Abstract
Background
to detect the role of procalcitonin, erythrocyte sedimentation rate to c-reactive protein (ESR/CRP) ratio, neutrophils-to-lymphocyte ratio (NLR), and platelets-to-lymphocyte ratio (PLR) in the diagnosis of infection in systemic lupus erythematosus (SLE) patients with fever, their diagnostic value to differentiate between infection and disease activity, and their correlation with disease activity.
Methods
Forty SLE patients and forty healthy control cases were included in the study. Disease activity was assessed by the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2 K), and quality of life was assessed by Lupus QoL. A bacterial infection was detected by clinical symptoms and positive culture results. Laboratory tests were done for all patients and controls: complete blood count (CBC), ESR, CRP, and procalcitonin (PCT). NLR, PLR, and ESR/CRP ratios were calculated.
Results
There was a statistically significant difference between infected SLE patients and non-infected SLE patients regarding PCT (p < 0.001), ESR (p = 0.002), CRP (p = 0.005), ESR/CRP ratio (0.002), and NLR (p = 0.023). PCT, ESR, CRP, and NLR were positively correlated with the presence of infection in SLE patients, while the ESR/CRP ratio was negatively correlated. There was no significant correlation with the SLEDAI-2 K score. Logistic regression analysis revealed that PCT was the best significant predictor of infection (OR 224.37, 95% CI 8.94–5631.35). PCT was a good predictor of infection, with a cut-off value of 0.90 ng/ml, which gave the best combination of sensitivity (84.62%) and specificity (85.71%).
Conclusion
PCT, ESR/CRP ratio, and NLR provide good diagnostic markers for the diagnosis of infection and can distinguish between infection and disease flare in SLE patients with fever.
Similar content being viewed by others
Background
Systemic Lupus Erythematosus (SLE) is a heterogeneous autoimmune disease [1] that causes immune system dysfunction [2]. SLE arises from antinuclear antibodies binding self-antigens, forming immune complexes that deposit in tissues, mediating inflammation and multi-organ damage [3]. Fever is a prevalent symptom of SLE, with 36–86% of individuals experiencing it, and may be the only indication of SLE in some circumstances, such as in individuals with fever of undetermined origin (FUO). Up to 5% of FUO patients develop SLE later in their lives [4]. Infection is a prevalent and serious complication in SLE, contributing significantly to mortality [5]. Around half of SLE patients will suffer major infections during their illness, with death rates ranging from 20 to 55% [6]. The majority of these infections are bacterial, commonly affecting the respiratory system, urinary tract, and soft tissues [7]. The high susceptibility to infections in SLE patients results from a combination of factors: the use of immunosuppressive treatments (like Corticosteroids, Cyclophosphamide, and newer biologic agents including Belimumab), active disease states, and inherent immune system abnormalities caused by SLE itself [8]. Differentiating between a lupus flare and an infection in SLE patients presenting with fever is often challenging, as both conditions can exhibit similar initial clinical signs, such as those seen in the acute febrile phase of infections like sepsis [9]. This makes accurate early diagnosis crucial, as treatment approaches for infections and active SLE differ markedly [8]. Consequently, much research has focused on identifying biomarkers for the early detection of infections and assessing disease activity in SLE patients [10]. Commonly used biomarkers for disease activity include anti-dsDNA antibodies, complement levels (C3 and C4), ESR, anti-C1q antibodies, and urinary sediment activity, incorporated into scales like the SLE Disease Activity Index (SLEDAI) [11]. For infections, an ideal biomarker should be sensitive, specific, easy and quick to use, cost-effective, and correlate with infection severity and prognosis [12].
Procalcitonin (PCT), a precursor peptide of calcitonin, is linked to invasive bacterial infections. Typically produced by parafollicular C cells, it is released in response to bacterial toxins and IL-1b stimulation. PCT levels rise within the first 24 h of infection, peak between 24 and 48 h, and rapidly decline after bacterial infection resolution. In healthy individuals [13], PCT is virtually undetectable [8]. This study investigates the role of PCT, the erythrocyte sedimentation rate/C-reactive protein ratio (ESR/CRP ratio), the neutrophils-to-lymphocyte ratio (NLR), and the platelets-to-lymphocyte ratio (PLR) in diagnosing infections in febrile SLE patients and their utility in differentiating between infection and disease activity.
Patients and method
Study population
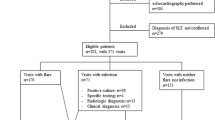
This was a cross-sectional, analytical case control study; it included forty SLE patients (39 females and only 1 male) who fulfilled the European League against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus [14]. All forty patients were recruited from the outpatient rheumatology clinic at Minia University Hospital in the period from June 2020 to March 2021. Forty apparently healthy, sex-matched individuals were served as a control group.
All SLE patients had fever within 24–48 h of presentation, while patients with juvenile lupus, patients with fungal, recent severe trauma, severe burn, major surgery, known malignancies, other autoimmune diseases, inflammatory bowel diseases, liver diseases, HCV positive, patients with angina, myocardial infarction or stroke, chronic kidney disease, and known chronic infection (e.g., osteomyelitis, endocarditis, HIV) were all excluded from the study. As we were within the era of Coved − 19 infection during the study, it was obligatory to exclude viral infection by PCR and CT chest especially for patients represented with respiratory manifestations or GIT manifestation associated with fever. Also, any patients with negative Covid-19 or had fever but associated with negative CRP and lymphocytosis (relative or absolute) with or without neutropenia were diagnosed as viral infection and also excluded from our study.
Ethical considerations
The nature of the study was explained to all patients. Their approval to share in the study was obtained. The study protocol was approved by Faculty of Medicine, Research Ethical Committee (FMREC), Minia University, Egypt (Approval No. 387:1/2020) and in accordance with the World Medical Association Declaration of Helsinki, and informed consent was obtained from all patients.
Methods
All patients underwent medical history, examination, and lab tests. Disease activity of SLE patients was evaluated using the SLEDAI-2k scale [15], which considers symptoms from the past 10 days, including 16 clinical and 8 laboratory measures, with total scores ranging from 0 to 105. Scores above 4 signified moderate-severe activity, while scores of 4 or less indicated mild activity.
The Lupus Quality of Life (Lupus QoL) questionnaire [16] was utilized in this study to evaluate quality of life across various domains in the enrolled patients. The Lupus QoL is a 34-item tool that measures eight aspects of quality of life: physical functioning, pain levels, emotional health, fatigue, body image perception, sexuality and intimacy, ability to plan activities, and the perceived burden on others. Patients respond to each item on a graded scale, with the total score ranging from 0 to 100. Higher total scores denote poorer quality of life.
Infection diagnosis was based on: (1) clinical symptoms like fever ≥ 38 °C, sore throat, cough, abdominal pain etc., and (2) positive bacterial cultures from blood, sputum, urine and other samples collected before starting anti-infection treatment. This approach allowed quantification of SLE disease activity, quality of life impact, and confirmation of infectious pathogens in the study cohort.
Laboratory investigations
About 10 ml of venous blood was collected from each patient by sterile venipuncture under complete aseptic conditions. This sample was divided into 2 ml for blood culture, 2 ml in a tube containing trisodium citrate for measurement of ESR, 2 ml in an EDTA-containing tube for CBC and WBC differentiation count, and 4 ml in one plain tube. Blood was left to clot in the incubator, then centrifuged. The expressed serum was used for measurement of serum levels of ANA and anti-ds-DNA, CRP, C3 and C4, procalcitonin, urea, and creatinine. Midstream urine collection and 24-hour collection in a sterile urine container for simple urine analysis and 24-hour protein urine.
Before receiving anti-infection treatment, blood culture, sputum culture, urine culture, or discharge from local suppurations according to presentation were collected. For the blood culture process, 2 ml of blood was aseptically inoculated into specialized media. These culture bottles were then incubated at a temperature of 37 °C for a duration of 5 to 7 days. In cases where blood cultures yielded positive results, further subculturing was conducted on blood agar plates. The microorganisms isolated from these cultures were then identified through standard bacteriological techniques Sputum and urine were collected in sterile containers and cultured in specific media. CBC was measured by an automated cell counter (CelltacES, Nihon Kohden, Germany). Erythrocyte sedimentation rate was measured by the Westergren method (Kongros et al., 2019), while CRP was measured by a quantitative turbidimetric method (CRP-turbilatex, SPINREACT, Spain), and the ESR/CRP ratio was calculated. Simple urine analysis was tested chemically by using a dry chemistry strip for the presence of albumin and microscopically for casts, RBCs, and pus in urine. 24 h urine collection protein was detected by automated chemistry analysis (ELITech Group Clinical Systems, France) for the presence of proteinuria. Serum anti-nuclear antibody (ANA) and serum anti-double-stranded DNA (anti-dsDNA) levels were detected by EIA (QUANTA Lite, INOVA DIAGNOSTICS, USA). Serum urea, creatinine, complement 3, and complement 4 (C3, C4) levels were assessed by an automated chemistry analyzer (Mindray BS-800 M, China). In suspected cases for detection of viral infection, we measured HCVAb, HBsAg and HIVAb by chemiluminescence (Cobas e411 analyzer, HITACHI, Germany). Finally, serum procalcitonin (PCT) was measured by EIA (Shanghai Korain Biotech Co., Ltd., China). The normal serum PCT range was 0.5–1 ng/ml; more than 1 ng/ml was considered the cutoff value for the presence of infection.
Statistical analysis
The Statistical Program for Social Science (SPSS) software version 26 (IBM Corp., 2019) was utilized for statistical analysis. Quantitative data was described through mean, standard deviation (SD) and range, while qualitative data was presented as number (no.) and percentage (%). Comparative analyses between the two study groups were done using chi-square tests (for qualitative variables), Fisher exact and Mann-Whitney tests (for quantitative parameters), and Spearman rho correlations. P values less than 0.05 were considered statistically significant. Logistic regression assessed bivariate predictors. Receiver operating characteristic (ROC) curves were constructed to determine sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) alongside optimal cut-off points for the evaluated biomarkers.
Results
Demographic data of SLE patients, clinical findings, disease activity, quality of life, and laboratory investigations are presented in Table 1. The mean age of SLE patients was 31.8 ± 8.5 years, and the range was 17–50 years. A strong predominance of female patients was found: 39 females (97.5%) and 1 male (2.5%).
Regarding clinical manifestation of SLE patients, 28 patients have muco-cuteaneous manifestations (70%), 27 patients having musculoskeletal manifestations (67.5%), 10 patients have cardiac manifestations (25%), 12 patients have pulmonary manifestations (30%) ,14 patients have renal manifestations (35%), 8(20%) have GIT manifestations, 15 patients have CNS manifestations (37%), serositis was found in 18 patients (45%), 6 patients having pleurisy (15%), 8 patients having pericarditis (20%) and 4 patients having peritonitis (10%).
As regard medication, we found 34 (85%) of SLE patients received hydroxychloroquine and corticosteroids, 6 (15%) received non-steroidal anti-inflamatory drugs (NSAIDS), in addition to disease modifying antirheumatic drugs (DMARDs), (23 (57.5%) of SLE patients received azathioprine, 7 (17.5%) received cyclophosphamide and 4 (10%) received mycophenolate mofetil (MMF)),
The mean total SLEDAI-2k was 17.5 ± 10.2, ranging from (1–37). SLE patients were graded as having moderate to severe activity if SLEDAI > 4. If the score of SLEDAI 2 K was ≤ 4, it was considered mild activity. HQoL was 98.7 ± 5.3, ranging from 87 to 107).
ANA was positive in all of the SLE patients, and anti-ds DNA was positive in 16 patients (40%). The mean value of C3 was 117.5 ± 26.3; the range was 50–187. C3 was consumed in 10 (25%) of patients, and the mean value of C4 was 10.3 ± 16.4, and the range was (7–42). C4 was consumed in 8 (20%) of SLE patients.
Pyuria was present in 17 (42.5%) of patients, hematuria in 22 (55%), there were urinary casts in 4 (10%) of patients, and 24 h urinary protein was higher than the normal range in 14 (35%) of them. In SLE patients, the range of urea was (15–85) mg/dl with a mean value of 30.6 ± 11.5, and the range of creatinine was (0.5–1.6) mg/dl with a mean value of 0.9 ± 0.2 (Table 1).
All 40 SLE patients were feverish (≥ 38 °C); 26 (65%) SLE patients had symptoms suggestive of infection, while 14 (35%) SLE patients had no symptoms suggestive of infection. Symptoms suggestive of infection were: upper respiratory tract infection (URTI) in the form of sore throat (acute pharyngitis) in 14 (53.8%) and lower respiratory tract infection (LRTI) in the form of cough and expectorations in 19 (73.1%) patients; renal manifestations (dysuria, loin pain, frequent urination, and urgent urination) in 14 (53.8%) patients; GIT symptoms (in the form of diarrhea, abdominal pain, and vomiting) in 17 (65.4%) patients; and local suppuration in 7 (26.9%) patients. Most of those patients had more than one symptom suggesting an infection. There was a statistically significant difference between SLE patients and control cases regarding the level of Hb, neutrophils, lymphocytes, NLR, platelets, PLR, ESR 1st hour, CRP, ESR/CRP ratio, and procalcitonin (p < 0.0001) for all of them. Also, there were significant differences in the mean value of neutrophil cells (p = 0.008), while there was no significant difference between SLE patients and controls in the mean value of WBCs and platelets between the two groups (p > 0.05). None of the control cases were positive for neither CRP nor procalcitonin.
SLE patients are divided according to the presence of clinical symptoms suggestive of infection and culture into two groups: SLE patients with infection (n = 26) and SLE patients without infection (n = 14). As regard demographic characteristics, there was no statistically significant difference between the two groups regarding their ages, sexes, or disease durations (p ≥ 0.05). There was no statistically significant difference between patients in both subgroups regarding the clinical manifestations, There was no significant difference between both groups as regard Type of medication administered. the mean total SLEDAI-2 K, and the score of HQoL. There were statistically significant differences between patients in both groups regarding the mean value of neutrophils (p = 0.008), neutrophils to lymphocyte ratio (NLR) (p = 0.023), ESR 1st hour (p = 0.002), CRP value (p = 0.005), and number of positive CRP (p = 0.002), the mean value of the ESR/CRP ratio (p = 0.029), the number of patients that were positive for procalcitonin (PCT) (p = 0.002), and its mean value (p < 0.001) (Fig. 1). The culture was positive for blood growth of bacteria in 9 (22.5%) and for urine culture in 17 (47.5%) of infected SLE patients, and all non-infected cases had a negative culture (p = 0.012) (Table 2).
Regarding the disease activity score in SLE patients with infection (n = 26), 20 infected SLE patients had moderate to severe disease activity (SLEDAI-2k score > 4), and 6 infected SLE patients had mild disease activity (SLEDAI-2k score ≤ 4) (6 patients). There were no significant differences between infected SLE patients with moderate to severe activity (20 patients) and infected SLE patients with mild activity (6 patients) regarding CBC and inflammatory markers (p > 0.05). Our results show a significant positive correlation between procalcitonin and ESR (p = 0.033), a negative correlation with the ESR/CRP ratio (p = 0.029), and a significant positive correlation between procalcitonin and symptoms and signs suggestive of infection, such as fever, respiratory rate, pulmonary manifestations (upper respiratory tract infection and lower respiratory tract infection), and urinary tract infection (dysuria, urgent urination, frequent urination) (p < 0.05). The presence of manifestations of infection was significantly positively correlated with neutrophils (p = 0.007), neutrophils to lymphocyte ratio (p = 0.021), 1st h of ESR (p = 0.001), CRP (p = 0.002), and procalcitonin (p < 0.001), and negatively correlated with the ESR/CRP ratio (p = 0.001), but not significantly correlated with other inflammatory markers such as WBCs, lymphocytes, platelets, and platelets to lymphocytes ratio (p > 0.05) (Table 3).There was no significant correlation between disease activity score (SLEDAI-2 K) and inflammatory markers in SLE patients (p > 0.05).
Logistic regression analysis revealed that the best significant predictor of infection was procalcitonin with the highest odds ratio (OR 224.37, 95% CI 8.94–5631.35, p = 0.001), the ESR–CRP ratio was also positively associated with infection (OR 2.996, 95% CI 1.05–8.57, p = 0.041), and the neutrophil–lymphocyte ratio was also a significant predictor when handled as a continuous measure (OR 2.034, 95% CI 1.08–3.80, p = 0.027) (Table 4).
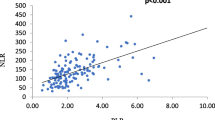
The sensitivity and specificity of inflammatory markers for the diagnosis of infection were evaluated using ROC curve analysis. Among the parameters, procalcitonin showed the highest sensitivity and specificity as a diagnostic tool for diagnosing infection in SLE patients; the area under the curve (AUC) was 0.861 (95% CI 0.715–0.950). A cut-off value of 0.9 ng/ml for PCT gave the best combination of sensitivity (84.62%) and specificity (85.71%), with a positive predictive value (PPV) of 91.7% and a negative predictive value (NPV) of 75%. For neutrophils, ROC curve analysis showed that the area under the curve (AUC) was 0.755 (95% CI 0.594–0.877). A cut-off value of > 2976 cells/mm for neutrophils gave the best combination of sensitivity (88.46%) and specificity (57.14%), with a positive predictive value (PPV) of 79.3% and a negative predictive value (NPV) of 72.7%. For neutrophil-to-lymphocyte ratio (NLR), ROC curve analysis showed that the area under the curve (AUC) was 0.720 (95% CI 0.556–0.850). A cut-off value of > 2 for NLR gave the best combination of sensitivity (69.23%) and specificity (57.14%), with a positive predictive value (PPV) of 75% and a negative predictive value (NPV) of 50%. ROC curve analysis of ESR showed that the area under the curve (AUC) was 0.802 (95% CI 0.646–0.911). A cut-off value of > 20 mm/h for ESR gave the best combination of sensitivity (84.62%) and specificity (57.14%), with a positive predictive value (PPV) of 78.6% and a negative predictive value (NPV) of 66.7%. ROC curve analysis of CRP showed that the area under the curve (AUC) was 0.761 (95% CI 0.6–0.881). A cut-off value of ≥ 6 mg/dl for CRP gave the best combination of sensitivity (65.38%) and specificity (85.71%), with a positive predictive value (PPV) of 89.5% and a negative predictive value (NPV) of 57.1%. ROC curve analysis of the ESR/CRP ratio showed that the area under the curve (AUC) was 0.772 (95% CI 0.612–0.889). A cut-off value of < 2 for the ESR/CRP ratio gave the best combination of sensitivity (65.38%) and specificity (85.71%), with a positive predictive value (PPV) of 89.5% and a negative predictive value (NPV) of 57.1%. The ROC curve analysis of variables predicting infection in SLE patients is presented in (Table 5; Fig. 2).
Discussion
Infections are a leading complication in SLE patients, responsible for substantial mortality and morbidity [17]. Distinguishing serious infections from lupus flares poses an urgent clinical challenge [18, 19]. Accurately diagnosing the condition is therefore critical to guide appropriate management. This study aimed to evaluate procalcitonin (PCT), ESR/CRP ratio, NLR and PLR in diagnosing infections among febrile SLE patients. It assessed their ability to differentiate between infection and inflammatory disease activity. The diagnostic value and correlations with SLEDAI disease activity scores were also examined. Prior evidence shows PCT and CRP levels significantly differ between infected and non-infected SLE groups [13, 20,21,22,23,24,25,26,27,28].
In contrast to our findings, some studies like Liu et al. [29] and El-Serougy et al. [30] did not find significant differences in PCT levels between infected and non-infected SLE patients. The discrepancies may stem from heterogeneity of infectious organisms or timing of PCT measurement in their cohorts. PCT elevation can be delayed in some severe sepsis cases [31] or decline prior to patient demise after initial peak [32]. Our study did reveal a significant difference in PCT between infected and non-infected SLE groups. We also noted significant variation in ESR/CRP ratio between the groups, aligning with past research by Littlejohn et al. [9] that proposed diagnostic thresholds for this ratio. While prior evidence is mixed, our data supports PCT and the ESR/CRP ratio as markers that discriminate between infected and non-infected SLE.
Our study revealed a significant difference in NLR between infected and non-infected SLE groups (p = 0.023). However, no significant variation was observed in PLR. Prior research by Kim et al. [19] noted higher PLR in infected SLE patients, contrasting our findings. These discrepancies could stem from differences in cohort sizes, study design, and patient disease severity between the investigations. Additionally, we did not find significant differences in PCT levels between SLE patients with moderate-severe versus mild disease activity. Nor was PCT correlated with SLEDAI-2 K scores assessing disease activity.
Our findings align with multiple studies demonstrating no correlation between procalcitonin (PCT) and systemic lupus erythematosus (SLE) disease activity [8, 23, 25, 26, 33,34,35,36]. Conversely, Wang et al. [10] observed significantly higher PCT in active infected versus inactive infected SLE patients, with PCT correlating to SLEDAI. Differing disease stages, continuous monitoring, sample sizes, and inclusion factors may explain variances. Retrospective analyses prohibit dynamically tracking biomarkers over illness courses, limiting diagnostic valuations without mechanistic characterizations of PCT elevation. Furthermore, some infections in Wang et al. lacked pathogen examinations, relying upon symptoms and imaging, restricting precision of diagnostic metrics.
We found no significant NLR/PLR differences between mild and moderate-severe SLE nor correlations to activity. However, Qin et al. [37] and others [19, 38] reported associations between these ratios and SLEDAI in non-infected cohorts.
In our research we discovered that PCT is the predictor of infection, with a high odds ratio (OR) of 224.37 (95% Confidence Interval [CI]; 8.94–5631.35). Furthermore we observed an inverse relationship between ESR/CRP ratio and infection occurrence (OR 2.996 95% CI; 1.05–8.57, p = 0.001). When considering it as a variable the NLR also emerged as a predictor (OR 2.034 95% CI; 1.08–3.80).
In comparison to AlJarhi et al.s study [27] logistic regression analysis demonstrated that serum PCT is a marker strongly associated with infection occurrence (p < 0.001, OR 1.059 95% CI; 1.031–1.087). Similarly Li et al.s research [18] revealed that PCT, CRP blood cells (WBC) and NLR are risk factors, for SLE combined with bacterial infection each having significant odds ratios; however CRP alone did not prove to be a reliable predictor (p > 0.05).
Furthermore our study conducted an analysis, on the Receiver Operating Characteristic (ROC) curves of factors including PCT, ESR, CRP, ESR/CRP ratio, neutrophils and NLR. The results showed that PCT had the sensitivity and specificity combination with 84.6% sensitivity and 85.7% specificity. In our study we found that a PCT value of 0.90 ng/ml was the cut off point with these sensitivity and specificity values. These findings are similar to the study by Echeverri et al. [39] who used a cut off value of 0.96 ng/ml but reported sensitivity and specificity compared to our results. Ho et al. [40] used a cut off value of 0.74 ng/ml which had sensitivity but higher, than our specificity values.
Additionally we identified a CRP cut off value of 16.1 mg/dl with a sensitivity of 68.4% and specificity of 90%. Li et al. [18] used a PCT cut off value at 0.705 ng/ml. Achieved a sensitivity of 78.3% and specificity of 98%.They also evaluated the blood cells (WBCs) and the neutrophil, to lymphocyte ratio (NLR) achieving sensitivities of 43.5% and 69.6% and specificities of 91.5% and 56.8% respectively.
Bador et al. [23] reported comparable AUC values for PCT (0.797) and CRP (0.755) in distinguishing infected versus flared SLE patients, with a PCT cutoff < 0.17 ng/ml excluding infection at 94% NPV. Lower thresholds likely stemmed from predominantly localized infections in their cohort. AlJarhi et al. [27] identified cutoffs of 0.145 ng/ml for PCT and 2.07 mg/L for CRP optimized for diagnosis.
Kim et al. [22] also established significantly higher CRP in infected SLE groups, declining after treatment. ROC analysis yielded CRP superior to PCT, with 1.35 mg/dl maximizing sensitivity and specificity. Our elevated thresholds may reflect higher background infection rates in developing nations. An NLR cutoff > 2 demonstrated moderate sensitivity (69.23%) and specificity (57.14%), lagging behind the > 5.70 NLR threshold proposed by Kim et al. (75%, 90%) [19].
Conclusion
This study demonstrates procalcitonin (PCT), ESR/CRP ratio, and neutrophil-to-lymphocyte ratio (NLR) as promising diagnostic markers for differentiating infections from inflammatory disease flares in febrile SLE patients. Our analysis aligns with a growing body of research indicating combinations of biomarkers can effectively discriminate between these challenging syndromic presentations to guide appropriate, prompt treatment decisions. While some variability exists across studies regarding optimal thresholds and utility of individual markers, broad consensus supports simultaneous examination of PCT alongside other factors like CRP ratios and NLR. Applying a multi-marker panel, tailored to the clinical scenario, maximizes sensitivity and specificity for diagnosing serious bacterial infections complicating SLE cases where both states exhibit similar initial features. Standardization of testing approaches and cutoffs may further refine performance.
Data availability
The data generated during the current study are available from the corresponding author upon reasonable request.
References
Hermansen M-LF, Hummelshø L, Lundsgaard D, L Hornum L, Keller P. Increased serum β2- microglobulin is associated with clinicaland immunological markers of disease activity in systemic lupus erythematosus patients. Volume 21. Lupus; 2012. pp. 1098–104.
Williams JN, Chang SC, Sinnette C, Malspeis S, Parks CG, Karlson EW, et al. Pesticide exposure and risk of systemic lupus erythematosus in an urban population of predominantly African-American women. Lupus. 2018;27(13):2129–34.
Tony EA, Mohammed HS, Fathi N, Tony AA, Afifi OA, Abdou MA et al. (2016). Serum and urinary biomarkers Endothelin-1, Beta-2 Microglobulin, Cystatin C, Galectin-3 and Alpha-1-acid Glycoprotein; can they surrogate clinical and histological staging in Lupus Nephritis patients? J Arthritis; (5):1–9.
Lunchenkov N, Filippov E, Prihodko O, Volchkova E. Classical fever of unknown origin: Retrospective Study in Infectious Clinical Hospital № 2. Open Forum Infect Dis. 2017;4(1):S343–343.
Rúa-Figueroa Í, López-Longo J, Galindo-Izquierdo M, Calvo-Alén J, Del Campo V, Olivé-Marqués A, et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2017;47(1):38–45.
Wang Z, Wang Y, Zhu R, Tian X, Xu D, Wang Q, et al. Long-term survival and death causes of systemic lupus erythematosus in China: a systemic review of observational studies. Medicine. 2015;94(17):e794.
Barber C, Gold WL, Fortin PR. Infections in the lupus patient: perspectives on prevention. Curr Opin Rheumatol. 2011;23(4):358–65.
Ospina FE, Echeverri A, Zambrano D, Suso JP, Martinez-Blanco J, Canas CA, et al. Distinguishing infections vs flares in patients with systemic lupus erythematosus. Rheumatology. 2017;56(1):i46–54.
Littlejohn E, Marder W, Lewis E, Francis S, Jackish J, McCune WJ, et al. The ratio of erythrocyte sedimentation rate to C-reactive protein is useful in distinguishing infection from flare in systemic lupus erythematosus patients presenting with fever. Lupus. 2018;27(7):1123–9.
Wang J, Niu R, Jiang L, Wang Y, Shao X, Wu M, et al. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine. 2019;98(33):e16798.
Kang, Park SH. Infectious complications in SLE after immunosuppressive therapies. Curr Opin Rheumatol. 2003;15(5):528–34.
Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351(1–2):17–29.
Yu J, Xu B, Huang Y, Zhao J, Wang S, Wang H, et al. Serum procalcitonin and C-reactive protein for differentiating bacterial infection from disease activity in patients with systemic lupus erythematosus. Mod Rheumatol. 2014;24(3):457–63.
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–12.
Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
McElhone K, Abbott J, Shelmerdine J, Bruce IN, Ahmad Y, Gordon C, et al. Development and validation of a disease-specific health‐related quality of life measure, the Lupus Qol, for adults with systemic lupus erythematosus. Arthritis Care Res. 2007;57(6):972–9.
Teh CL, Wan SA, Ling GR. Severe infections in systemic lupus erythematosus: disease pattern and predictors of infection-related mortality. Volume 37. Clinical rheumatology; 2018. pp. 2081–6.
Li Z, Xiao Y, Zhang L. Application of procalcitonin, white blood cell count and neutrophil-to-lymphocyte ratio in the diagnosis of systemic lupus erythematosus with a bacterial infection. Ann Palliat Med. 2020;9(6):3870–6.
Kim HA, Jung JY, Suh CH. Usefulness of neutrophil-to-lymphocyte ratio as a biomarker for diagnosing infections in patients with systemic lupus erythematosus. Clin Rheumatol. 2017;36(11):2479–85.
Shin KC, Lee YJ, Kang SW, Baek HJ, Lee EB, Kim HA, et al. Serum procalcitonin measurement for detection of intercurrent infection in febrile patients with SLE. Ann Rheum Dis. 2001;60(10):988–9.
Ho WL, Lan JL, Chen DY, Chen YH, Huang WN, Hsieh TY, et al. Procalcitonin may be a potential biomarker for distinguishing bacterial infection from disease activity in febrile patients with systemic lupus erythematosus. Formos J Rheumatol. 2009;23(2):52–8.
Kim HA, Jeon JY, An JM, Koh BR, Suh CH. C-reactive protein is a more sensitive and specific marker for diagnosing bacterial infections in systemic lupus erythematosus compared to S100A8/A9 and procalcitonin. J Rhuematol. 2012;39(4):728–34.
Bador KM, Intan S, Hussin S, Gafor AH. Serum procalcitonin has negative predictive value for bacterial infection in active systemic lupus erythematosus. Lupus. 2012;21:1172–7.
Pyo JY, Park JS, Park YB, Lee SK, Ha YJ, Lee SW. Delta neutrophil index as a marker for differential diagnosis between flare and infection in febrile systemic lupus erythematosus patients. Lupus. 2013;22(11):1102–9.
Serio I, Arnaud L, Mathian A, Hausfater P, Amoura Z. Can procalcitonin be used to distinguish between disease flare and infection in patients with systemic lupus erythematosus: a systematic literature review. Clin Rheumatol. 2014;33(9):1209–15.
Gao J, Zhang L, Zhang X, Zhao SL, Liu SY, Xing LH. Levels of serum procalcitonin and C-reactive protein for evaluating pulmonary bacterial infection in patients with lupus erythematosus. J Huazhong Univ Sci Technol [Medical Sciences]. 2016;36(5):653–8.
AlJarhi UM, Sadek KM, Darwish EM, Elmessiery RM, Salem K, Khalil SA, et al. Evaluation of serum presepsin, procalcitonin, copeptin, and high-sensitivity C-reactive protein for differentiating bacterial infection from disease activity in Egyptian patients with systemic lupus erythematosus. Clin Rheumatol. 2021;40(5):1861–9.
He C, Wang B, Li D, Xu H, Shen Y. Performance of procalcitonin in diagnosing parapneumonic pleural effusions: a clinical study and meta-analysis. Med (Baltim). 2017;96:e7829.
Liu LN, Wang P, Guan SY, Li XM, Li BZ, Leng RX, et al. Comparison of plasma/serum levels of procalcitonin between infection and febrile disease flare in patients with systemic lupus erythematosus: a meta-analysis. Rheumatol Int. 2017;37(12):1991–8.
El-Serougy E, Zayed HS, Ibrahim NM, Maged LA. Procalcitonin and C-reactive protein as markers of infection in systemic lupus erythematosus: the controversy continues. Lupus. 2019;28(11):1329–36.
Karlsson S, Heikkinen M, Pettilä V, Alila S, Väisänen S, Pulkki K, et al. Predictive value of procalcitonin decrease in patients with severe sepsis: a prospective observational study. Crit Care. 2010;14(6):1–10.
Dahaba AA, Hagara B, Fall A, Rehak PH, List WF, Metzler H. Procalcitonin for early prediction of survival outcome in postoperative critically ill patients with severe sepsis. BJA: Br J Anaesth. 2006;97(4):503–8.
Eberhard OK, Haubitz M, Brunkhorst FM, Kliem V, Koch KM, Brunkhorst R. Usefulness of procalcitonin for diff erentiation between activity of systemic autoimmune disease (systemic lupus erythematosus systemic antineutrophil cytoplasmic antibodyassociated vasculitis) and invasive bacterial infection. Arthritis Rheu. 1997;40(7):1250–6.
Schwenger V, Sis J, Breitbart A, Andrassy K. CRP levels in autoimmune disease can be specified by measurement of procalcitonin. Infection. 1998;26(5):274–6.
Quintana G, Medina Y, Rojas C. The use of procalcitonin determinations in evaluation of systemic lupus erythematosus. J Clin Rheum. 2008;14:138–42.
Lanoix JP, Bourgeois AM, Schmidt J, Desblache J, Salle V, Smail A, et al. Serum procalcitonin does not differentiate between infection and disease flare in patients with systemic lupus erythematosus. Lupus. 2011;20(2):125–30.
Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H et al. (2016). Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Modern rheumatology; 26(3):372–6.
Ma L, Zeng A, Chen B, Chen Y, Zhou R. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with systemic lupus erythematosus and their correlation with activity: a meta-analysis. Int Immunopharmacol. 2019;76:105949.
Echeverri A, Naranjo-Escobar J, Posso-Osorio I, Aguirre-Valencia D, Zambrano D, Castaño GL, et al. Neutrophil CD64 expression, procalcitonin and presepsin are useful to differentiate infections from flares in SLE patients with SIRS. Lupus. 2018;27(7):1130–9.
Ho KM, Lee KY, Dobb GJ, Webb SA. C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: a prospective cohort study. Intensive Care Med. 2008;34(3):481–7.
Funding
Open Access funding was granted by Minia University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. R.A.A, N.W.M, N.M.A and A.M.A performed material preparation, data collection, and analysis. R.A.A and N.W.M wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The nature of the study was explained to all patients. Their approval to share in the study was obtained. The study protocol was approved by the Local Ethics Committee of the Faculty of Medicine (Approval No. 387:1/2020) and in accordance with the World Medical Association Declaration of Helsinki, and informed consent was obtained from all patients. Informed consent was collected from all participants in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abdel-Magied, R.A., Mokhtar, N.W., Abdullah, N.M. et al. Infection versus disease activity in systemic lupus erythematosus patients with fever. BMC Rheumatol 8, 34 (2024). https://doi.org/10.1186/s41927-024-00395-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-024-00395-6