Abstract
Introduction
Prep-4-RT is a co-designed stepped-care multimodal prehabilitation program for people scheduled to receive radiotherapy for head and neck cancer (HNC). Prehabilitation, which occurs between diagnosis and treatment commencement, aims to improve a patient’s health to reduce the incidence and severity of current and future impairments. HNC treatment can be distressing and has detrimental impacts on function and quality of life. HNC patients have increased social vulnerabilities including higher rates of socio-economic disadvantage and engagement in lifestyle habits which increase cancer risk. High levels of physical and psychological impacts of HNC treatment and increased social vulnerabilities of this population warrant investigation of optimal pathways of care, such as prehabilitation. This paper describes a research protocol to evaluate the feasibility of Prep-4-RT, which was designed to prepare HNC patients for the physical and psychological impacts of radiotherapy.
Methods and analysis
At least sixty adult HNC patients, scheduled to receive radiotherapy (with or without chemotherapy), will be recruited over a five-month period. All participants will receive access to Prep-4-RT self-management resources. Participants identified through screening as high-risk will also be offered individualised interventions with relevant allied health professionals prior to the commencement of radiotherapy (psychologists, dietitians, speech pathologists and physiotherapists). Participants will complete evaluation surveys assessing their experiences with Prep-4-RT resources and interventions. Clinicians will also complete program evaluation surveys. Primary feasibility outcomes include adoption (uptake and intention to try) and fidelity (adherence to the specialist prehabilitation pathway). Secondary feasibility outcomes include acceptability (patient and clinician) of and satisfaction (patient) with Prep-4-RT as well as operational costs. Feasibility outcome data will be analysed using exact binomial and one-sample t tests, as appropriate.
Ethics and dissemination
Ethics approval has been obtained at the Peter MacCallum Cancer Centre in Melbourne, Australia. Results will be presented at national conferences and published in peer-reviewed journal(s) so that it can be accessed by clinicians involved in the care of HNC patients receiving radiotherapy. If the model of care is found to be feasible and acceptable, the transferability and scalability to other cancer centres, or for other cancer types, may be investigated.
Registration details
ANZCTA (Australian New Zealand Clinical Trials Registry) ACTRN12623000770662.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Strengths and limitations of this study
-
The model of care is multimodal in design supporting both physical and psychological aspects of care.
-
Prehabilitation is provided with a stepped-care approach enabling a patient-centred and resource sensitive model.
-
This study is being conducted in a real-world environment.
-
Participants will be English speaking and recruited from a single institution, potentially limiting uptake and applicability of findings to the broader HNC population.
-
Participants will self-select to participate in the evaluation, causing potential for high attrition.
Introduction
Head and neck cancer (HNC) has been described as the most “traumatic” cancer due to the toxicity of intensive treatment regimens [1, 2]. Radiotherapy and surgery can cause difficulties with speaking, swallowing and breathing, and consequences such as aspiration pneumonia, malnutrition and sarcopenia are common [3,4,5,6]. Additionally, many patients undergoing HNC radiotherapy treatment experience high levels of distress, including anxiety, depression, fear of cancer recurrence/progression and report lower quality of life and high rates of suicide [7,8,9,10,11]. HNC patients may also have increased social vulnerabilities, including higher rates of socio-economic disadvantage, engagement in lifestyle habits which increase cancer risk, such as alcohol and tobacco use, and those from regional areas have poorer survival outcomes [12]. The significant physical and psychological impacts of HNC treatment and increased social vulnerabilities of this population warrant the investigation of optimal models of care, such as prehabilitation.
Optimal care pathways for cancer care recommend prehabilitation for HNC patients [13, 14]. Prehabilitation in cancer care has been defined as a process on the continuum of care that occurs between the time of cancer diagnosis and the beginning of acute treatment, in which targeted interventions aim to improve a patient’s health to reduce the incidence and severity of current and future impairments [15]. Prehabilitation has been shown to facilitate improved clinical outcomes in surgical cohorts, such as improved physical functioning, mental health and health-related quality of life [14, 16,17,18]. Prehabilitation is now being implemented in clinical practice nationally and internationally, primarily with patients planned for major surgery [14, 19, 20]. As prehabilitation has been effective in surgical populations, it may offer similar benefits in the lead up to other intensive treatments, such as radiotherapy. Prehabilitation for patients prior to HNC radiotherapy has the potential to bolster physical and psychological health between diagnosis and treatment commencement [14, 21]. However, research exploring the impacts of prehabilitation for patients with HNC undergoing radiotherapy is scarce.
Literature reporting on surgical prehabilitation demonstrates that multimodal programs are being increasingly implemented into standard care [14, 22]. Recommendations for establishment of prehabilitation models advocate for stepped-care [23]. A stepped-care approach enables a patient-centred and resource sensitive model with an opportunity to be more cost effective by providing universal, generalised interventions to low risk patients (e.g. education) and intensive, individualised interventions to high-risk patients (e.g. one-on-one clinical assessment and intervention) [23].
Prep-4-RT is a new model of care designed to provide multimodal prehabilitation prior to radiotherapy to prepare people with HNC and their carers for the physical and psychological impacts of radiotherapy. This model includes a stepped-care approach whereby standardised screening is completed in order to determine the level of prehabilitation intervention required. The aim of this study is to evaluate Prep-4-RT in a large specialist cancer centre.
The specific objectives are:
-
To evaluate the adoption and fidelity of Prep-4-RT
-
To evaluate patient-reported acceptability of, and satisfaction with, Prep-4-RT
-
To evaluate health professionals’ acceptability of Prep-4-RT
Methods and analysis
Study design
This is a single-site feasibility study designed to investigate the feasibility of a new model of care, entitled ‘Prep-4-RT’. Recommendations for Interventional Trials (SPIRIT) guidelines were used to guide the reporting of the current study (see Additional File 1). The study duration is approximately nine months with participant recruitment spanning approximately five months.
Study population
The study population included adults diagnosed with HNC, living in regional and metropolitan settings in Victoria, Australia, who have been offered and accepted radiotherapy but not yet commenced at the study site.
Inclusion criteria
Participants must meet all the following criteria to participate in the study:
-
Aged at least 18 years at the time of recruitment
-
Able to speak and read English
-
Able to give informed consent (i.e. no psychiatric or cognitive condition that would impact informed consent, as determined by treating clinician)
-
Planned for definitive or post-operative radiotherapy (with or without chemotherapy) for HNC
Exclusion criteria
Participants will be excluded if they:
-
Are scheduled to receive palliative-intent radiotherapy
-
Report extreme distress (e.g. risk of harm to themselves or psychotic symptoms)
Recruitment and consent
Participants will be recruited from the HNC radiotherapy service at an Australian comprehensive cancer centre between March and July 2023. When attending the new patient clinic, eligible participants will be invited by the HNC nurse consultant to participate in the study. The nurse consultant will introduce the Prep-4-RT prehabilitation program, explain the evaluation component and gain verbal consent to participate in the evaluation. After verbal consent has been obtained, participants will be provided with the patient information and consent form (PICF) and screening survey via electronic device or paper copy (if device not available). After reading the plain language statement, participants will be asked to provide written consent for (a) use of their data in the study and (b) participation in the evaluation component of the study. The study team will monitor the patient clinic list to identify eligible participants who are missed through the recruitment strategy.
Health professionals
All clinical staff involved in fulfilling the Prep-4-RT model of care in practice will be invited to complete an acceptability survey regarding the model of care. These clinicians may include nurse coordinators, dietitians, psychologists, speech pathologists and physiotherapists. It is anticipated that approximately ten staff will be invited. Staff will be emailed a link to the online survey in Research Electronic Data Capture (REDCap; Tennessee, USA) at the end of the study and consent is implied by survey completion.
Intervention
Prep-4-RT self-management resources were developed through a co-design process with consumers who had undergone radiotherapy treatment for HNC and clinicians who worked in HNC radiotherapy. Resources were designed to educate, prepare and optimise the psychological and physical health of HNC patients planned for radiotherapy. The suite of resources developed were hosted on the hospital website as five webpages which included videos, fact sheets and links to existing key credible resources. This co-design process will be reported separately.
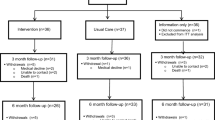
After providing consent, participants will be directed to complete the screening measures for physical and psychological issues to identify low or high-risk status. All patients (both low and high risk) will be provided with self-management resources (described below). Participants identified through screening as high-risk will be offered individualised interventions with the required allied health professional(s) (i.e. psychologist, dietitian, speech pathology, physiotherapy) for one-on-one consultations prior to the commencement of radiotherapy (see Fig. 1).
Screening measures
To determine what level of prehabilitation intervention participants require, multiple screening measures will be used. See Fig. 1 and the descriptions below. Small changes were made to the screening as part of an iterative and continuous improvement cycle early in the pilot period. Figure 1 describes the model of care applied from approximately six weeks into the pilot until pilot end.
The Kessler Psychological Distress Scale (K-10)
The K-10 is a 10-item screening tool designed to identify levels of non-specific psychological distress and likelihood of having a mental disorder. Responses are recorded using a 5-point scale (all of the time [5], most of the time [4], some of the time [3], a little of the time [2], and none of the time [1]). Scores range from 10 to 50 with higher scores indicting a higher severity of psychological distress. A score ≥ 20 indicates a mild to severe level of psychological distress and likely to have a mild to severe disorder [24, 25].
Malnutrition Screening Tool (MST)
The MST 2-item measure is used to identify patients at risk of malnutrition [26]. A score of 2 or more indicates the subject is at risk of malnutrition and subjects with a score of 0 or 1 are deemed not at risk of malnutrition. A higher score indicates a greater risk [26]. In this model of care, an MST score of 3 is being used in line with this cancer centres usual practice, based on a stepped-care approach and resource constraints.
Strength, Assistance with walking, Rising from a chair, Climbing stairs, and Falls (SARC-F)
The SARC-F is a self-reported 5-item screening tool that identifies probable sarcopenia. Each item receives a minimum and maximum score of 0 (none) and 2 (a lot or unable), respectively, with the greatest score being 10. A SARC-F score of ≥ 4 best predicts the need for further, more comprehensive evaluation [27].
Duke Activity Status Index (DASI)
The DASI is a 12-item self-administered questionnaire that can determine functional capacity [28]. Each question is weighted and answered as “yes” or “no”. Scores are summed (range: 0–58.2), and higher scores indicate the more physically active a person is (high functional capacity) according to this set of activities of daily living. A score < 35 indicates a patient with low functional capacity [28].
The Eating Assessment Tool (EAT-10)
The EAT-10 is a 10-item self-administered, symptom-specific questionnaire for dysphagia. Each of the 10 symptoms are rated from 0 (no problem) to 4 (severe problem). The total score ranges from 0 to 40 and a score ≥ 3 indicates that a patient is at risk of dysphagia and warrants further examination [29].
Self-management prehabilitation
All participants will receive access to the Prep-4-RT self-management resources. Participants will be emailed instructions to access the online self-management resources and encouraged to use them in their own time and at their own pace. Resources include the following topics: (1) Introducing prehabilitation for radiotherapy, (2) What is it like to go through radiotherapy?, (3) Preparing for Radiotherapy: How do you do it?, (4) Your guide to tube feeding and (5) Guidance for carers and support people. Each topic comprises one webpage and includes a video, written text, links to other useful websites and information and fact sheets. See Table 1 for further details.
Specialist prehabilitation
Participants identified as high-risk will be offered individualised interventions (phone, telehealth or face-to-face) with the relevant allied health professional(s) (psychologist, dietitian, speech pathologist, physiotherapist) prior to the commencement of radiotherapy. Participants will receive a minimum of one consultation with the professional(s) prior to commencing radiotherapy, with additional consultations determined based on clinical need and in negotiation with the participant. Consultations will be based on a comprehensive assessment with interventions tailored to their needs.
Data collection and evaluation measures
Participant demographic and clinical data
After providing consent, participants will be asked the following information: date of birth, gender, postcode at primary residence, country of birth, current employment situation, occupation, highest level of educational attainment and relationship status. Clinical data, including diagnosis and date of diagnosis, will be collected from the participant’s medical record. Table 2 outlines the time points of clinical data collection.
Acceptability and satisfaction measures
Patient-reported acceptability of, and satisfaction with, self-management and specialist prehabilitation will be assessed by evaluation against the constructs of the Theoretical Framework of Acceptability (TFA) [30], via the Client Satisfaction Questionnaire (CSQ) [31], and a study-specific satisfaction survey. Clinician-reported acceptability of the Prep-4-RT model of care will be measured against the constructs of the TFA as well as a study-specific satisfaction survey. Non-returned surveys will be followed up using a standard schedule of phone, mail and/or email reminders to minimise missing data. Table 2 outlines the time points of evaluation measures. The time point ‘After prehabilitation (pre radiotherapy)’ refers to the 5 days prior to radiotherapy commencing. This time was chosen to create the least amount of burden on patients, by avoiding contact with them in their first week of radiotherapy when patient burden can be particularly high.
Theoretical Framework of Acceptability (TFA)
The TFA was developed to explore multiple aspects of acceptability that can identify characteristics of interventions that may be improved [30]. The questionnaire consists of 6 items reflecting each of the following TFA constructs: affective attitude, burden, perceived effectiveness, intervention coherence, self-efficacy and general acceptability. Items are rated on a 5-point Likert scale. To generate a single acceptability score, (a) compute the total mean score of the 6 TFA items, or (b) use the score for the general acceptability item [30].
Client Satisfaction Questionnaire (CSQ)
The CSQ is a validated 8-item measure of the quality of an intervention, the extent to which the program meets participant’s needs, perceives increases in skills, and whether participants would recommend the program to others. Items are rated with a score of 1–4; total scores range from 8 to 32, with higher scores indicating greater satisfaction [31].
Study-specific survey
There were study-specific questions assessing the use of the Prep-4-RT resources, positive and negative aspects of the resources, continued use of the resources and recommendation for the model of care.
Criteria for drop out
Participant drop out will be recorded. Participants will be removed from the study if their treatment plans change, and they do not proceed with radiotherapy. Participant drop-out for other reasons, including lost to follow up, withdrawal of consent or being too unwell to participate, will also be recorded.
Outcomes
Primary outcomes
Primary feasibility outcomes include adoption (uptake and intention to try) and fidelity (adherence to the specialist prehabilitation pathway). Outcomes, objectives, measurement and feasibility criteria are summarised in Table 3. A case report form (CRF) will be used by researchers and clinicians to document screening and consent rates and uptake of the intervention. Reasons for declining to participate will be noted. In particular, the data collected will include the number of participants who commence HNC radiotherapy during the study period, are screened, are identified as low and high risk, accept referrals to specialist prehabilitation, consent to evaluation, complete evaluation surveys and receive specialist prehabilitation prior to commencing radiotherapy and attendance of specialist prehabilitation appointments. Regarding the self-management resources, the data collected will include the number of participants who access the resources, time spent accessing resources, barriers to access and number of participants that accessed each webpage. Clinicians will complete a CRF for each specialist prehabilitation consultation to document attendance, reason for non-attendance (if known), method of delivery (face to face, phone, telehealth), if goals are being achieved and, if not, the reasons for this.
Primary feasibility outcomes will be judged against pre-specified criteria. In this case, Prep-4-RT will be considered feasible if at least 70% of participants consent to screening and, of those deemed high risk, at least 70% accept requisite referrals, receive specialist prehabilitation services prior to the commencement of radiotherapy and attend at least 70% of scheduled appointments. Additionally, at least 60% of participants will need to utilise self-management resources. More detailed information on progression criteria is provided in Table 4.
Secondary outcomes
Secondary feasibility outcomes include acceptability (patient and clinician) of and satisfaction (patient) with Prep-4-RT (see Table 3 for details and Table 4 for progression criteria) as well as operational costs (clinician time and materials). Again, relevant details will be documented in the CRF. Documentation of clinical activities employed including time taken to complete activities (e.g. screening, provision of specialist prehabilitation) x hourly rate of the relevant clinician, in addition to costs of materials per participant will be recorded.
Statistical considerations
Sample size
The target sample for this study is at least 60 patients. While largely pragmatic, based on study resources and timeframes, clinical throughput and numbers of patients likely to require specialist prehabilitation from at least one allied health professional (approximately 65%), recommended sample sizes for testing binary feasibility outcomes were also considered [32]. For primary feasibility outcomes with ‘stop’ and ‘go’ cut-points set at 50% and 70%, respectively, a sample of 37 patients is required, assuming 80% power and a one-sided α = 0.05 exact binomial test. A sample of this size is sufficient for assessing the progression criteria with the lowest expected denominator (i.e. patients requiring specialist prehabilitation). Power will be greater for tests including larger numbers of patients. A sample of 17 patients only is required for the outcome with ‘stop’ and ‘go’ cut-points set at 30% and 60%, respectively, assuming the same parameters and test.
Statistical analysis
Screening measures will be scored according to author guidelines. Responses to the TFA general acceptability item (item 6) will also be recoded to discrete variables comprising two categories (≥ 4 and < 4). Counts and percentages will be used to summarise missing data, including missing items and forms, for each measure at baseline and pre-radiotherapy assessments. Values for missing forms will not be imputed. Descriptive statistics will be used to summarise participant demographic and clinical characteristics of all participants screened. Binary feasibility outcome data will be analysed using the exact binomial test (one-sided, α = 0.05). Continuous feasibility outcome data will be analysed using a one-sample t test (one-sided, α = 0.05). Analysis will include all available data and be performed in R (reference index version 4.3.1 or higher) [33]. Free text items in study-specific surveys will be summarised using content analysis, whereby the content of free responses will be coded and grouped, where applicable.
Data management
REDCap databases will be used to record and manage data and stored securely in password-protected folders on secure servers. Only Prep-4-RT research team members will have access to this data, in accordance with the National Statement on Ethical Conduct in Human Research 2018 and the Australian Code for Responsible Conduct of Research 2018. Five years after publication or dissemination of project outcomes, electronic data will be destroyed.
Ethics and dissemination
This study has been approved by the institutional ethics committee prior to commencement (HREC approval: HREC/86559/PMCC); all patient participants will provide written informed consent prior to participation and clinician participants will provide implied consent by survey completion.
The outcomes of this study will be published and presented at national and/or international conferences. Participants will not be identifiable in publications or presentations. The resources developed in this study will be made publicly available to other health services. If the Prep-4-RT model of care is feasible, it will be transferable and scalable to other cancer centres and provide a potential model to be utilised for other cancer types.
Discussion
It is well established that HNC treatment, including radiotherapy, causes significant physical and psychological trauma. Complications including dysphagia, malnutrition, sarcopenia and significant psychological distress are common. Evidence regarding multi-modal prehabilitation for HNC patients prior to radiotherapy remains limited and therefore clinical practices prior to radiotherapy commencement for this cohort remain variable across health services. Other barriers to implementing optimal care prior to radiotherapy include a lack of routine screening and limited resources to provide one-on-one prehabilitation to those at high risk. The stepped-care approach included is this Prep-4-RT model of care may provide a possible solution for this.
To our knowledge, the Prep-4-RT study is the first to explore the adoption and fidelity of a comprehensive multimodal intervention supporting both physical and psychological aspects of care, for HNC patients prior to radiotherapy. The Prep-4-RT model encompasses four allied health disciplines key to HNC care, including nutrition and dietetics, speech pathology, psychology and physiotherapy. The model provides a framework for screening and risk stratification of HNC patients to receive an appropriate level of prehabilitation intervention, to allow more intensive treatment to be reserved for individuals with greater physical and/or psychological need. This study will evaluate our co-designed self-management resources to determine if our suite of resources is acceptable and satisfactory to patients and provide a signal as to whether the model of care is cost-effective and acceptable to patients and health professionals. This study will inform a future larger study to test the effectiveness and implementation strategies of this model of care, for example using a hybrid type-2 implementation effectiveness methodology. If feasible and acceptable, the Prep-4-RT model of care has the potential to be transferable and scalable to other health services and settings. Consistent delivery of prehabilitation would encourage routine screening of all HNC patients, and a stepped-care approach would enable a cost-effective use of resources, providing one-on-one prehabilitation to those at greatest risk.
Availability of data and materials
This is not applicable as no datasets were generated or analysed during this study.
References
Moschopoulou E, Hutchison I, Bhui K, Korszun A. Post-traumatic stress in head and neck cancer survivors and their partners. Support Care Cancer. 2018;26(9):3003–11.
Threader J, McCormack L. Cancer-related trauma, stigma and growth: the ‘lived’ experience of head and neck cancer. Eur J Cancer Care (Engl). 2016;25(1):157–69.
Capuano G, Gentile PC, Bianciardi F, Tosti M, Palladino A, Di Palma M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support Care Cancer. 2010;18(4):433–7.
Findlay M, White K, Stapleton N, Bauer J. Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin Nutr. 2021;40(4):1711–8.
Marshall KM, Loeliger J, Nolte L, Kelaart A, Kiss NK. Prevalence of malnutrition and impact on clinical outcomes in cancer services: a comparison of two time points. Clin Nutr. 2019;38(2):644–51.
Xu B, Boero IJ, Hwang L, Le QT, Moiseenko V, Sanghvi PR, et al. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer. 2015;121(8):1303–11.
Alias A, Henry M. Psychosocial effects of head and neck cancer. Oral Maxillofac Surg Clin North Am. 2018;30(4):499–512.
Dunne S, Mooney O, Coffey L, Sharp L, Desmond D, Timon C, et al. Psychological variables associated with quality of life following primary treatment for head and neck cancer: a systematic review of the literature from 2004 to 2015. Psychooncology. 2017;26(2):149–60.
Kam D, Salib A, Gorgy G, Patel TD, Carniol ET, Eloy JA, et al. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1075.
McQuestion M, Fitch M, Howell D. The changed meaning of food: physical, social and emotional loss for patients having received radiation treatment for head and neck cancer. Eur J Oncol Nurs. 2011;15(2):145–51.
Badr H, Gupta V, Sikora A, Posner M. Psychological distress in patients and caregivers over the course of radiotherapy for head and neck Cancer. Oral Oncol. 2014;50(10):1005–11.
Daniell JR, Dolja-Gore X, McDowell L, Udovicich C, Rowe D, Iseli TA, et al. The impact of travel distance to treatment centre on oral tongue squamous cell carcinoma survival and recurrence. Int J Oral Maxillofac Surg. 2022;51(7):854–61.
Cancer Council Victoria and Department of Health Victoria. Optimal care pathway for people with head and neck cancer, second edition. Melbourne: Cancer Council Victoria; 2021.
MacMillan Cancer Support. Principles and guidance for prehabilitation within the management and support of people with cancer. 2017.
Silver JK, Baima J. Cancer Prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92(8):715–27.
Bolshinsky V, Li MHG, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Dis Colon Rectum. 2018;61(1):124–38.
Daniels SL, Lee MJ, George J, Kerr K, Moug S, Wilson TR, et al. Prehabilitation in elective abdominal cancer surgery in older patients: systematic review and meta-analysis. BJS Open. 2020;4(6):1022–41.
Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg. 2017;39:156–62.
Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am. 2017;28(1):49–64.
Santa Mina D, van Rooijen SJ, Minnella EM, Alibhai SMH, Brahmbhatt P, Dalton SO, et al. Multiphasic prehabilitation across the cancer continuum: a narrative review and conceptual framework. Front Oncol. 2021;11:10.
Loewen I, Jeffery CC, Rieger J, Constantinescu G. Prehabilitation in head and neck cancer patients: a literature review. J Otolaryngol Head Neck Surg. 2021;50(1):2.
Waterland JL, McCourt O, Edbrooke L, Granger CL, Ismail H, Riedel B, et al. Efficacy of prehabilitation including exercise on postoperative outcomes following abdominal cancer surgery: a systematic review and meta-analysis. Front Surg. 2021;19:8.
MacMillan Cancer Support. Prehabilitation evidence and insight review. 2017.
Andrews G, Slade T. Interpreting scores on the Kessler Psychological Distress Scale (K10). Aust N Z J Public Health. 2001;25(6):494–7.
Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60(2):184.
Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15(6):458–64.
Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–2.
Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol. 1989;64(10):651–4.
Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24.
Sekhon M, Cartwright M, Francis JJ. Development of a theory-informed questionnaire to assess the acceptability of healthcare interventions. BMC Health Serv Res. 2022;22(1):279.
Attkisson CC, Zwick R. The client satisfaction questionnaire. Eval Program Plann. 1982;5(3):233–7.
Lewis M, Bromley K, Sutton CJ, McCray G, Myers HL, Lancaster GA. Determining sample size for progression criteria for pragmatic pilot RCTs: the hypothesis test strikes back! Pilot Feasib Stud. 2021;7(1):40.
R Core Team. A language and environment for statistical computing. Foundation for Statistical Computing. Vienna, Austria; 2021. Available from: https://www.R-project.org/. Cited 2023 Jul 30.
Acknowledgements
Assistance provided by Karla Gough was greatly appreciated. The authors would like to thank the Prep-4-RT steering committee comprising clinicians and consumers and the wider PMCC multidisciplinary head and neck team for supporting this study. Finally, we would like to thank Western and Central Melbourne Integrated Cancer Services (WCMICS) for funding and supporting this study.
Funding
This work was supported by Western and Central Melbourne Integrated Cancers Service (WCMICS). WCMICS has no role in the study design, data collection, analysis and interpretation or in the writing of the manuscript. There is no study sponsor.
Author information
Authors and Affiliations
Contributions
JL and MF are co-principal investigators of this study. JL and MF lead the planning and development of this study with input from all members of the study steering committee. HR and ES are the joint project managers and wrote this manuscript. JL, MF, JF, KG, SA and ST were the contributors in writing and editing the manuscript. The authors have read and approved this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study, protocol (V5 as of writing) and all instruments, including the informed consent document, have been approved by the Human Research Ethics Committee (HRECV) of the Peter MacCallum Cancer Centre in Melbourne, Australia (HREC reference number: HREC/86559/PMCC; Project number: 22/100). Protocol modifications will be communicated to the reviewing HREC, steering committee and principal investigators.
Consent for publication
This is not applicable since the manuscript does not contain any person’s data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ray, H., Sexton, E., Frowen, J. et al. Study protocol for a single-site feasibility study evaluating the adoption and fidelity of Prep-4-RT: prehabilitation for head and neck cancer patients undergoing radiotherapy. Pilot Feasibility Stud 10, 106 (2024). https://doi.org/10.1186/s40814-024-01531-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-024-01531-2