Abstract
Systemic lupus erythematosus (SLE) patients are vulnerable to infections. We aim to explore the approach to differentiate active infection from disease activity in pediatric SLE patients. Fifty pediatric SLE patients presenting with 185 clinical visits were collected. The associations between both clinical and laboratory parameters and the outcome groups were analyzed using generalized estimating equations (GEEs). These 185 visits were divided into 4 outcome groups: infected-active (n = 102), infected-inactive (n = 11), noninfected-active (n = 59), and noninfected-inactive (n = 13) visits. Multivariate GEE (generalized estimating equation) analysis showed that SDI, SLEDAI-2K, neutrophil‐to‐lymphocyte ratio (NLR), hemoglobin, platelet, RDW-to-platelet ratio (RPR), and C3 are predictive of flare (combined calculated AUC of 0.8964 and with sensitivity of 82.2% and specificity of 90.9%). Multivariate GEE analysis showed that SDI, fever temperature, CRP, procalcitonin (PCT), lymphocyte percentage, NLR, hemoglobin, and renal score in SLEDAI-2k are predictive of infection (combined calculated AUC of 0.7886 and with sensitivity of 63.5% and specificity of 89.2%). We can simultaneously predict 4 different outcome with accuracy of 70.13% for infected-active group, 10% for infected-inactive group, 59.57% for noninfected-active group, and 84.62% for noninfected-inactive group, respectively. Combination of parameters from four different domains simultaneously, including inflammation (CRP, ESR, PCT), hematology (Lymphocyte percentage, NLR, PLR), complement (C3, C4), and clinical status (SLEDAI, SDI) is objective and effective to differentiate flares from infections in pediatric SLE patients.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease caused by autoreactive B cells in combination with T cell dysregulation and cytokine abnormalities1. The presentation, disease course, and outcomes of SLE are unpredictable. Approximately 60–70% of patients exhibit relapsing–remitting and active disease patterns2. Pediatric lupus patients typically have a severe disease course. Additionally, in comparison to adults, a significantly higher percentage of children with SLE continue to have a status of high disease activity3. Several indices have been designed to assess disease activity. The most commonly used disease activity score is the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)4. SLE can also be complicated by chronic multiorgan damage. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) is a reliable instrument for the assessment of the degree of disease-related damage in children with SLE5.
SLE patients are highly susceptible to infections due to the combined effects of immunosuppressive therapy and immune system abnormalities. In Taiwan, infections are among the leading causes of death in pediatric SLE6. Moreover, fever is a common symptom in pediatric SLE, and it is difficult to distinguish between an SLE flare and febrile infection7. Some infections may produce systemic manifestations mimicking SLE, either superimposed upon or triggering a flare8, making the diagnosis and therapeutic approach challenging. In one study, a delay in antimicrobial therapy of > 24 h reportedly increased the mortality of hospitalized SLE patients 12-fold; therefore, early identification and treatment of infections are essential9. The interaction between infection and SLE is complicated, as viral, bacterial, parasitic, and fungal pathogens can trigger SLE disease activity through molecular mimicry10. The establishment of a causative link between infection and autoimmunity has been studied in detail, confirming the role of infectious agents in the induction as well as the progression or exacerbation of SLE11. In general, clinicians have to make treatment decisions based on clinical judgment and laboratory parameters to distinguish between active disease and infection. Most such studies to date have been performed in adult populations, whereas data regarding pediatric SLE are lacking.
There have been a number of studies on predictive biological markers of SLE flares, including anti-double-stranded DNA antibodies (anti-dsDNA Ab), the complement system, anti-extractable nuclear antigen antibodies (anti-ENA Ab), cytokines, and chemokines12,13. In addition, conventional biomarkers (C-reactive protein [CRP], erythrocyte sedimentation rate (ESR), procalcitonin [PCT])14,15,16,17,18 and new markers have been developed for the prediction of infection in SLE patients19. Although several recent studies have focused on markers for differentiating between disease flare and infection in febrile SLE patients20,21,22, most physicians agree that no single biomarker has sufficient predictive value for both events8,19. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are significantly higher in SLE patients than in healthy controls and correlate positively with the SLEDAI score23. However, the NLR might be a good additive marker for diagnosing infection in patients with SLE24. New scores, which include combinations of different biomarkers, may represent better solutions for differentiation19.
Overall, it is possible that the use of only one biomarker would not be sufficient to distinguish infection from disease activity. We aimed to identify useful parameters for the differential diagnosis of disease flares and infections in pediatric-onset SLE patients and to develop predictive calculators that might assist in decision-making in daily clinical practice.
Results
Patient and clinical characteristics
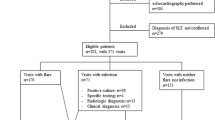
Fifty patients who accounted for a total of 185 clinical visits were included in the study (Table 1). Among these 50 patients, 7 (14%) were male and 43 (86%) female; the mean age at enrollment was 13.9 ± 4.4 years old. The type of infections and positive culture results were recorded in Table 2. The most common fungal infections of our study include Candida species and Pneumocystis jirovecii25. These 185 visits were divided into 4 groups: infected-active visits as group A (n = 102; 55%), infected-inactive visits as group B (n = 11; 6%), noninfected-active visits as group C (n = 59; 32%), and noninfected-inactive visits as group D (n = 13; 7%) (Table 3). Categorization of outcomes was performed in a fashion similar to that in previous studies18,26. The trend of our CRP results resembled those reported by others18,26,27, as did the trends of our ESR and PCT results18,27 (Supplement Fig. S1). The infected-active group (group A) had the highest PLR values among all four groups26. Without infection, CRP levels are higher in active SLE than in inactive SLE18,28.
Parameters predictive of activity flare
Among all the parameters analyzed, we found SDI score, SLEDAI 2K score, NLR, RDW-to-platelet ratio (RPR), ANA level, anti-dsDNA level, antiphospholipid Ab level, and urine cast to be positive predictors of activity flare. Conversely, Hb levels (g/dL), platelet levels, C3 levels (mg/dL), C4 levels (mg/dL), and urine nitrate were negatively associated with the occurrence of disease activity (Supplementary Table S1a). Multivariate GEE (generalized estimating equation) analysis showed that SDI score, SLEDAI 2K score, NLR, Hb level (g/dL), platelet level, RPR, and C3 level (mg/dL) were independent parameters for predicting SLE activity flares (Table 4). In this study, we confirmed that the NLR, PLR, and RPR are useful markers for the assessment of disease activity in pediatric SLE patients23,29, and the combination of these seven parameters resulted in a model with a calculated AUC of 0.8964 and a sensitivity of 82.2% and specificity of 90.9% (Fig. 1a).
Receiver Operating Characteristic (ROC) curves for prediction of (a) activity flares and (b) acute infections. In (a), ROC for SDI score, SLEDAI 2K score, NLR, Hb levels, platelet levels, RPR, and C3 levels according to univariate GEE result (dashed line), and their combination (multi flare GEE) according to multivariate GEE result (solid line) to predict activity flare is shown. The area under curve (AUC) were shown within parentheses. In (b), ROC for SDI score, fever temperature, CRP levels, PCT levels, lymphocyte percentage, NLR, Hb levels, and SLEDAI 2 K renal score according to univariate GEE result (dashed line), and their combination (multi infection GEE) according to multivariate GEE result (solid line) to predict acute infection is shown. The area under curve (AUC) were shown within parentheses.
We thus propose an Activity Predict Score formula:
We obtained the largest Youden Index when the cutoff point of the Activity Predict Score was 0.76652; that is, an Activity Predict Score greater than 0.76652 indicates an activity flare, whereas as score less than 0.76652 indicates no activity flare.
Parameters predictive of acute infection
Using GEE, we found that acute infection was associated with SDI score, fever temperature (°C), CRP level (mg/dL), PCT level (ng/mL), NLR, PLR, and renal score of SLEDAI 2 K but that lymphocyte percentage, Hb level (g/dL), and urine nitrate were negative predictors of infectious events (Supplementary Table S1b). Multivariate GEE analysis showed that SDI score, fever temperature (°C), CRP level (mg/dL), PCT level (ng/mL), lymphocyte percentage, NLR, Hb level (g/dL), and SLEDAI 2 K renal score were independent parameters for predicting acute infection in SLE patients (Table 5). Renal disease, despite being associated with infections in the univariate analysis, did not retain statistical significance in the multivariate analysis in some series30,31. However, our result resembled a previous report that renal involvement is significantly associated with active infection, as based on multivariate GEE analysis32. Of note, our data were consistent with a previous report that any increase in the SDI was associated with the occurrence of serious infection31. We also showed that compared to PCT, CRP is a more sensitive and specific marker for diagnosing bacterial infection in SLE33. Regardless, some reports have shown that PCT is more specific and has better diagnostic accuracy than CRP for infection in SLE15,34,35. The combination of these eight parameters resulted in a model with a calculated AUC of 0.7886 and a sensitivity of 63.5% and specificity of 89.2% (Fig. 1b).
Predicted by multiple GEE results, we also obtained the Infection Predict Score:
There will be the largest Youden Index will occur at cutoff value of 0.58286; that is, when the Infection Predict Score is greater than 0.58286, acute infection will be classified, whereas no acute infection will be classified at a score less than 0.58286.
Development of a calculator model to simultaneously differentiate flares from infections
Multinomial logistic regression, which describes the probability of being in a specific group, was used to analyze the individual effects of covariates (independent variables) on discrete nominal outcomes36. We selected a total of 10 variables (SDI, SLEDAI 2K, fever temperature, PCT, lymphocyte percentage, NLR, Hb, PLT, RPR, C3) to establish multinomial logistic regression. The regression formula obtained by multinomial logistic regression is as follows:
-
(1)
\({\text{ln}}\left( {\uppi {\text{A}}/\uppi {\text{D}}} \right) = 11.932 - 1.3896 \times {\text{SDI score}} + 0.4166 \times {\text{SLEDAI 2K score}} + 9.9529 \times {\text{fever}}\;{\text{temperature}} + 36.4342 \times {\text{PCT}} + 1.028 \times {\text{lymphocyte}}\;{\text{percentage}} + 1.3915 \times {\text{NLR}} - 1.0061 \times {\text{Hb}} - 0.00784 \times {\text{PLT}} - 21.2367 \times {\text{RPR}} - 0.0564 \times {\text{C}}3\)
-
(2)
\({\text{ln}}\left( {\uppi {\text{B}}/\uppi {\text{D}}} \right) = 6.1895-1.5815 \times {\text{SDI score}}+0.2968 \times {\text{SLEDAI 2K score}} + 10.9273 \times {\text{fever}}\;{\text{temperature}} + 36.1573 \times {\text{PCT}} + 0.9752 \times {\text{lymphocyte}}\;{\text{percentage}} + 1.3517 \times {\text{NLR}} - 0.9872 \times {\text{Hb}} - 0.0229 \times {\text{PLT}} - 56.2559 \times {\text{RPR}} - 0.0434 \times {\text{C}}3\)
-
(3)
\({\text{ln}}\left( {\uppi {\text{C}}/\uppi {\text{D}}} \right) = 12.5014 - 1.7698 \times {\text{SDI score}} + 0.3917 \times {\text{SLEDAI 2K score}} + 10.0168 \times {\text{fever}}\;{\text{temperature}} + 35.6598 \times {\text{PCT}} + 1.0357 \times {\text{lymphocyte}}\;{\text{percentage}} + 1.3925 \times {\text{NLR}} - 0.8858 \times {\text{Hb}} - 0.00679 \times {\text{PLT}} - 18.9057 \times {\text{RPR}} - 0.0646 \times {\text{C}}3\)
By inputting the value of the selected parameters into these three equations, we can obtain the ratio values of πA, πB, πC and πD. If the value obtained is larger, the probability of being classified into that nominal group is greater (the group divided into D is the reference group), and we will classify particular visits into that group (groups A, B, C). That is, if the calculated ln(πA/πD) is greater than 1 and is the largest number compared with others (ln(πB/πD) and ln(πC/πD)), the cases is categorized as in group A. If all three calculated numbers [ln(πA/πD), ln(πB/πD), and In(πC/πD)] are below 1, the case is categorized as in group D. With a combination of these ten parameters, we can simultaneously predict four groups with an accuracy of 70.13% for the infected-active group, 10% for the infected-inactive group, 59.57% for the noninfected-active group, and 84.62% for the noninfected-inactive group. From our multinomial logistic regression analysis, we identified SDI, SLEDAI 2K, fever temperature (°C), PCT, lymphocyte percentage, NLR, Hb (g/dL), PLT (K/μL), RPR, and C3 (mg/dL) as influencing factors for simultaneously differentiating activity flares from acute infections. By knowing the values of the observed parameters, we can predict the group classification of any specific visit for an individual patient.
Evaluation of possible associated interaction between acute infection and activity flare
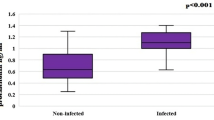
To observe whether there is an associated interaction between acute infection and activity flare, we sought to compare parameters from combined groups with or without infection (Fig. 2). We found that CRP (mg/dL), PCT (ng/mL), lymphocyte percentage, NLR, PLR, SLEDAI 2K and SDI from the combined groups with infection were significantly higher than those of the combined groups without infection. However, ESR (mm/h), C3 (mg/dL) and C4 (mg/dL) were not significantly different between the two combined groups. Our results (elevated SLEDAI 2K, SDI, NLR, and PLR under noninfected conditions) indicate that acute infection might play a triggering role in flare activity10,37,38. On the other hand, for proteins that participate in both SLE disease inflammation and acute-phase inflammation, no significant difference in ESR (mm/h), C3 and C4, with or without infection, was observed.
Comparison for mean values of major parameters from infected (infected-active plus infected-inactive; A + B) groups vs noninfected (noninfected-active plus noninfected-inactive; C + D) groups. Parameters from four different domains, including (a) inflammation (CRP, ESR, PCT), (b) hematology (NLR, PLR, lymphocyte percentage), (c) complement (C3, C4), and (d) clinical status (SLEDAI, SDI), are depicted.
Trend analysis of parameter changes over time through hospitalization
The results of the mean baseline level and changes per time interval of the different groups are shown in Supplement Table S2. There were significant differences in ESR (mm/h), NLR, lymphocyte percentage, C3 (mg/dL), and C4 (mg/dL), as shown in Fig. 3. According to Fig. 3 (a), ESR (mm/h) decreased with time, but the decreasing trend was more prominent in group A than in group C. ESR (mm/h) appears to be a useful biomarker for SLE activity assessment. Indeed, an elevated ESR (mm/h) is included in three of five validated SLE activity scores28. Our trend analysis indicated that a higher initial ESR level (mm/h) might reflect the effect of both activity and infection in group A14. From the trend difference between groups A and C, we could differentiate noninfected-active SLE visits (group C) from infected-active SLE visits (group A) by the change patterns of ESR (mm/h), NLR, lymphocyte percentage, C3 (mg/dL), and C4 (mg/dL) over time.
The average changing levels with time of (a) ESR, (b) NLR, (c) lymphocyte percentage, (d) C3, and (e) C4 during hospitalization are shown from a subset of 24 patients accounting for 29 times of admission. The trend for NLR showed that the NLR in group C were significantly decreased with time while those of group A were significantly increased with time. For lymphocyte percentage trend, the trend in group C was gradually increased with time while those of group A was sharply decreased with time. From (d,e), we found that the C3 and C4 levels in group C were significantly increased with time while those of group A were significantly decreased with time.
Discussion
In contrast to previous reports, the most important parameters used to establish the predictive model in our study consisted of four domains under simultaneous evaluation: inflammation (CRP, ESR, PCT), hematology (WBC, PLT, Hb, lymphocyte percentage, NLR, PLR, RPR), the complement system (C3, C4), and clinical status (SLEDAI, SDI) (Fig. 4). This classification approach is conceptually similar to the latest version of the SLE classification criteria39. Obtaining several parameters simultaneously remains necessary to differentiate flares from infections27. Attribution of clinical manifestations to SLE often requires a comprehensive, multidisciplinary approach to rule out mimics (e.g., infections), taking into account the presence of risk factors (e.g., immunosuppressive therapy), as well as other factors favoring alternative diagnoses (e.g., hematological malignancy)40. Cognitive bias might result in diagnostic errors41. It seemed that subspecialty expertise does not attenuate this bias. The use of our predictive models may aid in the diagnostic process. We hope to include more molecular biomarkers and genetic signatures in our model in the future, and the related mechanisms will be further explored.
Investigation scheme and brief summary of our study. When SLE patients encounter clinical indications, through the evaluation model based on the parameters of the four main domains, we can make a correct evaluation and appropriate treatment. CRP C-reactive protein; ESR erythrocyte sedimentation rate; GEE generalized estimating equation; NLR neutrophil-to-lymphocyte ratio; PCT procalcitonin; PLR platelet-to-lymphocyte ratio; SLEDAI Systemic Lupus Erythematosus Disease Activity Index; SDI Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index.
This was a rare study focusing on distinguishing flares from infection in pediatric-onset SLE patients, and the study design closely approximated real-world clinical practice. Studies with designs similar to ours have been reported previously18,20,21,26,27. Overall, our study design was similar to those for disease registries derived from the systematic collection of information from patients diagnosed with a particular disease (in this case, SLE). The management of pediatric SLE requires ongoing monitoring of patients, with the collection of data for many parameters/markers. We suggest that this composite predict score can be used in everyday clinical practice to improve the discrimination between activity flare and acute infection in pediatric patients with SLE, who have a greater risk of more fulminant SLE and/or more severe infection, and to take prompt interventions to improve clinical outcomes in pediatric SLE patients.
The NLR and PLR have been used as prognostic indicators for malignancy and are associated with morbidity and mortality in chronic diseases42. Previous studies have shown that the NLR is associated with rheumatoid arthritis and psoriasis. Recently, higher NLR and PLR both correlated positively with SLEDAI-2K score and disease activity23,42,43. On the other hand, the NLR is associated with infection, and it has been used as an indicator of bacteremia23,24,26. Our results showed that the NLR is associated with both disease flare and infection. In adult SLE cohorts, blood cell count ratios appeared to be more informative than blood cell counts per se because pancytopenia and thrombocytopenia tend to occur in SLE44. We observed the same tendency in our pediatric-onset SLE patients. Ideally, PLR values should be combined with values for NLR and other inflammatory markers to facilitate a more holistic determination44. Here, we demonstrate that the NLR and PLR are important complementary hematological indices that provide additional information about disease activity, the presence of neutrophilic inflammation, infectious complications, disease severity, and organ damage in SLE.
The relationship between infection and SLE disease damage is difficult to evaluate. Infection itself is reported to both potentially facilitate or protect against the development of SLE11,45. The results from an adult lupus cohort study in Latin America also showed that increased disease activity and damage accumulation are predictive of infection31. One study demonstrated a positive correlation between SDI score and the number of recurrent major infections in pediatric-onset SLE46. Sit et al. also reported that disease damage was significantly associated with a greater number of episodes of major infection47. These findings are consistent with our study of the association between SDI and infection (P < 0.0001) using multivariate GEE. Our results also indicate that SDI and SLEDAI-2K renal scores can be used to accurately predict acute infection, consistent with previous reports.
The utility of traditional markers (e.g., CRP, ESR, PCT) for detecting infection in SLE patients has been discussed for several decades. CRP is an acute-phase reactant synthesized by the liver during IL-6 regulation and is known as an inflammatory biomarker28. CRP levels reportedly increase during infection, arthritis, and serositis in SLE patients14. One study showed that the CRP level is more sensitive and specific in diagnosing bacterial infection than the PCT level33. Immune complexes induce severe inflammation via conventional pro-inflammatory pathways, including those for cytokines such as TNF-α and IL-6 (which leads to CRP production). On the other hand, the same immune complexes induce the production of type I IFNs and various immunoregulatory cytokines. The simple consequence is reduced production of CRP in active SLE (group B > group A), despite increased IL-6 levels, which are visible in concomitant infection18,26,27,28. PCT is a precursor peptide of calcitonin associated with invasive bacterial infections. Normally produced by parafollicular C cells, PCT is released in response to bacterial toxins and IL-1β8, and PCT has good specificity for distinguishing acute bacterial infection from disease flare in patients with autoimmune diseases, regardless of steroid use. The mechanism underlying PCT production after inflammation and its role are still not completely understood19. Furthermore, limited information is available regarding plasma PCT levels in patients with active SLE. Patients with active SLE may have slightly increased PCT levels15,18. However, Garvand et al. noted an unusual phenomenon, whereby PCT levels were high during macrophage activation syndrome (MAS) episodes in lupus flares48, and a systematic literature review suggested that PCT level and SLE disease activity do not correlate15. Our comparison of 4 groups showed that the active disease group had higher PCT levels (group A > group B; group C > group D), regardless of the presence or absence of infection, suggesting that activity flares are associated with elevated PCT levels.
In the complement system, C3 and C4 have traditionally been used to assess SLE disease activity. However, studies of C3 and C4 consumption in SLE flares indicate that as markers, C3 and C4 exhibit low sensitivity and a wide range of specificity12. One reason for these inconsistent results may be because complement proteins participate in both autoimmune (SLE activity) and inflammation (infection) responses. As a consequence, inflammation due to infection increases, but immune complex consumption during disease activity decreases complement protein levels. Hence, levels of C3 and C4 are regulated by both mechanisms28. Interestingly, decreases in C3 or C4 levels are not detected in some patients. Instead, levels can increase relative to the baseline during flare visits49. Our results (shown in Fig. 3) indicate that C3 and C4 levels in group C increased significantly over time, which may be related to this phenomenon.
Conclusions
Infections are a major cause of morbidity and mortality in SLE patients. Infections might mimic and even trigger SLE flares. To distinguish acute infection from activity flare always remains a clinical challenge. The proposed approach (Activity predict score, Infection predict score, and multinomial logistic regression formula) could differentiate flares from infections in pediatric SLE patients. Clinicians could make appropriate judgement and treatment decisions based on the combination of parameters from four different domains simultaneously, including inflammation (CRP, ESR, PCT), hematology (Lymphocyte percentage, NLR, PLR), complement (C3, C4), and clinical status (SLEDAI, SDI) in daily clinical practice (Fig. 4).
Limitations
This study was preliminary and was limited by that there were only 50 different patients with 185 visits. Further validation, replication and use of the calculator algorithm in larger populations would be indicated and useful to prove its reliability beyond this study. Repeated measures in a single patient may introduces potential bias. More molecular biomarkers and genetic signatures should be included and evaluated in our model to explore related underlying mechanisms. Because autoantibodies are not routine laboratory inspections, so that autoantibody profiles are included in missing data due to insufficient data quantities, which may have some impact on our evaluation results. The differentiation of flares versus infection due to microorganism types and infection sites needs further investigation.
Methods
Patients
This was a retrospective study conducted by reviewing the medical records of 50 pediatric-onset (≤ 18-year-old) SLE50 patients presenting with 185 clinical visits for any clinical condition from August 1, 2015, to September 1, 2019, at the Department of Pediatrics, National Taiwan University Hospital (NTUH). Patients who did not meet the 1997 ACR criteria for SLE diagnosis and those with overlapping autoimmune diseases or other chronic inflammatory diseases/infections or malignancies were excluded. This study was approved by the Institutional Review Board and Research Ethics Committee of the National Taiwan University Hospital and was conducted in compliance with the protocol for good clinical practices and the principles of the Declaration of Helsinki. Informed consent was obtained from all participants and/or their legal guardians.
Data encompassing both laboratory and clinical parameters, including general laboratory testing for pediatric SLE (indicators of inflammation, autoantibodies, complement, urinalysis, antiphospholipid antibodies)51, biomarkers of infection in SLE19, SLEDAI, SDI and markers based on previous reports23,26,29,43,52, were collected at each visit as a standardized clinical protocol to ensure that the resulting predicting model can be used in everyday clinical practice (detailed items in Supplement Table S1).
Outcome definitions
Disease flare was defined according to the SELENA-SLEDAI Flare index (SFI)53:
-
Mild or moderate flares were defined as 1 or more of the following: (a) change in SELENA-SLEDAI instrument score of 3 points or more (but not to more than 12); (b) new or worsening discoid, photosensitive, or other rash attributable to lupus (including lupus profundus, cutaneous vasculitis, or bullous lupus), nasopharyngeal ulcers, pleuritis, pericarditis, arthritis, or fever not attributable to infection; c) increase in prednisone but not to > 0.5 mg/kg/day; d) addition of nonsteroidal anti-inflammatory drugs (NSAIDs) or hydroxychloroquine for SLE activity; and (e) ≥ 1.0 increase in physician’s global assessment (PGA) score but not to more than 2.5.
-
Severe flares were defined as 1 or more of the following: a) change in SELENA-SLEDAI instrument score to greater than 12; b) new or worsening central nervous system involvement, vasculitis, nephritis, myositis, thrombocytopenia (platelet count < 60 × 109 cells/L), or hemolytic anemia (hemoglobin level < 7 g/dL or decrease in hemoglobin level > 3 g/dL over a 2-week period), each requiring doubling of corticosteroid dosage to a final dosage greater than 0.5 mg/kg per day or hospitalization; (c) any SLE manifestation requiring an increase in dosage of prednisone or equivalent drug to greater than 0.5 mg/kg per day, or initiation of therapy with cyclophosphamide, azathioprine, mycophenolate mofetil, or methotrexate; (d) hospitalization for lupus activity; and (e) increase in PGA score to > 2.5.
Acute infection was defined based on the following: (1) microbiological culture/isolation evidence that explained clinical symptoms; (2) clinical symptoms and/or inflammatory syndrome and/or laboratory/serological results that rapidly regressed after starting antimicrobial (antibiotic, antiviral, or antifungal) therapy; (3) confirmation by radiological and/or imaging study; and/or (4) confirmation via an infectious specialist consultation20,21,27,35.
Statistical analysis
The clinical and laboratory characteristics of the four groups were analyzed. Quantitative variables are presented as means ± standard deviations and ranges. Qualitative variables are shown as numbers (n) and percentages. The Mann–Whitney test was used instead of the t-test for the analysis of nonparametric data. Associations between parameters and the four outcome groups were analyzed using univariate and multivariate generalized estimating equations (GEEs) with an autoregressive (AR) model, as the GEE approach facilitates the analysis of data collected in longitudinal, nested, or repeated-measures designs and produces more efficient and unbiased regression estimates for analyzing measures with non-normal response variables54.
For both laboratory and clinical parameters, univariate logistic regressions were then fit to specific outcome models. A multivariate logistic stepwise regression was performed with variables that were significant according to both P value and AUC in univariate analysis. Pearson pairwise correlation coefficients were applied to preclude highly correlated parameters in the univariate model55. We excluded parameters with data not missing completely at random56. Measures for model adequacy were performed using the Akaike information criterion (AIC)56. Through univariate analysis and multivariate logistic stepwise regression, the remaining parameters were designated as “significant effectors” of activity flare or acute infection and were used to derive a Predict Score equation. The estimates of individual significant effectors were combined to generate the Predict Score formula. After obtaining the Predict Score equation, we calculated the score for each visit of an individual patient according to the times of repeated measurements. At this time, the "estimated score" is regarded as x, and the "activity flare" or “acute infection” is regarded as y. We used logistic regression to find the best cutoff point at which the largest Youden Index (that is, the sum of sensitivity plus specificity is the largest) would be obtained.
SLE is characterized by a relapsing–remitting course between at least two discrete clinical/laboratory episodes, separated by periods of clinical quiescence57. Therefore, we assumed that discrete episodes were all conceptually independent. We selected parameters with significant P values in univariate analysis and excluded highly correlated parameters using Pearson pairwise correlation analysis. A total of 10 variables were used for modeling. We thus used a multinomial logistic regression (MLR) model for nominal outcomes by setting group D (noninfected-inactive group) as the reference group and developed three regression equations for simultaneous prediction36. The relationship between the other outcome (for group A, B, C) and any particular explanatory variable (clinical and laboratory parameters) was captured using particular parameters that define the log odds of response jumping from the reference outcome (group D) to the otherwise outcome (group A, B, C)36.
Twenty-four hospitalized patients presented 29 admissions with serial measurement of parameters during admission. The evaluation at the first time point provided baseline data, and the data collected at each ensuing time point were assigned into one of the following 10 time periods: baseline/day 0 (assigned as time point 0), < 3 days (time point 1), 3–5 days (time point 2), 6–10 days (time point 3), 11–15 days (time point 4), 16–20 days (time point 5), 21–25 days (time point 6), 26–30 days (time point 7), 31–35 days (time point 8), and 36–40 days (time point 9). To identify potential markers, we analyzed group-specific parameter trends, i.e., baseline data and data from subsequent assessments in groups A vs C using a multivariate GEE with the AR model and the GLIMMIX procedure. To track trends in changes in laboratory and clinical parameters in groups A and C over time, we excluded the parameters that had estimates not statistically significant, and we utilized the GLIMMIX procedure using 13 variables (WBC, Hb, PLT, lymphocyte, NLR, PLR, RPR, SLEDAI 2K, C3, C4, CRP, PCT, and ESR), employing verification steps such as the type III tests of fixed effects to provide a solution for fixed effects. The default optimization technique for generalized linear mixed models is the quasi-Newton method. Dimensions included G-side covariance parameters. Fit statistics were calculated using generalized chi-square tests. Covariance parameter estimates and fixed-effect solutions were calculated. The analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Abbreviations
- AR:
-
Autoregressive
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- GEE:
-
Generalized estimating equation
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PCT:
-
Procalcitonin
- PLR:
-
Platelet-to-lymphocyte ratio
- RPR:
-
RDW-to-platelet ratio
- SLE:
-
Systemic lupus erythematosus
- SLEDAI:
-
Systemic Lupus Erythematosus Disease Activity Index
- SDI:
-
Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index
References
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121. https://doi.org/10.1056/NEJMra1100359 (2011).
Ceccarelli, F. et al. Assessment of disease activity in Systemic Lupus Erythematosus: Lights and shadows. Autoimmun. Rev. 14, 601–608. https://doi.org/10.1016/j.autrev.2015.02.008 (2015).
Tucker, L. B. et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 17, 314–322. https://doi.org/10.1177/0961203307087875 (2008).
Gladman, D. D., Ibanez, D. & Urowitz, M. B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 29, 288–291 (2002).
Gladman, D. D. et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 40, 809–813. https://doi.org/10.1002/art.1780400506 (1997).
Huang, J. L. et al. Pediatric lupus in Asia. Lupus 19, 1414–1418. https://doi.org/10.1177/0961203310374339 (2010).
Aggarwal, A. & Srivastava, P. Childhood onset systemic lupus erythematosus: how is it different from adult SLE?. Int. J. Rheum. Dis. 18, 182–191. https://doi.org/10.1111/1756-185x.12419 (2015).
Sciascia, S. et al. Systemic lupus erythematosus and infections: clinical importance of conventional and upcoming biomarkers. Autoimmun. Rev. 12, 157–163. https://doi.org/10.1016/j.autrev.2012.03.009 (2012).
Feng, P. H. et al. Inadequate antimicrobial treatment for nosocomial infection is a mortality risk factor for systemic lupus erythematous patients admitted to intensive care unit. Am. J. Med. Sci. 340, 64–68. https://doi.org/10.1097/MAJ.0b013e3181e0ef9b (2010).
Jung, J. Y. & Suh, C. H. Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J. Intern. Med. 32, 429–438. https://doi.org/10.3904/kjim.2016.234 (2017).
Rigante, D. & Esposito, S. Infections and systemic lupus erythematosus: binding or sparring partners?. Int. J. Mol. Sci. 16, 17331–17343. https://doi.org/10.3390/ijms160817331 (2015).
Gensous, N. et al. Predictive biological markers of systemic lupus erythematosus flares: a systematic literature review. Arthritis Res. Ther. 19, 238. https://doi.org/10.1186/s13075-017-1442-6 (2017).
Thanadetsuntorn, C. et al. The model of circulating immune complexes and interleukin-6 improves the prediction of disease activity in systemic lupus erythematosus. Sci. Rep. 8, 2620. https://doi.org/10.1038/s41598-018-20947-4 (2018).
Dima, A., Opris, D., Jurcut, C. & Baicus, C. Is there still a place for erythrocyte sedimentation rate and C-reactive protein in systemic lupus erythematosus?. Lupus 25, 1173–1179. https://doi.org/10.1177/0961203316651742 (2016).
Serio, I., Arnaud, L., Mathian, A., Hausfater, P. & Amoura, Z. Can procalcitonin be used to distinguish between disease flare and infection in patients with systemic lupus erythematosus: a systematic literature review. Clin. Rheumatol. 33, 1209–1215. https://doi.org/10.1007/s10067-014-2738-4 (2014).
Limper, M., de Kruif, M. D., Duits, A. J., Brandjes, D. P. & van Gorp, E. C. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J. Infect. 60, 409–416. https://doi.org/10.1016/j.jinf.2010.03.016 (2010).
Shaikh, M. M., Hermans, L. E. & van Laar, J. M. Is serum procalcitonin measurement a useful addition to a rheumatologist’s repertoire? A review of its diagnostic role in systemic inflammatory diseases and joint infections. Rheumatology (Oxford) 54, 231–240. https://doi.org/10.1093/rheumatology/keu416 (2015).
Wang, J. et al. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine 98, e16798. https://doi.org/10.1097/md.0000000000016798 (2019).
Ospina, F. E. et al. Distinguishing infections vs flares in patients with systemic lupus erythematosus. Rheumatology (Oxford) 56, i46–i54. https://doi.org/10.1093/rheumatology/kew340 (2017).
Pyo, J. Y. et al. Delta neutrophil index as a marker for differential diagnosis between flare and infection in febrile systemic lupus erythematosus patients. Lupus 22, 1102–1109. https://doi.org/10.1177/0961203313499957 (2013).
Beca, S., Rodriguez-Pinto, I., Alba, M. A., Cervera, R. & Espinosa, G. Development and validation of a risk calculator to differentiate flares from infections in systemic lupus erythematosus patients with fever. Autoimmun. Rev. 14, 586–593. https://doi.org/10.1016/j.autrev.2015.02.005 (2015).
Littlejohn, E. et al. The ratio of erythrocyte sedimentation rate to C-reactive protein is useful in distinguishing infection from flare in systemic lupus erythematosus patients presenting with fever. Lupus 27, 1123–1129. https://doi.org/10.1177/0961203318763732 (2018).
Ma, L., Zeng, A., Chen, B., Chen, Y. & Zhou, R. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with systemic lupus erythematosus and their correlation with activity: a meta-analysis. Int. Immunopharmacol. 76, 105949. https://doi.org/10.1016/j.intimp.2019.105949 (2019).
Kim, H. A., Jung, J. Y. & Suh, C. H. Usefulness of neutrophil-to-lymphocyte ratio as a biomarker for diagnosing infections in patients with systemic lupus erythematosus. Clin. Rheumatol. 36, 2479–2485. https://doi.org/10.1007/s10067-017-3792-5 (2017).
Danza, A. & Ruiz-Irastorza, G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 22, 1286–1294. https://doi.org/10.1177/0961203313493032 (2013).
Broca-Garcia, B. E. et al. Utility of neutrophil-to-lymphocyte ratio plus C-reactive protein for infection in systemic lupus erythematosus. Lupus 28, 217–222. https://doi.org/10.1177/0961203318821176 (2019).
Schafer, V. S., Weiss, K., Krause, A. & Schmidt, W. A. Does erythrocyte sedimentation rate reflect and discriminate flare from infection in systemic lupus erythematosus? Correlation with clinical and laboratory parameters of disease activity. Clin. Rheumatol. 37, 1835–1844. https://doi.org/10.1007/s10067-018-4093-3 (2018).
Aringer, M. Inflammatory markers in systemic lupus erythematosus. J. Autoimmun. https://doi.org/10.1016/j.jaut.2019.102374 (2019).
Xie, S. & Chen, X. Red blood cell distribution width-to-platelet ratio as a disease activity-associated factor in systemic lupus erythematosus. Medicine 97, e12342. https://doi.org/10.1097/md.0000000000012342 (2018).
Ruiz-Irastorza, G. et al. Predictors of major infections in systemic lupus erythematosus. Arthritis Res. Ther. 11, R109. https://doi.org/10.1186/ar2764 (2009).
Pimentel-Quiroz, V. R. et al. Factors predictive of serious infections over time in systemic lupus erythematosus patients: data from a multi-ethnic, multi-national, Latin American lupus cohort. Lupus 28, 1101–1110. https://doi.org/10.1177/0961203319860579 (2019).
Feldman, C. H. et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 67, 1577–1585. https://doi.org/10.1002/art.39070 (2015).
Kim, H. A., Jeon, J. Y., An, J. M., Koh, B. R. & Suh, C. H. C-reactive protein is a more sensitive and specific marker for diagnosing bacterial infections in systemic lupus erythematosus compared to S100A8/A9 and procalcitonin. J. Rheumatol. 39, 728–734. https://doi.org/10.3899/jrheum.111044 (2012).
Song, G. G., Bae, S. C. & Lee, Y. H. Diagnostic accuracies of procalcitonin and C-reactive protein for bacterial infection in patients with systemic rheumatic diseases: a meta-analysis. Clin. Exp. Rheumatol. 33, 166–173 (2015).
Yu, J. et al. Serum procalcitonin and C-reactive protein for differentiating bacterial infection from disease activity in patients with systemic lupus erythematosus. Mod. Rheumatol. 24, 457–463. https://doi.org/10.3109/14397595.2013.844391 (2014).
El-Habil, A. M. An application on multinomial logistic regression model. Pak. J. Stat. Oper. Res. 8, 271–291 (2012).
Fernandez, D. & Kirou, K. A. What causes lupus flares?. Curr. Rheumatol. Rep. 18, 14. https://doi.org/10.1007/s11926-016-0562-3 (2016).
Francis, L. & Perl, A. Infection in systemic lupus erythematosus: friend or foe?. Int. J. Clin. Rheumtol. 5, 59–74. https://doi.org/10.2217/ijr.09.72 (2010).
Aringer, M. EULAR/ACR classification criteria for SLE. Semin. Arthritis Rheum. 49, S14–S17. https://doi.org/10.1016/j.semarthrit.2019.09.009 (2019).
Fanouriakis, A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745. https://doi.org/10.1136/annrheumdis-2019-215089 (2019).
Trowbridge, R. et al. Diagnostic Error in Medicine. (2017).
Qin, B. et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod. Rheumatol. 26, 372–376. https://doi.org/10.3109/14397595.2015.1091136 (2016).
Wu, Y., Chen, Y., Yang, X., Chen, L. & Yang, Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int. Immunopharmacol. 36, 94–99. https://doi.org/10.1016/j.intimp.2016.04.006 (2016).
Gasparyan, A. Y., Ayvazyan, L., Mukanova, U., Yessirkepov, M. & Kitas, G. D. The Platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann. Lab. Med. 39, 345–357. https://doi.org/10.3343/alm.2019.39.4.345 (2019).
Esposito, S., Bosis, S., Semino, M. & Rigante, D. Infections and systemic lupus erythematosus. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1467–1475. https://doi.org/10.1007/s10096-014-2098-7 (2014).
Lee, P. P., Lee, T. L., Ho, M. H., Wong, W. H. & Lau, Y. L. Recurrent major infections in juvenile-onset systemic lupus erythematosus–a close link with long-term disease damage. Rheumatology (Oxford) 46, 1290–1296. https://doi.org/10.1093/rheumatology/kem102 (2007).
Sit, J. K. K. & Chan, W. K. Y. Risk factors for damage in childhood-onset systemic lupus erythematosus in Asians: a case control study. Pediatr. Rheumatol. Online J. 16, 56. https://doi.org/10.1186/s12969-018-0271-8 (2018).
Gavand, P. E. et al. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome: a study of 103 episodes in 89 adult patients. Autoimmun. Rev. 16, 743–749. https://doi.org/10.1016/j.autrev.2017.05.010 (2017).
Birmingham, D. J. et al. The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus 19, 1272–1280. https://doi.org/10.1177/0961203310371154 (2010).
Hochberg, M. C. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725–1725. https://doi.org/10.1002/art.1780400928 (1997).
Hollander, M. C. et al. International consensus for provisions of quality-driven care in childhood-onset systemic lupus erythematosus. Arthritis Care Res. 65, 1416–1423. https://doi.org/10.1002/acr.21998 (2013).
Bertoli, A. M. et al. Systemic lupus erythematosus in a multiethnic US cohort LUMINA LI: anaemia as a predictor of disease activity and damage accrual. Rheumatology (Oxford) 46, 1471–1476. https://doi.org/10.1093/rheumatology/kem153 (2007).
Buyon, J. P. et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann. Intern. Med. 142, 953–962. https://doi.org/10.7326/0003-4819-142-12_part_1-200506210-00004 (2005).
Liang, K.-Y. & Zeger, S. L. Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22 (1986).
Vatcheva, K. P., Lee, M., McCormick, J. B. & Rahbar, M. H. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology https://doi.org/10.4172/2161-1165.1000227 (2016).
Hardin, J. W. Generalized estimating equations (GEE). Encyclopedia of Statistics in Behavioral Science (2005).
Zen, M. et al. Disease activity patterns in a monocentric cohort of SLE patients: a seven-year follow-up study. Clin. Exp. Rheumatol. 30, 856–863 (2012).
Author information
Authors and Affiliations
Contributions
Conception and design of the studies: J-H.L. and B-L.C. Executed the studies and acquired the data: K-L.L. and J-H.L. Analyzed and interpreted the data: K-L.L., Y-H.Y., Y-T.L., L-C.W., H-H.Y., Y-C.H., and J-H.L. Wrote the manuscript draft, which was reviewed and revised by all authors: K-L.L. and J-H.L. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, KL., Yang, YH., Lin, YT. et al. Differential parameters between activity flare and acute infection in pediatric patients with systemic lupus erythematosus. Sci Rep 10, 19913 (2020). https://doi.org/10.1038/s41598-020-76789-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76789-6
- Springer Nature Limited