Abstract
Background
Atori Reservoir, located in the heart of southwestern Nigeria, is a tribute to the region's historical significance and natural splendor. Its establishment as a reservoir in 1935 marked a water resource management watershed in the region, transforming Atori into one of Nigeria’s oldest and most important waterbodies. Despite its ancient age and indisputable importance, the exact ecological state of Atori Reservoir has been shrouded in mystery for decades. This study was necessitated by a paucity of information on the ecological status of Atori Reservoir.
Results
The study identified 953 macroinvertebrates belonging to four classes, distributed across nine orders and nineteen families within Atori Reservoir. Among these, the class Insecta exhibited the highest diversity, while Melanoides tuberculata, a member of the Mollusca class, emerged as the dominant species. Despite the diverse macroinvertebrate community, the physicochemical parameters of the water raised concerns. Elevated values of total dissolved solids and conductivity indicated poor water quality, which was further reflected in the predominance of pollution-tolerant species and the absence of pollution-sensitive ones. Canonical correspondence analysis highlighted potential correlations between macroinvertebrates and water quality variables, yet statistical significance was lacking, as demonstrated by the Monte Carlo permutation test.
Conclusion
This study sheds light on the ecological state of Atori Reservoir, revealing a diverse macroinvertebrate community but highlighting concerns regarding water quality. The study also emphasizes the pressing need for improved management practices to safeguard the ecological health of Atori Reservoir, given the critical role it plays in the region's ecosystem and local communities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Reservoirs are generally considered essential components of the freshwater ecosystem as they constitute a major habitat for critical aquatic resources (Schofield et al., 2018; Zwahlen, 2022). However, many reservoirs are being threatened by the growing human population, urbanization and other anthropogenically induced stressors. This has brought about a growing concern about the sustainability of the natural, biological and functional dynamics of freshwater ecosystems. The situation is especially critical in developing countries like Nigeria, where little or no control measures are in place to manage and regulate activities around important waterbodies (Garba et al., 2022). Natural habitat destruction and the introduction of pollutants into aquatic environments are common and widespread phenomena in Nigeria. This has not only affected the ecological balance of many important waterbodies but has led to a great loss of biodiversity within such habitats (Ghali et al., 2020). Furthermore, these activities have practically led to the loss of the capacity of such waterbodies to self-purify to a large extent. As such, water quality monitoring is essential for ensuring public health and maintaining the ecological balance of waterbodies.
One of the major causes of degradation in the aquatic environment is the intensification of agricultural activities (Umara et al., 2019). The constant growth of the human population has placed a huge burden on the necessity of increased production and improvements in the yield of agricultural products. This has informed the conventional use of synthetic fertilizers and chemicals for improved yield as well as for weed and pest control (Yung-Chul et al., 2016). However, these activities have serious ecological implications for surrounding waterbodies as the residues from the chemicals are dispersed indiscriminately into the aquatic environment. This catalyzes excessive nutrient enrichment and pollution in the waterbodies. Pollution in the aquatic environment causes degradation and fragmentation of local habitats, which ultimately alter the equilibrium and community structure of aquatic organisms in such waterbodies. Pollutants such as nitrogen and phosphorus from runoff may also cause increased biological productivity, resulting in the depletion of dissolved oxygen (DO) and eutrophication in reservoirs and other lentic waterbodies. The direct consequence of this is the reduction of habitat heterogeneity and the alteration of the ecological equilibrium of such waterbodies (Saleh et al., 2021).
In assessing the ecological status of waterbodies, one method that has become globally acceptable is bio-assessment using metrics of the community structure of aquatic organisms (Makumbe et al., 2022). The species richness and community assemblage of macroinvertebrates in a waterbody can provide useful and reliable information on the health of the waterbody. The species richness and community assemblage of macroinvertebrates reflect the number of species and individuals in a community and how evenly spread they are in such a community. Pollution leads to a decrease in the number of sensitive species, while hardy species thrive and outcompete less resilient ones. Assessment of water quality using physicochemical parameters of water is one of the traditional methods that have also been commonly used. The objectivity of this method is based on the fact that the physicochemical qualities of the substratum on which the macroinvertebrates are found determine their occurrence and distribution. Variation in the physicochemical parameters of water may lead to distortions in biological activities, the transfer of energy and the migration of aquatic insects (Amusan & Balogun, 2019). Furthermore, the nature of the distribution of physicochemical parameters of water provides firsthand information on the ecological processes and environmental variables that govern the populations and community structure of macroinvertebrates in a given waterbody (Kumar et al., 2022). Therefore, a combination of bio-assessment and the determination of the physicochemical properties of water will capture the whole spectrum of ecological stressors, thus providing a more reliable estimate of the health of waterbodies (Gouissi et al., 2019).
Most studies on water quality assessment in Nigeria have focused on either the use of physicochemical parameters or the macroinvertebrate community structure. However, this study aimed at assessing the health of Atori Reservoir using a combination of macroinvertebrate community structure and physicochemical parameters of the waterbody as indicators.
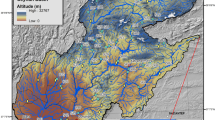
Atori Reservoir, one of the oldest in Nigeria, was impounded in 1935, yet no documented effort has been made to assess its health status since then. Located in Iseyin, southwestern Nigeria, the reservoir lies between Longitude 003036’E and Latitude 07058’N, at an altitude of 329 m amsl (Fig. 1). It boasts a capacity of about 580 million m3, covering a surface area of 4.7 km2, with a mean depth of 5.2 m. The embankment stretches approximately 120 m in length and 40 m in width, featuring a mechanical spillway that automatically releases water when it exceeds the maximum operational capacity. Unique to Atori Reservoir is its water source, which primarily comes from seepage from the ground. The reservoir serves various purposes, including providing potable water and other ancillary functions to the people in Iseyin and neighboring communities. Iseyin, an ancient city in Oyo State, lies approximately 100 km north of Ibadan, encompassing a total land mass of 1,419 km2 (Adewuyi et al., 2018). Despite its historical significance, the reservoir faces varying degrees of anthropogenic disturbances, mainly from runoff from agricultural lands, fishing activities and domestic waste. In addition to its physical attributes, Atori Reservoir's surrounding landscape is characterized by extensive agricultural lands, where food and cash crops such as maize, cassava, yam, citrus, cashew and cocoa are cultivated. The area also supports animal husbandry, with abundant grasslands to sustain livestock. Notable tree species like Elaeis guineensis (Oil palm), Cocos nucifera (Coconut), Adonsonia digitata (Baobab) and Albizia lebbeck (Ayunre) are found in the vicinity, contributing to the region's biodiversity. Overall, while Atori Reservoir plays a vital role in providing water resources to the local communities and supporting agricultural activities, it faces environmental challenges due to human activities (Oladimeji et al., 2024). Understanding its health status is crucial for effective management and conservation efforts in the region.
Hence, this study aims to evaluate the health status of the waterbody by examining macroinvertebrate community structure and the physicochemical properties of water as indicators. The specific objectives include analyzing the composition, diversity and abundance of macroinvertebrate species, monitoring variations in physicochemical properties during the wet and dry seasons across various sampling locations and assessing potential relationships between physicochemical properties and macroinvertebrate composition in the reservoir.
Methods
Sampling program and selection of sampling stations
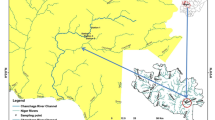
The study area lies within the tropical monsoon climate zone, characterized by two distinct seasons: the wet (May–October) and the dry (November–April). As such, the sampling program was designed to cover both the wet and dry seasons of an annual cycle. Sampling was carried out between December 2019 and October 2020. The reservoir was visited once every two months, thus making a total of six sampling periods for the collection of macroinvertebrates and water samples. We selected five (5) sampling stations based on the reservoir reaches and to ensure that the numerous microhabitats within the reservoir are covered. Information on the grid location, elevation and mean depth for the sampling stations is presented in Table 1.
Collection, sorting and identification of macroinvertebrates
Sampling of macroinvertebrates was carried out three consecutive times per station during every sampling period aboard a canoe using an improvised grab sampler of 0.04 m2 (0.2 m × 0.2 m). The grabbed sediment samples were then gently sieved through a 0.5 mm mesh sieve using the reservoir water (Strayer, 2009). The macroinvertebrates retained in the sieve were picked up using a pair of long forceps. Apart from this method, macroinvertebrates were also collected thrice per sampling site using a long-handled D-frame net. This net was used to scoop the open water, submerged and emergent vegetation zones in the reservoir. The sorting of the collected specimens was done on a white enamel tray. Sorted specimens were preserved in specimen bottles containing 70% ethanol solution (APHA, 2005) and appropriately labeled. The specimens were identified using appropriate taxonomic keys and guides such as Brown (1980), Madsen (1985), Schneider (1990), Water Research Commission WRC (2003) and Verma (2006).
Collection of water samples
Two water samples per site were collected from clear water in sterilized 1-L plastic bottles for the analysis of the physicochemical properties of the water during every sampling period. Water temperature, dissolved oxygen (DO), pH, electrical conductivity (EC) and total dissolved solids (TDS) were determined on the field using a multi-3630 IDS digital meter, precision meters and a waterproof pH meter (version pH-689). However, the water samples were taken to the laboratory for the analysis of other parameters, including biological oxygen demand (BOD), acidity, total alkalinity, chloride, calcium, magnesium, nitrate and phosphate, according to APHA (2005).
Data analysis
All the data sets were subjected to a normality test. Based on the normality test, appropriate parametric and nonparametric statistical tests were used to analyze the data. Physicochemical parameters were analyzed with one-way analysis of variance (ANOVA), while a Kruskal–Wallis test was used to analyze the community structure of the macroinvertebrates. Margalef, Shannon–Wiener and Simpson's diversity index were used to measure diversity, and Pielou's evenness index was used to assess species evenness. Canonical correspondence analysis (CCA) was used to determine the relationship between the community structure of macroinvertebrates and physicochemical parameters. All statistical tests were carried out using Microsoft Excel and PAST (Hammer et al., 2001).
Results
Physicochemical parameters of water in Atori Reservoir
Spatial variations
There were variations in the values obtained for the physicochemical parameters of the water across the sampling stations, but the variations were not statistically significant (p > 0.05) as shown in Table 2. The pH and phosphate values were similar across the sampling stations. The highest values for EC, TDS and alkalinity were recorded in Station 1, while acidity, calcium and hardness were highest in Station 3. The highest BOD value was obtained in Station 2, while magnesium was highest in Station 4. A good number of the investigated parameters, such as DO, EC, TDS, alkalinity, calcium and hardness, had their minimum values in Station 2 (Table 2).
Seasonal variations
The mean values obtained for DO, TDS, pH, magnesium, calcium, water hardness, phosphate, nitrate and chloride were higher in the wet season, while water temperature, BOD, EC, acidity and alkalinity had higher values in the dry season (Table 3). Only six (6) parameters in the seasonal variations of physicochemical characteristics of water in Atori Reservoir were significantly different (T-test, p < 0.05, p < 0.01, p < 0.001), according to a paired-two sample T-test (p < 0.05, p < 0.01, p < 0.001). The physicochemical parameters that were significantly different include DO concentration, acidity, alkalinity, calcium, water hardness and phosphate (Table 3).
Taxonomic composition and distribution of macroinvertebrates
A total of 953 macroinvertebrate individuals (ind.) distributed in three phyla, four classes, nine orders, 19 families and 32 genera were reported in this study (Table 4). Four families comprised the non-insect group (Hirudidae, Phanorbidae, Thiaridae and Pisauridae), while the remaining fifteen (15) were insects, and they were the dominant macroinvertebrates group collected. The identified insect orders include Hemiptera, Coleoptera, Diptera, Ephemeroptera and Odonata. The dominant species recorded was Melanoides tuberculata (Muller, 1774), with 321 macroinvertebrate individuals (33.68%). Other species that were recorded in relatively high numbers include Notonecta sp. (Insecta) and Biomphalaria sp. (Gastropoda), with 191 (20%) and 138 (14%), respectively. More macroinvertebrates were recorded in the dry period (569) (59.7%) as against 384 (40.3%) macroinvertebrate individuals recorded in the wet (Table 4). The stations did not differ significantly with respect to the abundance of macroinvertebrates. However, the highest number of macroinvertebrates was recorded at Station 5, while the least was recorded at Station 2. In terms of taxa richness, Station 2 had the lowest, while Station 1 had the highest (Tables 5 and 6). Margalef and Shannon–Wiener (H) indices showed that Station 1 had the highest diversity of macroinvertebrates, while Station 2 had the least diversity (Table 5). Pielou’s evenness index was highest in Station 3, while the least was recorded in Station 2.
Relationship between the macroinvertebrates and physicochemical parameters
Canonical correspondence analysis (CCA) of the relationship between macroinvertebrates and water physicochemical parameters revealed that macroinvertebrate abundance in stations 2 and 4 was associated with increasing temperatures, magnesium and pH, whereas abundance in the other stations was associated with increasing values for all other parameters (Fig. 2). Furthermore, the distribution of macroinvertebrates in the triplot of the first and second CCA axes showed that macroinvertebrates (Hydrophilus sp., Bulinus sp. and Limnogonus sp.) that were sensitive to optimum concentrations of physicochemical variables of water were also observed to be associated with Stations 3 and 5 on the triplots (Fig. 2). Aside from this, Agriocnemis sp., Macrocoris sp., Rhagadotarus sp. and Anisops were associated with increases in magnesium and temperature in Station 2. However, Anax sp., Enithares sp. and Potamocloeon sp. were associated with Station 4 where pH was the only increased variable while Pseudagrion sp., Naucoris sp. and Agasicles sp associated with high levels of BOD.
Graph CCA representation of macroinvertebrates, water variables and sampling stations in Atori Reservoir, Nigeria (December 2019–October 2020). Keys: Tem = temperature, DO = dissolved oxygen, BOD = biological oxygen demand, EC = electrical conductivity, TDS = total dissolved solids, Aci = acidity, Alk = alkalinity, Mag = magnesium, Cal = calcium, WaH = water hardness, Pho = phosphate, Nit = nitrate, Chl = chloride, Bio = Biomphalaria, Bul = Bulinus, Mel = Melanoides tuberculata, App = Appasus, Limg = Limnogonus, Limp = Limnoporus, Neo = Neogerris, Rha = Rhagadotarus, Mac = Macrocoris, Nau = Naucoris, Ani = Anisops, Eni = Enithares, Not = Notonecta, Aga = Agasicles, Amp = Amphiops, Hyd = Hydrophilus, Chil = Chironomus, Pot = Potamocloeon, Anax = Anax, Dip = Diplacodes, Tetr = Tetrathemis, Agr = Agriocnemis, Pse = Pseudagrion
The percentage of variance that each axis of the CCA explains is that axes 1 and 2 accounted for 47.38% and 24.18%, respectively (Table 7).
Discussion
Physicochemical parameters of water
Atori Reservoir, a vital water source in Nigeria, serves multiple purposes including providing potable water, irrigation and domestic uses. However, its health is subject to scrutiny because the reservoir receives farm runoff from agricultural lands around the reservoir catchment basin while fishing, bathing and washing of agricultural equipment were also evident. These activities can potentially cause a deterioration of the water quality in the reservoir. As such, the high values reported for EC, TDS, turbidity, water hardness and chloride are markers of human-induced stress on the reservoir (Akamagwuna et al., 2019).
Although the measured water quality variables of Atori Reservoir were generally within the optimum range for the survival and growth of aquatic life in the tropics (Godswill, 2012; Mugo, 2010), the parameters were within the permissible limits of the National Environmental Standards and Regulatory Enforcement Agency (NESREA, 2007), the United States Environmental Protection Agency (USEPA, 2005) and the World Health Organization (WHO, 2004) standard for standing waters.
Spatially, variations in physicochemical parameters across sampled stations were observed but not statistically significant (p > 0.05), indicating microhabitat homogeneity. However, higher values in Station 2 may signal increased stress compared to others due to its closer proximity to agricultural farm lands. These findings align with studies on some selected similar waterbodies in Nigeria, such as Owalla Reservoir (Aduwo et al., 2019), Opa Reservoir (Aliu et al., 2020) and Aiba Reservoir (Atobatele & Ugwumba, 2008), in which there were no significant variations in the spatial distribution of the physicochemical parameters of the waterbodies.
Seasonal variations in physicochemical properties in Atori Reservoir were more pronounced and showed statistical significance for some properties such as DO, acidity, alkalinity, water hardness and nutrient levels. During the wet season, heightened precipitation and runoff introduced into the reservoir probably caused an increase in pH nutrient content, DO and water hardness (Zhang et al., 2020). Elevated temperatures enhance metabolic rates, influencing aquatic organism growth and reproduction. Conversely, the dry season brings increased temperatures, reduced water levels and flow rates, lowering dissolved oxygen.
Dissolved oxygen concentration is an important indicator of the ecological condition of aquatic ecosystems as it reflects the physical and biological processes taking place in such an environment (Kumar et al., 2011a; Ridanovic et al., 2010). The range of values obtained for the dissolved oxygen concentration in the reservoir was considered satisfactory. The relatively high values of DO could be attributed to the aeration rate and photosynthetic activities (Jaji et al., 2007). The distribution of DO in waterbodies has been known to be majorly influenced by a balance between some factors such as precipitation, photosynthesis activities, inputs from the atmosphere, losses by chemicals and biotic oxidations (Muralidharan et al., 2010; Nkwoji et al., 2010). The relatively higher concentration of DO in the wet season is not surprising, as it aligns with previous reports such as Amusan et al. (2018), Bhawsar and Vyas (2022), and Yusoff et al. (2002), in which higher concentrations of DO were reported. The observed higher concentration of DO in the wet season could be as a result of lower water temperature and increased algal productivity that produces oxygen in the season (Venkatesharaju et al., 2010).
The pH is an important parameter that not only measures the acid–base balance of water but also determines its suitability for various purposes (Sharma et al., 2011). The relatively high pH values recorded for the water indicated that the reservoir was slightly alkaline. The relatively high pH may be attributed to intense photosynthetic activities of aquatic plants, which reduce the free carbon dioxide concentrations in water (Gouissi et al., 2019). The reduction of free carbon dioxide concentration has been identified as one of the key indicators of higher pH in water bodies. Atori Reservoir had the majority of its surface area covered with emergent and submerged vegetation, as such a large volume of carbon dioxide is used up by the plants. Similar reports of slightly high pH values have been reported for Ikere Gorge Reservoir (Aiwerioghene & Ayoade, 2016), Opa Reservoir (Adedeji et al., 2020) and Watari Reservoir (Rabiu et al., 2018).
Water hardness is largely determined by the calcium and magnesium concentrations in the water. The hardness of water describes the effects of dissolved minerals and determines its suitability for drinking, domestic and industrial purposes (Kumar et al., 2011a). The relatively high values recorded for total hardness of Atori Reservoir water may be attributed to the discharge of sewage, washing and bathing with soaps and detergents as observed in this study.
Electrical conductivity (EC) is a measure of the capacity of the water to conduct electrical current and is chiefly determined by the concentrations of ions and nutrient load. The EC values reported in this study were considered relatively high when compared with similar water bodies in this part of the country. The high values recorded for conductivity in this study may be linked to the stagnant water in the reservoir. This is because conductivity has been reported to increase with stagnancy in water (Atobatele & Ugwumba, 2008). Aside from this, the circulation of water, which neutralizes salts in domestic and industrial wastes, could have also contributed to the high values of conductivity recorded in this study (Popoola et al., 2019).
Conductivity is also a good measure of the total dissolved solids (TDS) of water bodies. A water body with high TDS values is expected to have correspondingly high conductivity values. The TDS values recorded in this study could be considered relatively high for a tropical inland water body. The high values could possibly be due to high decaying vegetation, which would have increased the amount of dissolved solids in the water (Tanimu et al., 2011; Wang et al., 2007).
The nutrients were relatively low when compared with similar water bodies in tropical areas where low values have been reported for nutrients. The low concentration of the nutrients could be attributed to decreased organic matter due to mixing by runoff in the wet season, which reduced algal bloom in the water body (Popoola et al., 2019).
Taxonomic composition of macroinvertebrates
The abundance and diversity of macroinvertebrates recorded in Atori Reservoir can be considered relatively high and it compares favorably with studies on other water bodies in Nigeria such as Aduwo et al. (2019), Akindele et al. (2019), Aliu et al. (2020) and Amusan et al. (2018) in which high abundance and diversity of macroinvertebrates have been reported. The high diversity of macroinvertebrates reported in this study may be attributed to the stable substrata and nutrient availability in the reservoir (Aliu et al., 2020). The dominant taxon, Melanoides tuberculata, has been reportedly known to be dominant in water bodies around Nigeria. Previous studies such as Aduwo et al. (2019), Amusan et al. (2018), Anyanwu et al. (2019) and Aliu et al. (2020) have all reported M. tuberculata as the dominant taxon in their respective studies. The dominance of this taxon in most water bodies in the country has been attributed to their ability to survive in moderately impacted waters (Aliu et al., 2020).
The richness and abundance of macroinvertebrates exhibited notable seasonal variations, with considerably higher numbers recorded during the dry season. This trend aligns with previous findings by Ayoade and Aiwerioghene (2017), Makumbe et al. (2022), Kahlon et al. (2018) and Ogidiaka (2012). The lower abundance observed in the wet season could be attributed to several factors. The instability of bottom sediment due to increased rainfall and runoff during this season may have dislodged macroinvertebrates from their microhabitats (Yusuf, 2019). Additionally, higher levels of total suspended solids in the water column during the wet season reduce visibility, making macroinvertebrate collections more challenging.
Among various taxa, distinct differences in abundance were evident between the dry and wet seasons. For instance, Biomphalaria sp. displayed higher abundance during the dry season, while Notonecta sp. thrived more in the wet season. These patterns underscore the strong influence of seasonal environmental factors on macroinvertebrate distribution and abundance.
The observed variations suggest that increased precipitation during the wet season creates favorable conditions for certain species, leading to higher abundance. Conversely, fluctuations in water levels and nutrient availability during the dry season may impact macroinvertebrate communities differently. Moreover, the exclusive presence of certain species during specific seasons indicates their reliance on seasonal conditions for breeding or foraging, highlighting specialized adaptations to seasonal environmental cues.
The range of values for the Margalef and Shannon–Wiener diversity indices in clean water ranges between 3 and 4 and less than 1 in polluted water bodies (Akindele & Adeniyi, 2013; Akindele et al., 2013; Yusuf, 2019). The values recorded for the Margalef diversity index ranged from 2.77 to 4.16 while the Shannon–Wiener index values ranged from 1.77 to 2.22. This indicated that the reservoir was slightly stressed at the time the study was carried out.
Relationship between the macroinvertebrates and water parameters.
A total of 71.96% of the variables were explained by the first two axes, with Axis 1 of the CCA triplot explaining 47.39% of the total inertia and Axis 2 explaining 24.17% of the total inertia. Axis 1 of the CCA triplot accounted for six environmental variables, i.e., TDS, acidity, alkalinity, magnesium, calcium and water hardness. Axis 2, on the other hand, accounted for a total of three environmental variables, i.e., water temperature, nitrate and phosphate. A good number of the macroinvertebrates showed positive relationships with the physicochemical parameters of the water, but canonical correspondence analysis (CCA) revealed that the relationships were not statistically significant (Monte Carlo permutation test). The dominant species, Melanoides tuberculata, showed positive relationship with some of the physicochemical parameters such as electrical conductivity, alkalinity, TDS, DO and calcium. This implied that elevated concentrations of these factors positively impacted the population of this taxon. This is not shocking, as studies have reported positive correlation between these physicochemical parameters and Melanoides tuberculata (Efitre et al., 2001; Mwabvu & Sasa, 2009). These studies revealed that most tropical waters with moderately high conductivity, calcium and DO concentrations have recorded abundance of Melanoides sp. Conductivity and calcium are essential in shell development (Mitchell et al., 2007; Alkandari et al., 2020); as such, elevated concentrations are considered favorable to the Melanoides sp. This may as well explain their abundance and dominance in the tropical inland waters.
Pseudagrion sp., Naucoris sp. and Agasicles sp. were related to the elevated levels of BOD while Anax sp. was closely related to increased nitrate values. Anisops sp., Tetrathamis sp. and Enithares sp. were related to magnesium values while Biomphalaria sp. and Hydrophilus sp. were both related to organic carbon and organic matter values. Bulinus sp., Macrocoris sp. and Neogeris sp. were also related to manganese and Limnogonus chloride concentrations. Furthermore, the canonical correspondence analysis (CCA) revealed that the reservoir harbors a greater number of facultative species. These species are capable of thriving across a wide range of physicochemical concentrations found in the water quality parameters, including both higher and lower concentrations. It has previously been reported that facultative organisms can thrive in aquatic environments with both good and moderately impacted water quality (Akindele et al., 2019; Voshell, 2002).
Conclusions
This study discovered signs of water quality degradation in Atori Reservoir as a result of anthropogenic disturbances and a lack of adequate management practices within the water body's catchment basin. The dominance of pollution-tolerant species, the rarity of sensitive species and the values obtained for the water quality variables reflected that the reservoir was constantly subjected to certain stress factors. The baseline information on the ecological condition of Atori Reservoir provided in this study should serve as a basis for monitoring structures for policymakers as well as preventive measures to prevent further deterioration of the water quality in the reservoir.
Availability of data and materials
Data are available on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BOD:
-
Biological oxygen demand
- CCA:
-
Canonical correspondence analysis
- DO:
-
Dissolved oxygen
- EC :
-
Electrical conductivity
- TDS:
-
Total dissolved solids
References
Adedeji, A. A., Adesakin, T. A., & Bolawa, O. P. (2020). Assessment of the ecological status of OPA reservoir, ILE IFE, Nigeria based on a comparative study of its planktonic community and water quality parameters. Open Academic Journal of Advanced Science and Technology, 4(1), 1–14. https://doi.org/10.33094/5.2017.2020.41.1.14
Adewuyi, G., Adeyemo, A. K., & Adejumo, S. A. (2018). Use of GIS in production of soil series map in Oyo State, southwestern Nigeria. International Research Journal of Earth Sciences, 6(12), 12–21.
Aduwo, A. I., Adedeji, A. A., & Adeniyi, I. F. (2019). The benthic macro-invertebrate fauna of Owalla Reservoir, Osun State, southwestern Nigeria. Egyptian Journal of Aquatic Biology and Fisheries, 23(5), 341–356.
Aiwerioghene, A. O., & Ayoade, A. A. (2016). Evaluation of some physicochemical parameters and benthic macroinvertebrates of Ikere Gorge Reservoir in Oyo State, Nigeria. Journal of Applied Science and Environmental Management, 20(4), 1097–1103.
Akamagwuna, F. C., Mensah, P. K., Nnadozie, C. F., & Odume, O. N. (2019). Traits-based responses of Ephemeroptera, Plecoptera and Trichoptera to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. River Research and Applications, 35(7), 999–1012. https://doi.org/10.1002/rra.3458
Akindele, E. O., & Adeniyi, I. F. (2013). A study of the physico-chemical water quality, hydrology and zooplankton fauna of Opa Reservoir catchment area, Ile-Ife, Nigeria. African Journal of Environmental Science and Technology, 7(5), 192–203.
Akindele, E. O., Adeniyi, I. F., & Indabawa, I. I. (2013). Spatio-temporal assessment andwater quality characteristics of Lake Tiga, Kano, Nigeria. Research Journal of Environmental and Earth Sciences, 5(2), 67–77.
Akindele, E. O., Adetayo, I. O., Akinpelu, O. T., Amoo, T. & Adedapo, A. M. (2019). Biodiversity of benthic macroinvertebrates in the protected and unprotected areas of Osun River, Osogbo, Nigeria. In Proceedings of NTBA FUD 6th National Conference, 249–256
Aliu, O. O., Akindele, E. O., & Adeniyi, I. F. (2020). Biological assessment of the Headwater Rivers of Opa Reservoir, Ile-Ife, Nigeria, using ecological methods. Journal of Basic and Applied Zoology, 81(11), 981.
Al-Kandari, M., Oliver, P. G., Chen, W., Skryabin, V., Raghu, M., & Yousif, A. (2020). Diversity and distribution of the intertidal Mollusca of the State of Kuwait, Arabian Gulf. Regional Studies in Marine Science, 2020(33), 100905.
Amusan, B. O., & Balogun, I. O. (2019). Assessment of the aquatic insect assemblage and water quality of a tropical stream in Southwestern Nigeria. Journal of Entomology and Zoology Studies, 7(1), 1199–1205.
Amusan, B. O., Idowu, M. A., & Ogbogu, S. S. (2018). Comparative Study of the macroinvertebrate community composition and water quality of Ona and Opa Rivers, southwestern Nigeria. West African Journal of Applied Ecology, 26(1), 33–48.
Anyanwu, E. D., Okorie, M. C., & Odo, S. N. (2019). Macroinvertebrates as bioindicators of water quality of effluent-receiving Ossah River, Umuahia, Southeast Nigeria. Zanco Journal of Pure and Applied Sciences, 31(5), 9–17.
APHA, (2005). Standard Methods for the Examination of Water and Wastewater. American Public Health Association, American Water Works Association, Water Environment Federation, Denver, USA. Dubuque, Iowa. 1287.
Atobatele, O. E., & Ugwumba, O. A. (2008). Seasonal variation in the physico-chemistry of a small tropical reservoir (Aiba Reservoir, Iwo, Osun, Nigeria). African Journal of Biotechnology, 7(12), 62–171.
Ayoade, A. A., & Aiwerioghene, A. O. (2017). Evaluation of some physicochemical parameters and benthic macroinvertebrates of Ikere Gorge Reservoir in Oyo State, Nigeria. Journal of Applied Science and Environmental Management, 20(4), 1097.
Bhawsar, A., & Vyas, V. (2022). Correlation between macroinvertebrates and physicochemical parameters in the Barna Basin of the Narmada River. Research Square. https://doi.org/10.21203/rs.3.rs-1500556/v1
Brown, D. S. (1980). Taxonomic keys to freshwater snails of Africa. Danish Laboratory Manual Series, 7, 25–98.
Efitre, M. J., Chapman, L. J., & Makanga, B. (2001). The inshore benthic macro invertebrates of Lake Nabugabo, Uganda: Seasonal and spatial patterns. African Journal of Applied Zoology, 36(2), 205–216.
Garba, F., Ogidiaka, E., Akamagwuna, F., & Nwaka, A. (2022). Deteriorating water quality state on the structural assemblage of aquatic insects in a North-Western Nigerian River. Water Science, 36(1), 22–31. https://doi.org/10.1080/23570008.2022.2034396
Ghali, H. E., Osimen, E., Ogidiaka, E., Akamagwuna, F. C., Keke, U. N., & Edegbene, A. O. (2020). Preliminary assessment of the deteriorating state of a dam in north-western Nigeria using phytoplankton structural assemblage and environmental factors. Water Science, 34(1), 181–189. https://doi.org/10.1080/11104929.2020.1816152
Godswill, A. C. (2012). Crustacean ecology in opi lake (p. 126). University of Nigeria.
Gouissi, F. M., Koundjode, S. A., David, D. A., Zoulkanerou, O. P., & Okoya, J. G. (2019). Relationship between macroinvertebrates and physicochemical parameters to access water quality of the Affon River in Benin. Advances in Entomology, 7, 92–104.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9.
Jaji, M. O., Bamgbose, O., Odukoya, O. O., & Arowolo, T. A. (2007). Water quality assessment of Ogun River, Southwest Nigeria. Environmental Monitoring and Assessment, 133(1–3), 473–482.
Kahlon, S. K., Julka, J. M., Jaswal, A., & Devi, S. (2018). Impact of temperature and dissolved oxygen on the macroinvertebrate density of a hill stream in Western Himallaya. IJBPAS, 7(1), 18–22.
Kumar, T., Tamire, G., & Beneberu, G. (2022). Macroinvertebrate-based index of biotic integrity for assessing the ecological condition of Lake Wanchi, Ethiopia. F100 Research, 11, 544.
Kumar, V., Arya, S., Dhaka, A., Minakshi & Chanchal. (2011a). A study on physicochemical characteristics of Yamuna River around Hamirpur (UP), Bundelkhand region central India. International Multidisciplinary Research Journal 1(5), 14–16.
Madsen, H. (1985). Keys on tropical freshwater snails; Pulmonate Snails. Danish Bilharzias Laboratory Manual, 12, 89.
Makumbe, P., Kanda, A., & Chinjani, T. (2022). The relationship between benthic macroinvertebrate assemblage and water quality parameters in the sanyati Basin, Lake Kariba, Zimbabwe. The Scientific World Journal. https://doi.org/10.1155/2022/5800286
Mitchell, A. J., Hobbs, M. S., & Brandt, T. M. (2007). The effect of chemical treatments on red-rim melania Melanoides tuberculata, an exotic aquatic snail that serves as a vector of trematodes to fish and other species in the USA. North American Journal of Fisheries Management, 27(4), 1287–93.
Mugo, M. J. (2010). Seasonal changes in physicochemical status and algal biomass of Lake Naivasha. M. Sc Thesis, Kenyatta University, pp. 236
Müller, O.F. (1774). Vermium terrestrium et fluviatilium, seu animalium infusoriorum, helminthicorum, et testaceorum, non marinorum, succincta historia. In Volumen alterum. Heineck & Faber, Havniae et Lipsiae. pp. 214–215.
Muralidharan, M., Selvakumar, C., Sundar, S. & Raja, M. (2010). Macroinvertebrates as potential indicators of environmental quality. IJBT 0976–4313.
Mwabvu, T., & Sasa, A. (2009). The influence of environmental parameters on the abundance, distribution and species composition of macroinvertebrates in Fletcher Reservoir, Zimbabwe. African Journal of Ecology, 47, 502–508.
NESREA. (2007). National environmental standards and regulations enforcement agency. Act No. 25
Nkwoji, J. A., Onyema, I. C., & Igbo, J. K. (2010). Wet season spatial occurrence of phytoplankton and zooplankton in Lagos Lagoon, Nigeria. Scientific World Journal, 5(5), 7–14.
Ogidiaka, E. (2012). Physicochemical parameters and benthic macroinvertebrates of Ogunpa River at Bodija, Ibadan, Oyo State. European Journal of Science Research, 85(1), 89–97.
Oladimeji, A. S., Omokunle, A. B., & Funmilayo, A. A. O. (2024). Ecological status of a tropical inland water using macroinvertebrate feeding groups and sediment characteristics. Asian Journal of Research in Zoology, 7(1), 19–31.
Popoola, K. O. K., Sowunmi, A. A., & Amusat, A. I. (2019). Comparative Study of Physicochemical parameters with national and international standard and insect community of Erelu Reservoir in Oyo town, Oyo State, Nigeria. International Journal of Water Resources and Environmental Engineering, 11(3), 56–65.
Rabiu, H. D., Umar, L. M., Sulaiman, I., Madina, M., & Abubakar, A. I. (2018). Assessment of the water quality of watari dam, kano state using selected physicochemical parameters. International Journal of Advanced Academic Research Sciences, Technology & Engineering, 4(5), 45–59.
Ridanovic, L., Ridanovic, S., Jurica, D., & Spasojevic, P. (2010). Evaluation of water temperature and dissolved oxygen regimes in river Neretva. Republic of Macedonia.
Saleh, H. M., Mahmoud, H. H., Abdou, M. I., & Eskander, S. B. (2021). Health risk assessment based on metal analysis of soil and crops in Al-Dakhla Oasis. Arabian Journal of Geosciences, 14, 260. https://doi.org/10.1007/s12517-021-06597-3
Schneider, W. (1990). FAO species identification sheets for fishery purposes. Field guide to the commercial marine resources of the Gulf of Guinea Prepared and published with the support of the FAO Regional Office for Africa. Rome, FAO, pp. 268
Schofield, K. A., Alexander, L. C., & Ridley, C. E. (2018). Biota connect aquatic habitats throughout freshwater ecosystem mosaics. JAWRA Journal of the American Water Resources Association, 54(2), 372–399.
Sharma, S., Vishwakarma, R., Dixit, S., & Jain, P. (2011). Evaluation of water quality of armada River with reference to physcochemical parameters at Hoshangabad city, MP, India. Research Journal of Chemical Sciences, 1(3), 40–48.
Strayer, D. L. (2009). Benthic invertebrate fauna, lake and reservoirs. 191–204. In G. E. Likens (Ed.), Encyclopedia of inland waters (2) (p. 545). Elsevier.
Tanimu, Y., Bako, S. P., & Adakole, J. A. (2011). Effects of domestic waste water on water quality of three reservoirs supplying drinking water. Waste Water-Evaluation and Management, 32, 119–205.
Umara, D. A., Ramlib, M. F., Arisc, A. Z., Jamilb, N. R., & Tukur, A. I. (2019). Surface water resources management along Hadejia River basin, northwestern Nigeria. H2Open Journal, 2(1), 184–199. https://doi.org/10.2166/H2OJ.2019.010
United States Environmental Protection Agency (USEPA). (2005). Water quality standards Academy: Basic course. Office of water. Washington Distinct of Columbia, pp. 152
Venkatesharaju, K., Ravikumar, P., Somashekar, R. K., & Prakash, K. L. (2010). Physicochemical and Bacteriological Investigation on the river Cauvery of Kollegal Stretch in Karnataka. Journal of Science Engineering and Technology, 6(1), 50–59.
Verma, P. S. (2006). A manual of practical Zoology invertebrates (p. 497). S. Chand & Company Ltd.
Voshell, J. R. (2002). A guide to freshwater invertebrates of North America (p. 442). The McDonald and Woodland Publiahing Comp NY.
Wang, X., Pengkang, J., Zhao, H., & Meng, L. (2007). Classification of contaminants and treatability evaluation of domestic wastewater. Frontiers of Environmental Science & Engineering in China, 1(1), 57–62.
Water Research Commission (WRC). (2003). Freshwater invertebrates of Southern Africa. Hemiptera, Megaloptera, Neuroptera, Trichoptera and Lepidoptera. In: de Moor, I. J, Day, J. A. and de Moor, F. C (eds). WRC Report No. TT214/03, pp. 207
World Health Organization. (2004). Guidelines for drinking-water quality [electronic resource]: incorporating first addendum. Vol. 1, Recommendations. – 3rd ed., 20 Avenue Appia, 1211 Geneva 27, Switzerland.
Yung-Chul, J., Nan-Young, K., Sang-Hun, K., Young-Seuk, P., Dong-Soo, K., & Soon-Jin, H. (2016). Spatial-distribution of benthic macroinvertebrate assemblages in relation to environmental variables in Korean Nationwide stream. Water, 8(27), 1–20. https://doi.org/10.3390/w/8010027
Yusoff, F. M., Matias, H. M., & Khan, N. (2002). Changes of water quality, chlorophyll and zooplankton along the river-lucustrine continuum in a tropical reservoir. Verh Internat Verein Limnol, 28, 295–298.
Yusuf, Z. H. (2019). Benthic macroinvertebrates diversity as bioindicators of water quality of Nasarawa Reservoir, Katsina State, Nigeria. Bayero Journal of Pure and Applied Sciences, 12(1), 449–456.
Zhang, J., Li, S., & Jiang, C. (2020). Effects of land use on water quality in a River Basin (Daning) of the Three Gorges Reservoir Area, China: Watershed versus riparian zone. Ecological Indicators, 113, 106226.
Zwahlen, R. (2022). Reservoirs as habitats. In: Assessing the environmental impacts of hydropower projects. Springer Berling, vol. 45, pp. 369–385
Acknowledgements
The authors would like to acknowledge Mr Abiodun Adedapo of the department of Zoology Obafemi Awolowo University who helped in no small measure in the statistical analysis and interpretation of the results.
Funding
There was no funding for this research.
Author information
Authors and Affiliations
Contributions
SA performed the practical section, collected the samples and analyzed the data. SA, BA and AK conceived the idea, helped in designing the study, supervised the preparation of the experiment and helped in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable for this kind of research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amusan, B.O., Adeleke, S.O. & Koleosho, A.F. Assessment of the ecological status of an ancient reservoir using macroinvertebrate assemblage and water quality parameters. JoBAZ 85, 32 (2024). https://doi.org/10.1186/s41936-024-00384-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-024-00384-8