Abstract
Background
Melon fruit fly, Zeugodacus cucurbitae Coquillett (Diptera: Tephritidae), is a devastating polyphagous pest attacking large number of fruits and vegetables causing huge economic yield losses across the world. Management of this notorious pest is very challenging as the larvae feed inside the fruit. Hence, the present research study aimed to screen the indigenous Bacillus thuringiensis Berliner (Bacillales: Bacillaceae) strains causing toxicity to larvae and to identify the insecticidal toxicity-related genes present in respective strains. In the present study, 50 indigenous B. thuringiensis (Bt) strains along with one reference strain were screened against second-instar larvae Z. cucurbitae. All the strains were analyzed for presence of 21 dipteran active cry genes.
Results
Mortality in Z. cucurbitae larvae due to Bt strains ranged from 16 to 92%. PCR results revealed that each strain tested positive for a minimum of three cry genes to maximum of nine cry genes. Among the cry genes, cry1A, cry2A, cry1C, cry19, cry11 and cry70 were detected in high frequency of 100, 88, 84, 74, 58 and 54%, respectively. Bioassay studies revealed that ten out of fifty strains displayed more than 50% mortality. Hence, these ten strains, along with the reference strain, were further tested for mortality for the calculation of the median lethal concentration (LC50). The LC50 values ranged between 38.48 and 105.18 μg/ml. The lowest LC50 found for the strain NBAIR Bt107 was 38.48 μg/ml and was on par with the reference strain (Bti 4Q1) (31.3 μg/ml).
Conclusion
Indigenous Bt strains displayed a toxicity against the larvae of Z. cucurbitae. The probable dipteran active cry genes responsible for toxicity were interpreted. Thus, the Cry toxins from Bt can play a very important role in the management of Z. cucurbitae.
Similar content being viewed by others
Background
Zeugodacus cucurbitae Coquillett (Diptera: Tephritidae), commonly known as the melon fruit fly, is a serious pest belonging to the family Tephritidae of the order Diptera. This highly notorious species is capable of infesting over 100 different varieties of vegetables and fruits, making it a major concern for crop yield and food security (Dhillon et al. 2005). The level of damage caused by this pest can range from 30 to 100% (Subedi et al. 2021). Melon fruit fly not only causes direct damage by reducing the yield and marketability of fruits and vegetables but also presents substantial challenges to quarantine security. Thus, it poses a threat to the international trade of fruits and vegetables on a global scale (Vargas et al. 2015).
The management practices predominantly depend on the use of synthetic pesticides mainly as cover sprays due to their rapid knockdown effect (De Bon et al. 2014). These cover sprays not only trigger ecological repercussions in fruit fly populations, such as the development of insecticide resistance, but also lead to unintended harm to non-target beneficial arthropods and can induce phytotoxic effects on plants (Ankitha et al. 2023). There is a need for alternate management approaches that efficiently control this pest while keeping non-target organisms safe. So, the use of microbial control agents, in the form of biopesticides, could be a promising alternative (Ruqiya et al. 2023). In this context, the use of Bt provides a promising opportunity for the management of Z. cucurbitae. With its proven efficacy against a diversity of insect groups and considering its relatively underexplored status in testing toxicity against Z. cucurbitae, it provides a large scope for the identification of indigenous Bt strains effective against melon fruit fly.
Bacillus thuringiensis is a Gram-positive, spore-forming aerobic saprophytic bacterium of ubiquitous nature. Because of its high specificity, safety, quick degradation from soil, and consistent pest control, it is the most widely accepted commercial biopesticide across the globe (Manjunatha et al. 2023). The insecticidal property of Bt is due to the production of toxins during different phases of its life cycle. Bt strains can synthesize crystal and cytolytic toxins called Cry and Cyt toxins widely called as δ-endotoxins. Upon ingestion by insect pests, the activation of these toxins takes place within the midgut, facilitated by midgut proteases. Once activated, the toxins bind to specific receptors on the midgut epithelial cells, ultimately disrupting cell function and causing cell death (Bravo et al. 2007). The efficacy of these toxins extends beyond insect pests of the order Lepidoptera to Diptera, including those belonging to the Tephritidae family of agricultural importance. A few of these reports include the toxicity of Bt against the Mexican fruit fly, Anastrepha ludens Loew (Martinez et al. 1997), the peach fruit fly, Bactrocera zonata Saunders (Nisar et al. 2020) and the olive fruit fly B. oleae Rossi (Ilias et al. 2013). However, there are only limited studies on the evaluation of the toxicity of indigenous Bt strains against the melon fruit fly. Moreover, this is the first report from India to evaluate the toxicity of indigenous Bt against Z. cucurbitae.
Each strain of Bt may possess a diverse array of cry genes. Researchers have identified nearly 700 cry genes from 75 families to date (Crickmore et al. 2021). Each toxin exhibits high specificity toward one or a group of closely related insects. Furthermore, the presence of specific combinations of genes in each strain strongly influences its toxic activity against insect pests. Therefore, the identification of cry gene profiles within a strain offers a significant opportunity to understand and interpret its insecticidal activity. Hence, in the present study, the detection of dipteran active toxic genes using PCR was done in all indigenous strains of the study. The strains with different combinations of cry genes were screened to determine their potential toxicity against Z. cucurbitae.
Methods
Source of Bacillus thuringiensis strains
A total of fifty Bt is strains, along with the dipteran active strain Bt var. israelensis, were collected from the Insect Bacteriology Laboratory, Division of Genomic Resources, ICAR-National Bureau of Agricultural Insect Resources, Bengaluru, Karnataka, India. All the strains were revived on T3 broth then incubated for four days and plated on LB media and preserved in glycerol stock for further use.
Toxicity analysis of Bacillus thuringiensis against Zeugodacus cucurbitae
Insect rearing
Fruit fly culture was raised from the infested Cucumis sativus Linnaeus (Cucurbitales: Cucurbitaceae) fruits that were collected from cucumber fields in the Chikkaballapur district of Karnataka. Infested melon fruits were brought and kept in a glass jar (8″ × 6″) under controlled conditions of 25 ± 2° C, 70 ± 5% RH. The glass jar was filled with sterilized sand for pupation, allowing full-grown fourth-instar larvae to emerge from infested fruits and pupate in soil. Upon adult emergence, ten pairs of males and females were collected and released in a cage (30 × 30 × 30 cm). Adults were provided with a mixture of yeast hydrolysate and sugar (1:3) as a source of food and a water-soaked cotton swab as a source of water. This was used as a mother culture. Equal pairs of males and females were transferred from the mother culture to ovipositional cages provided with a piece of cucumber for oviposition. The eggs were collected and seeded over the artificial diet as recommended by Liu et al. (2020).
Preparation of spore-crystal suspension
Cells from each Bt isolate were inoculated into 100 ml of T3 broth and incubated at 30° C for 3 days. On the fourth day, a microscopic examination was conducted to identify the presence of crystals. From the isolates that tested positive for crystals, the spore-crystal mixture was harvested by centrifuging the inoculated broth separately at 15,000 rpm and 4° C for 10 min. The resultant pellet was washed three times with sterile distilled water before being re-suspended in sterile distilled water and used for bioassays as described by Ammouneh et al. (2011).
Screening of Bacillus thuringiensis isolates against Zeugodacus cucurbitae
Screening of Bt isolates against second-instar larvae of Z. cucurbitae was performed using the diet incorporation with a spore-crystal suspension. The mortality caused by the fifty Bt isolates was compared with reference strain, Bt var. israelensis (strain 4Q1). All isolates, along with the reference strain, were screened against second-instar larvae at a single concentration (250 µg/ml). Exactly, 100 µl of spore-crystal homogenate incorporated into 10 g of artificial diet and transferred to small plastic containers (3 × 3 cm), with each container serving as a replicate. Five larvae for each replicate, 25 larvae for each concentration and three replications were used for each treatment. The bioassay was carried out under controlled conditions of 25 ± 2° C and 70 ± 5% relative humidity. Mortality percentage was calculated at 24-h intervals till the fifth day of the experiment. Concentration- response bioassay using different concentrations (250, 125, 62.5, 31.25 or 15.62 µg/ml) was carried out in a similar manner to the single concentration assay. Based on the single concentration assay, the strains caused more than 50% mortality in Z. cucurbitae larvae were chosen for this bioassay. Five larvae were used for each replicate and 25 larvae for each concentration (250, 125, 62.5, 31.25 or 15.62 µg/ml). There were five replicates for each treatment.
PCR amplification of dipteran active cry genes
Total DNA was extracted from all Bt isolates using DNeasy Blood and Tissue Kit, following the manufacturer’s protocol (Qiagen, Hilden, Germany). The DNA from each strain individually used as template for amplification of the dipteran active cry gene in a PCR mixture of 25 µl containing 12.5 µl of 2 × EmeraldAmp PCR master mix (Emerald Amp GT PCR master mix, TaKaRa, Japan), 1 µl of forward primer, 1 µL of reverse primer, 5.5 µl of molecular biology grade water and 5 µl of template DNA (Valtierra-de-Luis et al. 2020; Nanditha et al. 2024). The reaction was set up at 94o C for 4 min as pre-denaturation, followed by 35 cycles of denaturation at 94o C for 1 min, annealing for 1 min at a temperature specific to each primer, extension at 72o C for 1 min and a final extension for 5 min at 72° C; annealing temperatures for each primer are given in (Table 1). The amplified products were visualized in 1.5% agarose gel and documented under the gel documentation system (DNR, MiniLumi, Israel).
Statistical analysis
The experiment was laid out as a completely randomized design. All the assays were performed three times with five replicates of each treatment. The concentration–response relationships for mortality of larvae were analyzed by subjecting data to probit analysis (PROC PROBIT) using SAS software (version 9.3, 2011; SAS Institute, Cary, NC, USA) and used as the basis for the calculation of LC50 and LC90 values and their corresponding 95% fiducial limit (95% FL).
Results
Bioassay of Bacillus thuringiensis strains against Zeugodacus cucurbitae
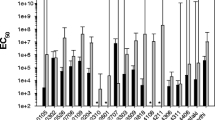
The evaluation of Bt strains for potential toxicity against second-instar larvae of Z. cucurbitae indicated that the mortality percentage ranged from 16 to 92% in comparison to the reference strain, which showed 95% mortality. Mortality of the larvae due to Bt confirmed by re-isolating Bt from dead larvae (Fig. 1). Ten out of fifty strains (NBAIR Bt101, NBAIR Bt103, NBAIR Bt104, NBAIR Bt107, NBAIR Bt112, NBAIR Bt113, NBAIR Bt114, NBAIR Bt119, NBAIR Bt120 and NBAIR Bt142) caused more than 50% mortality (Table 2). LC50 and LC90 values of all the ten strains ranged 38.48–105.18 (μg/ml) and 146–150 (μg/ml), respectively, at 120 h (five days) after treatment. The highest percent mortality and lowest LC50 value were observed for NBAIR Bt107, and it was on par with the mortality caused by the reference strain (Bti-4Q1) (Table 3).
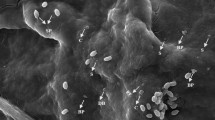
Bioassay and re-isolation of Bacillus thuringiensis from cadaver of Zeugodacus cucurbitae to prove Koch’s postulates a control: healthy larva not showing any discoloration of body; b B. thuringiensis-treated larva displaying complete discoloration of body; c Petri plates showing the colonies of B. thuringiensis reisolated from cadaver; d vegetative cells of B. thuringiensis; e spores and crystal proteins of B. thuringiensis re-isolated from dead larva under microscope
Frequency and distribution of dipteran active cry genes
To dwell into the cry gene profiles of each strain and correlate them with differences in mortality percentage, 50 strains for amplification of twenty-one dipteran active cry genes were tested (Table 2). The strains that displayed desired-size amplification products on agarose gel were regarded as positive for the corresponding genes (Additional file 1: Fig. S1). The frequency of cry genes present in all strains was identified, summarized and depicted in Fig. 2. The results showed each strain harbored at least one or other dipteran active cry genes, but none of the strains tested positive for cry4A, cry4B, cry29 and cry44 genes.
All the strains (50/50) tested positive for cry1A, followed by the cry2A gene in 88% (44/50) and cry1C gene in 84% (42/50) of strains that were found. Among the different cry1 subfamilies, cry1B was the least abundant, as only 12 strains harbored this gene. Similarly, the genes cry25 and cry27 were the least abundant among all the cry genes present, as only 0.0 2% (1/50) of the strains tested positive for those genes. Only three and ten strains among 50 tested positive for cry21 and cry39 genes, respectively. Also, twenty-seven and thirty-seven strains among 50 strains tested positive for cry70 and cry27 genes, respectively. Similarly, 28, 58, 74, 24, 16, 14 and 22% of strains tested positive for cry10, cry11, cry19, cry24, cry25, cry30 and cry32 in the same order, respectively.
Diversity of dipteran active cry gene combinations
The study on cry gene combinations in cry gene-positive strains revealed that every strain contained a minimum of three cry genes, with certain strains harboring a maximum of nine cry genes. Three out of 50 strains (6%) tested positive for nine cry genes, but just two percent (1/50) of strains only had three cry genes. A total of 16% (8/50), 12% (6/50), 44% (22/50) and 10% (4/50) of strains were positive for eight, seven, six, five and four cry genes, respectively, in different combinations. However, cry gene profiles of each strain were found to be distinct. So, for ease of comparison of cry gene combinations with possible toxicity, the strains were grouped, based on the number of cry genes (Fig. 3). All the fifty strains were classified into seven groups based on the number of cry genes present in each strain (Table 2).
Among the four strains that had the highest number of cry genes, 100% of them (4/4) harbored cry1A, cry1C, cry2A, cry11 and cry70, but all strains were found to be negative for gene cry30. But cry10 tested positive for only two strains (NBAIR Bt101 and NBAIR Bt104) that showed high toxicity. Three strains tested positive for the cry19 gene, only two strains tested positive for the cry27 gene, and another two strains tested positive for the gene cry25. The genes cry16 and cry27 combination was found only in NBAIR Bt111; similarly, cry32 and cry39 combination was found only in NBAIR Bt121.
In addition to the commonly observed genes cry1A, cry1C and cry2A, a few other genes, such as cry17 and cry19, were also prevalent among the thirteen strains that tested positive for either eight or seven cry genes. There were only two strains that carried either the cry21 or cry25 genes. The presence of cry24 was more abundant, and cry25 was the least abundant among the strains that tested positive for eight cry genes compared to those with seven cry genes. Ten strains showed positive results for cry11, and eleven strains are positive for cry19. Only a single strain harbored both cry30 and cry32.
The highest number of strains (23/50) tested positive for six dipteran active cry genes in different combinations. Among these strains, the cry gene profiles in combination with cry1A, cry1C, cry2A, cry11 and cry19 are found to be more abundant. The only strain that was positive for cry20 was also found positive for cry24, cry70, cry10, cry2A and cry1A. There was a huge correlation between cry11 and cry19, as most of the strains that are positive for cry11 were also found positive for cry 19. Within the strains that were positive for either three, four or five cry genes, association of cry 19 with cry1 was more frequent, and 60% of strains (6/10) had cry gene profiles of cry1A, cry1B and cry 19.
Comparison of cry gene profiles of Bacillus thuringiensis strains with toxicity against Zeugodacus cucurbitae larvae
Based on the PCR amplification of the dipteran active cry genes in all fifty strains, a diversity of cry gene combinations was identified. The cry gene combinations in a strain were compared to the mortality of second-instar larvae of Z. cucurbitae (Table 1). The number of cry genes present in the Bt strains that displayed more than 50% mortality tested positive for at least six active dipteran active cry genes to a maximum of nine cry genes. However, the greatest number of cry genes in a strain not necessarily causes greater mortality, but the all the Bt strains displayed more than 50% mortality showed a common combination of cry1A, cry2A, cry10A and cry70.
Discussion
Numerous effective Bt strains have been found worldwide, and their toxicity may vary depending on insect species and environmental factors. So, it is always essential to look for indigenous strains that are highly acquainted with the local environment from which they are isolated (Ma et al. 2023). India, being rich in a diversity of habitats, offers ample opportunities for the identification, characterization and exploration of native effective Bt strains. Several studies have documented the insecticidal toxicity of Bt against multiple insect orders, including Diptera (Fernández-Chapa et al. 2019).
In the present study, a total of fifty Bt strains were screened for dipteran active cry genes and toxicity against Z. cucurbitae. The strong insecticidal property of any Bt strain is highly correlated with the combined activity of different cry genes in a particular strain. Hence, the identification of cry gene content forms a basis for the prediction of possible toxicity against different species of insects. Based on PCR amplification analysis, dipteran active cry genes such as cry1A, cry1B, cry1C, cry2A, cry10, cry11, cry16, cry19, cry20, cry21, cry24, cry25, cry27, cry30, cry32, cry39 and cry70 were reported in native Bt strains at different frequencies. None of the isolates were tested positive for cry4A, cry4B, cry29 or cry44. In the present study, among cry1 subfamilies, cry1A was present in all strains, and only 24% (12/50) and 84% (42/50) cry1A gene-positive strains also harbored cry1B and cry1C, respectively. The cry1A and cry2A gene combinations were more frequently observed among strains than any other gene combination, as 88% (44/50) of strains were positive for both genes. So, cry1A, cry1C and cry2 were the most commonly found cry genes in indigenous Bt strains, and these results are consistent with other reports (Zhang et al. 2000). Among the 21 dipteran active cry genes evaluated in the current study, cry1A, cry 1C, cry2A, cry10, cry11, cry19 and cry70 were found to be more frequent in the native Bt strains.
Looking into the cry gene profiles of first groups of strains, NBAIR 101 had cry1 A-C, cry2A, cry10, cry11, cry19, cry25 and cry70, whereas NBAIR Bt104 also had a similar profile, except for cry19, but had a cry24. Various studies proved that strains with cry4, cry10, cry11 and cyt gene combinations, highly effective against mosquitoes (Ibarra et al. 2003) and also that high toxicity of strains due to the synergetic action of cry4 and cry11 is reported (Hayakawa et al. 2017). Similarly, the gene cry19 has been reported to increase the toxicity of strains in combinations with the orf2 gene, which is reported to have sequence similarity with cry4B, and this combo is similar to the operon structure of the cry10-orf2 format reported in Bti (Rosso et al. 1997). Beron and Salerna (2007) in cry24 family, Cry24Ca1 was reported to have high toxicity against Aedes aegypti Linnaeus (Diptera: Culicidae). In the same way, a study conducted by Fayad and co-workers showed novel cry genes, such as cry70B, reported to have an anti-dipteran activity (Fayad et al. 2021). Based on the present study, we may predict that cry genes such as cry10, cry11 and cry19 might interact with other cry genes present in a strain and these genes are probably interacting with other cry and cry70 as both the strains tested negative for cry4A and cry4B genes.
The cry gene profiles of strains NBAIR Bt111 and NBAIR Bt121 were found to be similar to those of NBAIR Bt101 and NBAIR Bt104, except for the gene cry10. It shows that cry10 may be responsible for enhanced toxicity in NBAIR Bt101 and NBAIR Bt104. The presence of cry16 and cry27 in strain NBAIR Bt111 may be responsible for more toxicity compared to NBAIR Bt121, as the cry16 gene, when co expressed with other genes, was found to cause increased toxicity (Qureshi et al. 2014), and cry27 also caused toxicity to Anopheles stephensi Listen (Diptera: Culicidae) (Saitoh et al. 2000).
Among the second group of strains that harbored eight dipteran active cry genes in different combinations, only the two strains NBAIR Bt114 and NBAIR Bt142 showed a combination of the cry10, cry11, cry19 and cry70 genes. Among the second group of strains that harbored eight dipteran active cry genes in different combinations, three strains, NBAIR Bt113, NBAIR Bt114 and NBAIR Bt142, the cry gene profiles of these two strains were very similar, and these three were the only strains that showed a combination of cry1A-C, cry2A, cry10, cry11, cry19 and cry70. This shows that interaction among these genes causes maximum mortality of melon fruit fly larvae. Similarly, among the strains that were positive for either seven, six, five, four or and three cry genes, only three strains, such as NBAIR Bt107, NBAIR Bt119 and NBAIR Bt120, caused the highest mortality. The cry gene profiles of these strains revealed that cry10 or cry70 was the most common cry genes that were found. The strains caused more than 50% mortality had a combination of cry1A, cry2A, cry10A, cry70. Moreover, the strains that are negative for either cry10 or cry70 showed a very low mortality compared to strains that were positive for both strains. However, further research has to be conducted on certain aspects, like cloning the cry1A, cry2A, cry 10A and cry70 to be done individually to evaluate the potential toxicity and synergistic activity with other cry genes of interest.
Interestingly, even though strain NBAIR Bt107 harbored only six dipteran active cry genes, it is the only strain that tested positive for cry20. In contrast, NBAIR Bt142 was almost equal to NBAIR Bt107, but instead of cry20, it had cry39. Similarly, strains such as NBAIR Bt112, NBAIR Bt113 and NBAIR Bt119 had more or less equal LC50 values, and all these strains were tested positively for cry16 and cry19, apart from cry 10 and cry70. NBAIR Bt114 strain tested positive for cry11 instead of cry16. Despite the similarity in mortality percentage and cry gene profiles, differences in LC50 were found between two strains, NBAIR Bt101 and NBAIR Bt104. This shows that not the greater number of cry genes and its mere presence of cry genes; expression levels of cry genes determine the toxicity. However, further studies have to be carried out to study the role of individual cry genes and the combination of cry genes causing mortality in larvae of Z. cucurbitae.
Conclusion
The frequency and distribution of dipteran active cry genes were detected in fifty indigenous Bt strains. All the strains tested positive for more than one cry gene. Ten out of 50 Bt strains displayed high toxicity against Z. cucurbitae. Among the cry genes, cry1A, cry1B, cry1C, cry2A, cry10A, cry11A, cry16A, cry19A, cry20, cry24, cry25, cry39A and cry70 were detected in high frequency. The probable cry genes responsible for causing toxicity in larvae were interpreted based on a comparison of the cry gene profiles of each strain and corresponding larval mortality. The common combination in all ten strains that displayed great toxicity included cry1A, cry2A, cry10A and cry70. Hence, this study paves the way for further studies in testing the toxicity of individual cry gene or interactions among genes and thus helps in identification of potential cry genes responsible for causing mortality. So, effective strains with potential cry genes can be used for the management of Z. cucurbitae. This further helps the development of an environmentally friendly bioformulation in anticipation of its possible application in the field and developed of Bt-based transgenic crops.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Bt :
-
Bacillus thuringiensis
- hrs. :
-
Hours
- 0 C :
-
Degree Celsius
- RH :
-
Relative humidity
- LC 50 :
-
Median lethal concentration
- μg :
-
Microgram
- min :
-
Minutes
- cry :
-
Crystal
- NCBI :
-
National Centre for Biotechnology Information
- mL :
-
Milliliter
References
Ammouneh H, Harba M, Idris E, Makee H (2011) Isolation and characterization of native Bacillus thuringiensis isolates from Syrian soil and testing of their insecticidal activities against some insect pests. Turk J Agric for 35(4):421–431. https://doi.org/10.3906/tar-1007-1117
Ankitha KS, Radha TK, Ruqiya S, Kukreti A, Aarthi N, Nanditha S, Rajagopal R, Kandan A, Sivkumar G, Shylesha AN, Girisha HC, Nagaraju K, Venkatesan K, Sushil SN, Manjunatha C (2023) Exploring the impact of cyclic lipopeptides from Bacillus subtilis NBAIR-BSWG1 through in vitro and in planta, studies against Sclerotium rolfsii. J Biol Cont 37(3):145–149. https://doi.org/10.18311/jbc/2023/35546
Barloy F, Lecadet MM, Delécluse A (1998) Distribution of clostridial cry-like genes among Bacillus thuringiensis and Clostridium strains. Curr Microbiol 36:232–237. https://doi.org/10.1007/s002849900300
Ben-Dov E, Zaritsky A, Dahan E, Barak ZE, Sinai R, Manasherob R, Margalith Y (1997) Extended screening by PCR for seven cry-group genes from field-collected strains of Bacillus thuringiensis. Appl Environ Microbio 63(12):4883–4890. https://doi.org/10.1128/aem.63.12.4883-4890.1997
Berón CM, Salerno GL (2007) Cloning and characterization of a novel crystal protein from a native Bacillus thuringiensis isolate highly active against Aedes aegypti. Curr Microbiol 54(4):271–276. https://doi.org/10.1007/s00284-006-0299-8
Bravo A, Gill SS, Soberón M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49(4):423–435. https://doi.org/10.1016/j.toxicon.2006.11.022
Bukhari DA, Kanwal T, Iftikhar S, Rehman A (2022) The potential mosquitocidal activity of cry4A toxic region crystal protein gene from local isolates of Bacillus thuringiensis against Aedes aegypti. J King Saud Univ Sci 34(6):102191. https://doi.org/10.1016/j.jksus.2022.102191
Crickmore N, Berry C, Panneerselvam S, Mishra R, Connor TR, Bonning BC (2021) A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J Invertebr Pathol 186:107438. https://doi.org/10.1016/j.jip.2020.107438
De Bon H, Huat J, Parrot L, Sinzogan A, Martin T, Malezieux E, Vayssières JF (2014) Pesticide risks from fruit and vegetable pest management by small farmers in sub-Saharan Africa A review. Agron Sustain Dev 34:723–736. https://doi.org/10.1007/s13593-014-0216-7
Dhillon MK, Singh R, Naresh JS, Sharma HC (2005) The melon fruit fly, Bactrocera cucurbitae: a review of its biology and management. J Insect Sci 5(1):40. https://doi.org/10.1093/jis/5.1.40
Ejiofor AO, Johnson T (2002) Physiological and molecular detection of crystalliferous Bacillus thuringiensis strains from habitats in the South-Central USA. J Ind Microbiol Biotechnol 28(5):284–290. https://doi.org/10.1038/sj/jim/7000244
Fayad N, Kambris Z, El Chamy L, Mahillon J, Kallassy Awad M (2021) A novel antidipteran Bacillus thuringiensis strain: unusual cry toxin genes in a highly dynamic plasmid environment. Appl Environ Microbio 87(5):1–21. https://doi.org/10.1128/AEM.02294-20
Fernández-Chapa D, Ramírez-Villalobos J, Galán-Wong L (2019) Toxic potential of Bacillus thuringiensis. In: Yulin Jia (ed) An overview protecting rice grains in the post-genomic era. IntechOpen. https://doi.org/10.5772/INTECHOPEN.85756.
Hayakawa T, Yoneda N, Okada K, Higaki A, Howlader MTH, Ide T (2017) Bacillus thuringiensis cry11Ba works synergistically with cry4Aa but not with cry11Aa for toxicity against mosquito Culex pipiens (Diptera: Culicidae) larvae. Appl Entomol Zool 52:61–68. https://doi.org/10.1007/s13355-016-0454-z
Ibarra JE, Del Rincón MC, Ordúz S, Noriega D, Benintende G, Monnerat R, Regis L, De Oliveira CM, Lanz H, Rodriguez MH, Sánchez J, Peña G (2003) A Diversity of Bacillus thuringiensis strains from Latin America with insecticidal activity against different mosquito species. Appl Environ Microbiol 69(9):5269–5274. https://doi.org/10.1128/AEM.69.9.5269-5274.2003
Ilias F, Gaouar N, Medjdoub K, Awad MK (2013) Insecticidal activity of Bacillus thuringiensis on larvae and adults of Bactrocera oleae Gmelin (Diptera: Tephritidae). J Environ Prot 4(5):1–6. https://doi.org/10.4236/jep.2013.45056
Ito T, Ikeya T, Sahara K, Bando H, Asano SI (2006) Cloning and expression of two crystal protein genes, cry30Ba1 and cry44Aa1, obtained from a highly mosquitocidal strain, Bacillus thuringiensis subsp. entomocidus INA288. Appl Environ Microbio 72(8):5673–5676. https://doi.org/10.1128/AEM.01894-05
Liu X, Lin X, Li J, Li F, Cao F, Yan R (2020) A novel solid artificial diet for Zeugodacus cucurbitae (Diptera: Tephritidae) larvae with fitness parameters assessed by two-sex life table. J Insect Sci 20(4):21. https://doi.org/10.1093/jisesa/ieaa058
Ma X, Hu J, Ding C, Portieles R, Xu H, Gao J, Borrás-Hidalgo O (2023) New native Bacillus thuringiensis strains induce high insecticidal action against mosquito larvae and adults. BMC Microbiol 23(100):1–23. https://doi.org/10.1186/s12866-023-02842-9
Manjunatha C, Velavan V, Rangeshwaran R, Mohan M, Kandan A, Sivakumar G, Shylesha AN, Kumar MK, Pramesh D, Sujithra M, Ranganath HK, Sushil SN (2023) Assessment of bio-formulations of indigenous strains of Bacillus thuringiensis, Metarhizium robertsii and Metarhizium majus for management of the rhinoceros beetle, Oryctes rhinoceros L., in field. Egypt J Biol Pest Control 33(1):1–6. https://doi.org/10.1186/s41938-023-00715-x
Martinez AJ, Robacker DC, Garcia JA (1997) Toxicity of an isolate of Bacillus thuringiensis subspecies darmstadiensis to adults of the Mexican fruit fly (Diptera: Tephritidae) in the laboratory. J Econ Entomol 90(1):130–134. https://doi.org/10.1093/jee/90.1.130
Nanditha S, Nanjundaiah SA, Thiruvengadam V, Channappa M, Thammayya SK, Aravindaram K, Sushil SN (2024) Development of recombinase polymerase amplification-based colorimetric detection assay for rapid identification of invasive cassava mealybug, Phenacoccus manihoti Matile-Ferrero. Saudi J Biol Sci 31(6):104005. https://doi.org/10.1016/j.sjbs.2024.104005
Nisar MJ, Gogi MD, Arif MJ, Sahi ST (2020) Toxicity and chemosterility impact of insect growth regulators baited diet on adult peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae). Pak J Agric Sci 57(4):1089–1099. https://doi.org/10.21162/PAKJAS/20.9962.
Porcar M, Juárez-Pérez V (2003) PCR-based identification of Bacillus thuringiensis pesticidal crystal genes. FEMS Microbiol Rev 26(5):419–432. https://doi.org/10.1016/S0723-2020(00)80042-9
Qureshi N, Chawla S, Likitvivatanavong S, Lee HL, Gill SS (2014) The Cry toxin operon of Clostridium bifermentans subsp. malaysia is highly toxic to Aedes larval mosquitoes. Appl Environ Microbio 80(18):5689–5697. https://doi.org/10.1128/AEM.01139-14
Rosso ML, Delécluse A (1997) Contribution of the 65-kilodalton protein encoded by the cloned gene cry19A to the mosquitocidal activity of Bacillus thuringiensis subsp. jegathesan. Appl Environ Microbio 63(11):4449–4455. https://doi.org/10.1128/aem.63.11.4449-4455.1997
Ruqiya S, Girisha H, Rangeshwaran R, Kandan A, Siva Kumar G, Shivakumar KT, Aditya K, Ankitha KS, Venu HS, Nanditha S, Aarthi N, Manjunatha C (2023) Identification of secondary metabolites biosynthetic genes, antagonistic activity and potential mechanism of Bacillus subtilis NBAIR-BSWG1 in suppression of Alternaria alternata. J Biol Cont 37(4):226–232. https://doi.org/10.18311/jbc/2023/35973.
Saitoh H, Hwang SH, Park YS, Higuchi K, Mizuki E, Ohba M (2000) Cloning and characterization of a Bacillus thuringiensis serovar higo gene encoding a novel class of the δ-endotoxin protein, cry27A, specifically active on the anopheles mosquito. Syst Appl Microbiol 23(1):25–30. https://doi.org/10.1016/S0723-2020(00)80042-9
Subedi K, Regmi R, Thapa RB, Tiwari S (2021) Evaluation of net house and mulching effect on cucurbit fruit fly (Bactrocera cucurbitae Coquillett) on cucumber (Cucumis sativus L.). J Agric Res 3:100103. https://doi.org/10.1016/j.jafr.2021.100103.
Thammasittirong A, Attathom T (2008) PCR-based method for the detection of cry genes in local isolates of Bacillus thuringiensis from Thailand. J Invertebr Pathol 98:121–126. https://doi.org/10.1016/j.jip.2008.03.001PMid:18407288
Valtierra-de-Luis D, Villanueva M, Berry C, Caballero P (2020) Potential for Bacillus thuringiensis and other bacterial toxins as biological control agents to combat dipteran pests of medical and agronomic importance. Toxins 12(12):773–788. https://doi.org/10.3390/toxins12120773
Vargas RI, Piñero JC, Leblanc L (2015) An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 6(2):297–318. https://doi.org/10.3390/insects6020297
Zhang HongYu ZH, Yu ZinIu YZ, Deng WangXi DW (2000) Composition and ecological distribution of cry proteins and their genotypes of Bacillus thuringiensis isolates from warehouses in China. J Invertebr Pathol 76(3):191–197. https://doi.org/10.1006/jipa.2000.4970
Acknowledgements
The authors would like to express their sincere gratitude to The Director, ICAR-NBAIR, Bangalore, The Head of the Department, Division of Entomology, I.G.K.V., Raipur, for the invaluable support provided during the successful execution of this research. The current research is funded through the ICAR-NBAIR project “Molecular Studies on Virulence of Bacillus thuringiensis and other Entomogenous Bacteria against Fall Armyworm and White grubs (CRSCNBAIRSIL202100300205)."
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CM, AN, ANS and VKD conceptualized the research. AN conducted experiment. AK, AK and RR monitored experiments and edited the manuscript. All the authors have read the manuscript and approved for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in the study are in accordance with the ethical standard of the institutional and/or national research committee. We further declare that no animal was harmed during the study.
Consent for publication
Informed consent was obtained from all the individual participants included in the study.
Competing interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Agarose gel electrophoresis of PCR products amplified with dipteran active cry genes in indigenous Bacillus thuringiensis strains.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aarthi, N., Shylesha, A.N., Dubey, V.K. et al. Screening of indigenous Bacillus thuringiensis for dipteran active cry gene profiles and potential toxicity against melon fruit fly, Zeugodacus cucurbitae (Coquillett). Egypt J Biol Pest Control 34, 45 (2024). https://doi.org/10.1186/s41938-024-00811-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-024-00811-6