.001, 0.005, 0.001, 0.001, 0.004, 0.001 and 0.001, respectively. It had a significant negative correlation with lowest O2 saturation, r = − 0.445, and p = 0.029, while there was no significant correlation between the sP-selectin serum level and age and BMI, r = 0.380, 0.147 and p = 0.067, 0.494, respectively (Table 6). The sP-selectin serum level and sex had no statistically significant relationship (p = 0.312) while it had a highly statistically significant relationship with the size of SBI (p = 0.001) (Table 7). Severe OSA was found to be highly associated with a silent brain infarction more than the mild and moderate OSA, odds ratio (OR): 24.75, [95% CI (2.17–350)] (Table 8).
Discussion
The association between OSA and SBI is not well understood, and past research findings have been confounded by comorbidities.
The current study investigated sP-selectin level as a marker of platelet activation and CRP as inflammatory marker and the development of SBI in OSA patients not suffering from associated comorbid conditions. The rationale of this paper regarding exclusion of other comorbid confounding conditions is to assess the independent effect of OSA as a risk factor for SBI which is potentially modifiable.
The study results showed that gender is equally distributed in study groups, also there no difference regarding the age and BMI in study groups to assess the independent effect of OSA.
In our study, the patients have a poor quality of sleep with shorter total sleep time and more arousals and nocturnal awakenings, lower minimal SpO2 with excessive daytime sleepiness in comparison with the control group. This is significant because cerebral small vessel disease is now believed to be the underlying etiology of most SBIs. High AHI indicates that these individuals experienced frequent episodes of hypoxia. Thus, our findings support the idea that a sleep study could be useful for identifying OSA as a risk factor for SBI.
According to this study, moderate and severe OSA patients had higher levels of sP-selectin than mild OSA patients and healthy controls, and this level significantly correlates with OSA severity. In agreement with these results, Minoguchi and colleagues [20] stated that OSA patients had high levels of sP-selectin, with the highest levels in severe OSA, followed by moderate OSA, and then mild OSA. Chin and colleagues [21] stated that sP-selectin was high in OSA patients, and CPAP treatment did play a role in lowering its level. Cofta and colleagues [22] reported that severe OSA had significantly higher levels of selectins than in moderate or mild OSA and plasma adhesion molecules levels predict severity of OSA and participate in occurrence of cardiovascular diseases. Patients with OSA had significantly higher levels of soluble vascular cell adhesion molecules and sP-selectin than the controls, according to Pilkauskaite and colleagues [23]. Chen and colleagues [24] disagreed with the previous results as they dicovered no significant variations in serum adhesion molecules, selectin levels in severe OSA patients compared to controls.
In this study, about 45% of moderate/severe OSA who had no previous stroke had SBI, which was found to be significantly linked to the severity of OSA, and that the majority of these SBI were in the subcortical white matter. This result was higher than that observed in previous studies as Minoguchi and colleagues [20], who stated that 25% moderate-to-severe OSA and 7.7% with mild OSA had SBI and 54% of SBI were in subcortical white matter followed by (43%) in basal ganglia and thalamus. Hulya and colleagues [25] said that severe OSA is a risk factor for SBI, and Cho and colleagues [26] discovered that 7.64% of moderate–severe OSA patients had SBI.
According to this study SBI was found to have a higher risk of developing if a person had severe OSA [odds ratio (OR): 24.75, 95% CI 2.17–350.07], which was higher than the results of Cho and colleagues [26] who found a lower positive association between moderate–severe OSA and SBI [odds ratio (OR): 2.44, 95% CI 1.03–5.80], and they concluded that moderate-to-severe OSA was linked to SBI and lacunar infarction.
After controlling for possible confounders, this study discovered that severe OSA was linked with lacunar SBI independently. This is significant since small vessel disease is assumed to be the cause of the bulk of SBI. These patients exhibited many bouts of intermittent hypoxia because of their high AHI. Small vessels rely on their own processes to adjust to barotrauma in this situation, and microvascular damage (remodeling of hypertrophic vessel wall, lumen constriction, rarefaction) may ensue, leading to cerebral infarction. Also, when intermittent hypoxia happens, sympathetic tone may increase, worsening vasoconstriction and leading to more ischemic lesions, particularly in the terminal arteries [27]. Other mechanisms that may all play a role in the link between OSA and SBI involve arterial stiffness [27], hypercoagulability [28], and arrhythmia, especially atrial fibrillation [29].
In the current study, serum CRP levels were found to be significantly correlated with sP-selectin levels and OSA severity in patients with OSA. Row and colleagues [30] reported that long-term persistent hypoxia in OSA resulted in activated inflammatory processes with higher levels of proinflammatory cytokines. Kanbay and colleagues [31], showed that OSA patients had significantly higher CRP levels than healthy controls. Furthermore, Kor Kmaz [32] discovered that AHI and CRP have positive associations. However, Guilleminault and colleagues [33] in less obese OSA patients, observed no link between OSA and serum CRP levels.
There are some limitations to this study that it should be repeated in long-term follow-up and independent prospective larger studies with ideal representation of all severity groups of OSA to evaluate if the therapeutic measures of OSA can prevent development of SBI. Also, the possibility of using sP-selectin level as a complementary diagnostic or therapeutic biomarker in OSA patients needs to be assessed.
Conclusion
Moderate and severe OSA were associated independently with elevated serum sP-selectin reflecting increased platelet function, elevated inflammatory marker CRP and an increased risk of silent brain infarctions, so it is essential to be meticulous for early diagnosis of OSA. It is mandatory to conduct randomized controlled studies determine if therapeutic measures are effective in preventing new vascular insults and the possibility of development of stroke and cognitive impairment in these high-risk patients.
Availability of data and materials
The data can be publicly available at the Faculty of Medicine, Suez Canal University.
Abbreviations
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- EDS:
-
Excessive daytime sleepiness
- ESR:
-
Erythrocyte sedimentation rate
- ESS:
-
Epworth Sleepiness Scale
- FLAIR:
-
Fluid-attenuated inversion recovery
- OSA:
-
Obstructive sleep apnea
- PSG:
-
Polysomnography
- SBI:
-
Silent brain infarction
- sP-selectin:
-
Soluble P-selectin
- T1WI:
-
T1-weighted images
- T2WI:
-
T2-weighted images
References
Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–50.
Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169(3):207–14.
Li M, Hou WS, Zhang XW, Tang ZY. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. Int J Cardiol. 2014;172(2):466–9.
Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation. 2000;101(19):2290–5.
Margetic S. Inflammation and hemostasis. Biochem Medica. 2012;22(1):49–62.
Angiari S, Constantin G. Selectins and their ligands as potential immunotherapeutic targets in neurological diseases. Immunotherapy. 2013;5(11):1207–20.
Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood J Am Soc Hematol. 2011;118(26):6743–51.
Tan TY, Liou CW, Friedman M, Lin HC, Chang HW, Lin MC. Factors associated with increased carotid intima-media thickness in obstructive sleep apnea/hypopnea syndrome. Neurologist. 2012;18(5):277–81.
Koo DL, Kim JY, Lim JS, Kwon HM, Nam H. Cerebral microbleeds on MRI in patients with obstructive sleep apnea. J Clin Sleep Med. 2017;13(1):65–72.
Song TJ, Park JH, Choi KH, Chang Y, Moon J, Kim JH, Choi Y, Kim YJ, Lee HW. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017;30:36–42.
Del Brutto OH, Mera RM, Zambrano M, Castillo PR. Relationship between obstructive sleep apnea and neuroimaging signatures of cerebral small vessel disease in community-dwelling older adults. The Atahualpa Project. Sleep Med. 2017;37:10–2.
Epping-Jordan JE, Galea G, Tukuitonga C, Beaglehole R. Preventing chronic diseases: taking stepwise action. Lancet. 2005;366(9497):1667–71.
Ahmed AE, Fatani A, Al-Harbi A, Al-Shimemeri A, Ali YZ, Baharoon S, Al-Jahdali H. Validation of the Arabic version of the Epworth sleepiness scale. J Epidemiol Glob Health. 2014;4(4):297–302.
Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993;103(1):30–6.
Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, and colleagues. For the American Academy of sleep medicine. The AASM manual for scoring of sleep and associated events: rules, terminology, and technical specifications. (Version 2.4). Darien, IL: American Academy of Sleep Medicine; 2017. p. 1–89.
Sateia MJ. International classification of sleep disorders. Chest. 2014;146(5):1387–94.
Riba-Llena I, Jarca CI, Mundet X, Tovar JL, Orfila F, López-Rueda A, Nafría C, Fernández JL, Castañé X, Domingo M, Álvarez-Sabín J. Investigating silent strokes in hypertensives: a magnetic resonance imaging study (ISSYS): rationale and protocol design. BMC Neurol. 2013;13(1):1–8.
Yoshida M, Tomitori H, Machi Y, Katagiri D, Ueda S, Horiguchi K, Kobayashi E, Saeki N, Nishimura K, Ishii I, Kashiwagi K. Acrolein, IL-6 and CRP as markers of silent brain infarction. Atherosclerosis. 2009;203(2):557–62.
Zhu YC, Dufouil C, Tzourio C, Chabriat H. Silent brain infarcts: a review of MRI diagnostic criteria. Stroke. 2011;42(4):1140–5.
Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Oda N, Tanaka A, Yamamoto M, Ohta S, O’Donnell CP, Adachi M. Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Resp Crit Care. 2007;175(6):612–7.
Chin K, Nakamura T, Shimizu K, Mishima M, Nakamura T, Miyasaka M, Ohi M. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109(7):562–7.
Cofta S, Wysocka E, Dziegielewska-Gesiak S, Michalak S, Piorunek T, Batura-Gabryel H, Torlinski L. Plasma selectins in patients with obstructive sleep apnea. In: Respiratory regulation-the molecular approach 2013. Dordrecht: Springer. pp. 113–9.
Pilkauskaite G, Miliauskas S, Vitkauskiene A, Sakalauskas R. Vascular adhesion molecules in men with obstructive sleep apnea: associations with obesity and metabolic syndrome. Sleep Breath. 2014;18(4):869–74.
Chen HL, Lu CH, Lin HC, Chen PC, Chou KH, Lin WM, Tsai NW, Su YJ, Friedman M, Lin CP, Lin WC. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep. 2015;38(3):361–70.
Gunbatar H, Bulut MD, Ekin S, Sertogullarindan B, Bora A, Yavuz A, Tamac E. A silent pre-stroke damage: obstructive sleep apnea syndrome. Int J Clin Exp Med. 2016;9(2):3481–8.
Cho ER, Kim H, Seo HS, Suh S, Lee SK, Shin C. Obstructive sleep apnea as a risk factor for silent cerebral infarction. J Sleep Res. 2013;22(4):452–8.
Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131(5):1379–86.
von Ka R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124(5):1956–67.
Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–71.
Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Resp Crit Care. 2003;167(11):1548–53.
Kanbay A, Kaya E, Büyükoğlan H, Kaya MG, Şimşek ZÖ, Tutar N, Demir R. Correlation between pentraxin-3 and endothelial dysfunction in obstructive sleep apnea syndrome. Ann Thorac Med. 2015;10(3):199.
Korkmaz M, Korkmaz H, Küçüker F, Ayyıldız SN, Çankaya S. Evaluation of the association of sleep apnea-related systemic inflammation with CRP, ESR, and neutrophil-to-lymphocyte ratio. Med Sci Monit. 2015;21:477.
Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27(8):1507–17.
Acknowledgements
Not applicable.
Funding
The authors were responsible for the cost of this study including the design of the study and collection, analysis, and interpretation of data and in writing the manuscript and no funding.
Author information
Authors and Affiliations
Contributions
AR carried out the study conception and design, participated in its design, acquisition of data and coordination, and drafted the manuscript. AA carried out the design of the study, the analysis and interpretation of data and helped to draft the manuscript. OM participated in the sequence alignment, interpretation of data and drafting of manuscript. MS participated by acquisition of data and performed the statistical analysis. EM carried out the study conception and design, participated in its design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics committee of Suez Canal Faculty of medicine on November 16, 2016. Committee Number: 2958.
An informed written consent was taken from all the participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests (financial or non-financial).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El-Razek, R., Abou Hagar, A., Orabi, M. et al. Impact of obstructive sleep apnea on platelet activation and development of silent brain infarctions. Egypt J Neurol Psychiatry Neurosurg 58, 134 (2022). https://doi.org/10.1186/s41983-022-00575-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-022-00575-6