Abstract
Background
Post-extubation dysphagia (PED) emerges as a frequent complication following endotracheal intubation within the intensive care unit (ICU). PED has been strongly linked to adverse outcomes, including aspiration, pneumonia, malnutrition, heightened mortality rates, and prolonged hospitalization, resulting in escalated healthcare expenditures. Nevertheless, the reported incidence of PED varies substantially across the existing body of literature. Therefore, the principal objective of this review was to provide a comprehensive estimate of PED incidence in ICU patients undergoing orotracheal intubation.
Methods
We searched Embase, PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science, Technology Journal Database (VIP), and SinoMed databases from inception to August 2023. Two reviewers independently screened studies and extracted data. Subsequently, a random-effects model was employed for meta-statistical analysis utilizing the “meta prop” command within Stata SE version 15.0 to ascertain the incidence of PED. In addition, we performed subgroup analyses and meta-regression to elucidate potential sources of heterogeneity among the included studies.
Results
Of 4144 studies, 30 studies were included in this review. The overall pooled incidence of PED was 36% (95% confidence interval [CI] 29–44%). Subgroup analyses unveiled that the pooled incidence of PED, stratified by assessment time (≤ 3 h, 4–6 h, ≤ 24 h, and ≤ 48 h), was as follows: 31.0% (95% CI 8.0–59.0%), 28% (95% CI 22.0–35.0%), 41% (95% CI 33.0–49.0%), and 49.0% (95% CI 34.0–63.0%), respectively. When sample size was 100 < N ≤ 300, the PED incidence was more close to the overall PED incidence. Meta-regression analysis highlighted that sample size, assessment time and mean intubation time constituted the source of heterogeneity among the included studies.
Conclusion
The incidence of PED was high among ICU patients who underwent orotracheal intubation. ICU professionals should raise awareness about PED. In the meantime, it is important to develop guidelines or consensus on the most appropriate PED assessment time and assessment tools to accurately assess the incidence of PED.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Mechanical ventilation is the most common technological support, being required by 20–40% of adult in ICU [1]. Orotracheal intubation is the primary way of mechanical ventilation in ICU, which can increase the risk of post-extubation dysphagia (PED) [2, 3]. PED is any form of swallowing dysfunction that arises subsequent to extubation following endotracheal intubation, affecting the passage of food from the entrance to the stomach. The occurrence rate of PED within the ICU setting demonstrates considerable variation among different countries [4]. The incidence varied among countries, including 13.3–61.8% in the United States [5, 6], 25.3–43.5% in France, and 23.2–56% in China [7, 8], and the incidence ranging from 7 to 80% [9, 10]. Significantly, PED standing out as a prominent complication encountered in this particular context. For instance, See et al. have elucidated that patients afflicted with PED face an 11-fold higher risk of aspiration compared to those without PED [11]. McIntyre et al. have underscored that patients afflicted with PED endure double the length of stay in the ICU and the overall hospitalization period when compared to patients without PED [10]. Furthermore, it is essential to note that PED emerged as an independent predictor of 28-day and 90-day mortality [12]. This high incidence of PED places an immense burden not only on patients but also on the broader healthcare system. Therefore, a systematic review and meta-analysis is necessary to explore the incidence of PED in ICU patients. A systematic review and meta-analysis conducted by McIntyre et al. reported that the incidence of PED was 41%, but the main outcomes of their partly included studies was aspiration [12]. Although aspiration and PED are closely related, not all aspiration is caused by dysphagia. The incidence of aspiration was 8.80%-88.00% in ICU [13, 14], so the incidence of PED in that study may be overestimated. Moreover, there has been increasing literature on PED of ICU patients, and a new systematic review and meta-analysis is needed to obtain a more precise estimate of its incidence.
The incidence of PED may indeed vary depending on various covariates, including assessment time, mean intubation time, age and other relevant factors. First, there is no standard time for swallowing function assessment, which spans a range of intervals, including 3 h [6, 9, 12], 4–6 h [15, 16], 24 h [17,18,19], 48 h [20], 7 days [21], and discharge [22], and the incidence of PED was 80% [9], 22.62% [15], 56.06% [18], and 35.91% [20], 22.06% [21], and 28.78% [22], respectively. Second, the PED is closely tied to the time of orotracheal intubation. Skoretz et al. have demonstrated that the overall incidence of PED in the ICU ranges from 3 to 4%. However, upon re-analysis of patients subjected to orotracheal intubation for more than 48 h, the PED incidence can surge as high as 51% [23]. Third, the choice of assessment tool to evaluate PED in ICU patients plays a pivotal role. These assessment tools may include Video-fluoroscopic Swallowing Study (VFSS), Fiberoptic Endoscopic Evaluation of Swallowing (FEES), Standardized Swallowing Assessment (SSA), Bedside Swallowing Evaluation (BSE), Gugging Swallowing Screen (GUSS), Post-Extubation Dysphagia Screening Tool (PEDS), Water Swallowing Test (WST) and other assessment tools. FEES and VFSS are considered the gold standards, with a detection rate of approximately 80% [9]. SSA and BSE exhibit detection rates of 22% and 62%, respectively [5, 15]. Finally, age-related changes in laryngeal sensory and motor functions also influence PED risk [24]. Notably, there may not be a significant difference in the incidence of PED between elderly and young patients within the initial 48 h post-extubation. However, elderly patients exhibit a significantly slower rate of PED recovery compared to their younger counterparts over time (5.0 days vs 3.0 days; p = 0.006) [5]. Therefore, it is necessary to explore the potential source of heterogeneity in the incidence of PED in ICU patients from such covariates.
The purpose of this study was to estimate the incidence of PED among ICU patients who underwent orotracheal intubation and investigate potential sources of heterogeneity through the application of subgroup analyses and meta-regression.
Methods
This systematic review and meta-analysis was conducted adhering to the guidelines outlined in the Joanna Briggs Institute (JBI) Reviewers’ Manual and followed the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement (PRISMA 2020) [25] (see Additional file 1: Table S1). In addition, it was registered with PROSPERO under the registration number CRD42022373300.
Eligibility criteria
The study’s eligibility criteria were established in accordance with the PICOS principle. Inclusion criteria as follows: population (P): adult patients (≥ 18 years old) admitted to the ICU who underwent orotracheal intubation. Exposure (E): undergoing orotracheal intubation. Outcome (O): PED. Study design (S): observational study (cohort, case–control, cross-sectional study). In studies where multiple articles were derived from the same sample, only the article providing the most detailed data was included. Patients at high risk of dysphagia (such as those with head and neck cancer, who have undergone head and neck surgery, patients receiving palliative care, esophageal dysfunction, stroke, esophageal cancer and Parkinson’s disease) were excluded. Studies were excluded if they exhibited incomplete original data or data that could not be extracted. Studied were also excluded if their sample sizes fell below 30 participants or the full text was inaccessible.
Data sources and search strategy
Our comprehensive search multiple databases, including Embase, PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang, China Science and Technology Journal Database (VIP), and SinoMed, with the search period encompassing inception to August 18, 2023. Search language was Chinese and English. The limited number of studies retrieved initially, primarily attributed to the inclusion of the qualifier “ICU” in the initial search, prompted us to broaden the scope of our literature search. Consequently, we refined the search strategy by reducing the emphasis on “ICU” during the search process. After a series of preliminary searches, we finalized the search strategy, which combined subject headings and free-text terms while employing Boolean operators to enhance search precision. In addition, a manual hand-search of the reference lists of selected articles was carried out to identify any supplementary studies not originally identified through the electronic search. For a detailed presentation of our complete search strategies across all databases, please refer to Additional file 1: Table S2.
Quality evaluation
The evaluation of the risk of bias within the included studies was conducted by two trained investigators. Cross-sectional study was evaluated by the Agency for Healthcare Research and Quality (AHRQ) tool [26], which consisted of 11 items, resulting in a maximum score of 11. Scores falling within the ranges of 0–3, 4–7, and 8–11 corresponded to studies of poor, moderate, and high quality, respectively. Cohort study was evaluated by the Newcastle–Ottawa Scale (NOS) tool [27], which comprised three dimensions and eight items, allowing for a star rating ranging from 2 to 9 stars. In this case, 0–4, 5–6, and 7–9 stars were indicative of study of poor, moderate, and high quality, respectively. Any discrepancies or disagreements between the investigators were resolved through discussion, when necessary, consultation with a third expert specializing in evidence-based practice methodology.
Study selection and data extraction
Bibliographic records were systematically exported into the NoteExpress database to facilitate the screening process and the removal of duplicate citations. Initial screening, based on titles and abstracts, was conducted by two reviewers who possessed specialized training in evidence-based knowledge. To ascertain whether the studies satisfied the predefined inclusion and exclusion criteria, the full texts of potentially relevant articles were acquired. In the event of disagreements between the two reviewers, resolution was achieved through discussion or, when necessary, by enlisting the input of a third reviewer for arbitration.
After confirming the included studies, the two authors independently extracted data from the each paper, including the first author, year of publication, country, study design, ICU type, mean patient age, mean intubation time, assessment time, assessment tool, evaluator, sample size, and the PED event. Any disparities during the process of extracted data were addressed through thorough discussion and consensus-building among the reviewers.
Outcomes
The outcomes of this review were as follows: (1) incidence of PED in patients with orotracheal intubation in the ICU; (2) sources of heterogeneity of PED in patients with orotracheal intubation in ICU.
Statistical analyses
Meta-analysis was conducted using the ‘meta prop’ function from the meta package within STATA/SE (version 15.0, StataCorp, TX, USA). To approximate the normal distribution of the data, incidence estimates were transformed using the “Freeman-Tukey Double Arcsine Transformation”. Heterogeneity was assessed using the I2 statistic, and pooled analyses of PED were executed employing a random-effects model in the presence of significant heterogeneity (I2 ≥ 50%), with fixed-effects models utilized when heterogeneity was non-significant. A significance level of P < 0.05 was established for all analyses.
Subgroup analyses were undertaken to investigate the potential impact of various factors, including assessment tool (gold standard, SSA, GUSS, BSE, PEDS, WST, and other assessment tools), year of publication (2000–2010, 2011–2015, 2016–2020, 2021–2023), study design (cross-sectional study and cohort study), study quality (moderate quality and high quality), assessment time (≤ 3 h, 4–6 h, ≤ 24 h, ≤ 48 h, and after 48 h post-extubation), mean intubation time (≤ 24 h, 48 – 168 h, and > 168 h), mean patient age (≤ 44 years, 45–59 years, 60–74 years), evaluator (nurses, speech-language pathologist), ICU type (Trauma ICU, Cardiac surgery ICU, Mixed medical and surgical ICU), and sample size (N ≤ 100, 100 < N ≤ 200, 200 < N ≤ 300, N > 300) on the pooled estimate. In instances where no source of heterogeneity was identified in the subgroup analyse, we conducted meta-regression to further pinpoint the origins of heterogeneity, focusing on assessment time, mean intubation time, mean age, assessment tool, sample size, evaluator, ICU type, study design, study quality and year of publication. Sensitivity analysis by the “leave-one-out method” was employed to evaluate the random-effects model’s stability of the pooled incidence of PED. Publication bias was assessed by funnel plot and “Trim and Full” method.
Certainty of the evidence
The level of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) [28]. This tool classifies the certainty of evidence into four levels: very low, low, moderate, and high. “High quality” suggests that the actual effect is approximate to the estimate of the effect. On the other hand, “Very low quality” suggests that there is very little confidence in the effect estimate and the reported estimate may be substantially different from what was measured. Two reviewers judged the following aspects: risk of bias, inconsistency, imprecision, indirect evidence, and publication bias. Disagreements were resolved by consensus with the third reviewer.
Results
Study selection
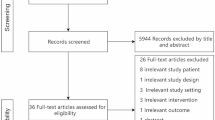
Out of the 4144 studies initially identified, 1280 duplicate studies were removed, and an additional 2864 studies that were deemed irrelevant were excluded based on title and abstract screening. Subsequently, a thorough examination of the full text was conducted for the remaining 122 studies. A manual hand-search of the reference lists of selected articles was 5 studies. Finally, 30 studies were chosen as they met the predetermined inclusion criteria for this systematic review and meta-analysis. The study selection flowchart is shown in Fig. 1.
General characteristics of the included studies
The characteristics of the included studies are shown in Table 1. The total sample size across these studies amounted to 6,228 participants. The earliest study in this review was conducted in 2003 [29], while the most recent study was conducted in 2023 [15], with 14 studies published after 2020. The study with the largest sample size was conducted by Schefold et al. [12], comprising 933 participants, while the study with the smallest sample size was carried out by Yılmaz et al. [19], including 40 participants. The methods employed to assess the incidence of PED exhibited variability among the studies. Specifically, one study employed VFSS [30], and four studies relied on FEES [9, 29, 31, 32], and seven studies utilized SSA assessment tools [7, 15, 16, 33,34,35,36]. Furthermore, six studies utilized BSE [5, 10, 17, 37,38,39], two studies employed WST [12, 40], two studies adopted PEDS [8, 18], two studies utilized GUSS [19, 41], and six studies employed other assessment tools [6, 20, 21, 22,43,, 42, 43] such as ASHA, FOIS, SSQ200, NPS-PED, MASA, and YSP.
Among all the studies, 23 studies recorded the assessment time for PED. Specifically, three studies assessed PED within ≤ 3 h post-extubation [6, 9, 12], four studies conducted assessments at 4–6 h post-extubation [15, 16, 33, 36], nine studies assessed PED within ≤ 24 h post-extubation [7, 8, 17,18,19, 31, 34, 40, 41], three studies assessed PED within ≤ 48 h post-extubation [5, 20, 37], and four studies evaluated PED at > 24 h post-extubation [21, 22, 29, 38]. In terms of study quality, eight of the included studies were categorized as high quality, while the remainder were deemed of moderate quality (see Additional 1: Tables S3, S4).
Meta-analysis results
Utilizing the random-effects model, the pooled incidence of PED was estimated to be 36% (95% CI 29.0%–44.0%, I2 = 97.06%, p < 0.001; Fig. 2), indicating a substantial degree of heterogeneity. Despite conducting additional subgroup analyses, the source of this high heterogeneity remained elusive. However, the results of the meta-regression analysis revealed that sample size (p < 0.001), assessment time (p = 0.027) and mean intubation time (p = 0.045) emerged as the significant factor contributing to the heterogeneity.
Subgroup analysis of incidence
The subgroup analyses yielded the following incidence rates of PED based on assessment time post-extubation: the incidence of PED within 3 h post-extubation was 31% (95% CI 8.0–59.0), 4–6 h was 28% (95% CI 22.0–35.0, I2 = 78.56%, p < 0.001), within 24 h was 41% (95% CI 33.0–49.0, I2 = 88.99%, p < 0.001), and within 48 h was 49%. In addition, the incidence of PED beyond 24 h post-extubation was 37% (95% CI 23.0–52.0, I2 = 91.73%, p < 0.001) (Additional file 1: Fig. S1). Furthermore, when analyzing studies based on sample size (N), the overall incidence of PED was found 51% (95% CI 39.0–63.0, I2 = 87.11%, p < 0.001) for studies with N < 100 participants, 37% (95% CI 31.0–43.0, I2 = 84.74%, p < 0.001) for studies with 100 < N ≤ 200 participants, 32% (95% CI 20.0–46.0, I2 = 97.16%, p < 0.001) for studies with 200 < N ≤ 300 participants, and 16% (95% CI 8.0–26.0, I2 = 97.07%, p < 0.001) for studies with N > 300 participants (see Additional file 1: Fig. S2). In addition, further analyses were conducted based on assessment tool, mean intubation time, mean age, ICU type, evaluator, publication year, study design and study quality (see Additional file 1: Figs. S3–S11).
Results of meta-regression analysis
In the meta-regression analysis, we examined PED assessment time, sample size, assessment tools, mean intubation time, mean age, ICU type, evaluator, publication year, study design and study quality as potential covariates to identify the source of heterogeneity (Table 2). The univariate meta-regression analysis revealed a statistically significant correlation between incidence and sample size, assessment time and mean intubation time. Bubble plots of meta-regression of covariates were shown in Additional (see Additional file 1: Figs. S12–S22).
Sensitivity analysis
Sensitivity analysis showed that the incidence of PED ranged from 29 to 44% (see Additional file 1: Fig. S23). The marginal variance between these results and the pooled incidence was minimal, suggesting that the result of the pooled incidence being stable and reliable.
Publication bias
In our study, publication bias was detected by the funnel plot (see Additional file 1: Fig. S24). We found that the adjusted effect size was similar to the original effect size (p < 0.01) (see Additional file 1: Fig. S25).
Certainty of the evidence
The certainty of evidence was very low for all comparisons performed according to the GRADE rating [28]. Thus, it can be considered that the certainty of the evidence regarding the incidence of PED in this review is very low (Table 3).
Discussion
This systematic review and meta-analysis aimed to estimate the incidence of PED in ICU patients. The study revealed an overall incidence of PED in ICU patients who underwent orotracheal intubation to be 36.0%. This incidence rate was comparable to the incidence of dysphagia resulting from stroke (36.30%) [45] and aligned with the incidence of PED observed in ICU patients (36%) [46]. However, it was slightly lower than the 41% reported in the meta-analysis conducted by McIntyre et al. [4]. The incidence of PED among ICU patients who underwent orotracheal intubation was high, ICU medical professionals, especially nurses should raise awareness about PED. However, the included studies were characterized by diversity and heterogeneity in assessment time and assessment tools signaled the need for obtaining consensus on a range of issues, including assessment time and assessment tools appropriate for ICU.
Sample size
This review identified sample size as a significant source of heterogeneity (p < 0.001). Notably, the incidence of PED demonstrated a gradual decrease as the sample size of the studies increased. In larger scale studies, such as those conducted by McIntyre et al. and Schefold et al., simpler assessment tools are employed, allowing for quick completion [10, 12]. However, the reliability and validity of some of these tools remain unverified. Conversely, certain studies are conducted by highly trained professionals using the gold standard for PED assessment [9, 29, 31], which, while more accurate, is also time-consuming and costly [47]. In addition, some ICU patients, due to their unstable conditions, are unable to complete the gold standard assessment, resulting in relatively smaller sample sizes for these studies.
In statistics, sample size is intricately linked to result stability, and the confidence intervals for subgroups with N < 100 in this study exhibited a wider range, this might diminish the result precision and lead to larger deviations from the true value. However, as the sample size increased to 100 < N ≤ 300, the confidence intervals narrowed in comparison to other subgroups. Consequently, when sample size was 100 < N ≤ 300, the PED incidence rates were more close with the overall PED rate. According to the central limit theorem, if the sampling method remains consistent, results obtained from larger samples are more stable and closer to the true value [48, 49]. It is worth noting that the confidence intervals for the subgroup with N > 300 in this study were wider and demonstrated a larger divergence from the total PED incidence. Therefore, in future studies, careful consideration of the sample size, based on the detection rate of the assessment tool used, is advisable to ensure both the stability and reliability of the results.
Mean intubation time
This review identified mean intubation time as a significant source of heterogeneity (p = 0.045). Variances in mean intubation time among ICU patients undergoing orotracheal intubation can lead to differing degrees of mucosal damage in the oropharynx and larynx [2, 50], thereby resulting in varying incidence rates of PED. For instance, Malandraki et al. have reported that prolonged intubation is associated with more than a 12-fold increased risk of moderate/severe dysphagia compared to shorter intubation durations, and this effect is particularly pronounced among elderly patients [51]. Moreover, studies have demonstrated that ICU patients with extended orotracheal intubation periods leading to PED also exhibit diminished tongue and lip strength, protracted oral food transportation, slower swallowing, and muscle weakness in swallowing-related muscles [24, 46]. In view of these findings, ICU medical professionals should routinely evaluate the need for orotracheal intubation, strive to minimize the duration of mechanical ventilation.
PED assessment time
This review identified assessment time as a significant source of heterogeneity (P = 0.027). It is important to note that there are currently no established guidelines recommending the optimal timing for the initial assessment of PED in ICU patients who have undergone orotracheal intubation. Consequently, the assessment time varies widely across studies, resulting in PED incidence rates ranging from 28 to 49% among subgroups. Interestingly, the incidence of PED assessed within ≤ 3 h post-extubation appeared lower than that assessed within ≤ 24 h and ≤ 48 h post-extubation. This difference may be attributed to the study by Schefold et al., which featured a shorter intubation duration [12]. Therefore, the incidence of PED assessed within ≤ 3 h post-extubation in ICU patients with orotracheal intubation may be underestimated. Moreover, it is essential to highlight that some ICU patients, particularly those with severe illnesses and extended intubation time, may face challenges in complying with post-extubation instructions provided by healthcare personnel. Paradoxically, this group of patients is at a higher risk of developing PED, subsequently increasing their susceptibility to post-extubation pneumonia [11]. ICU professionals should evaluate swallowing function in patients post-extubation; early identification of patients at risk for PED to reduce complications. If PED is identified, nurses should follow-up assessments at multiple time to obtain a thorough comprehension of PED recovery trajectory among PED patients, which can serve as a foundation for determining the timing of clinical interventions accurately.
PED assessment tools
Despite the subgroup analyses and meta-regression results indicating that PED assessment tools did not contribute to the observed heterogeneity, it is important to acknowledge the wide array of assessment tools employed across the studies included in this review. The study’s findings revealed that the results of the GUSS and BSE assessments were most closely aligned with the gold standard screening results. In contrast, the PEDS assessment results tended to be higher than those derived from the gold standard assessment. Furthermore, the results of other assessment tools generally yielded lower incidence rates of PED, possibly attributable to variations in specificity or sensitivity. FEES and VFSS assessments are recognized for their meticulous scrutiny of patients’ swallowing processes, including the detection of food residue and aspiration, which may not be as comprehensively addressed by other assessment methods [51]. Assessment tools such as BSE, SSA, GUSS, WST, and other clinical methods do not provide direct visualization of the swallowing process. Instead, assessors rely on the observation of overt clinical symptoms during the patient’s initial food or water intake to judge the presence of PED. However, these methods may overlook occult aspiration in patients, potentially resulting in an underestimation of PED incidence. In contrast, PEDS, which primarily assesses patients based on their medical history and plumbing symptoms without screening for drinking or swallowing, may overestimate PED incidence. Considering the varying strengths and limitations of existing assessment tools, ICU professionals select appropriate PED assessment tool based on the characteristics of the critically ill patient. Early and rapid identification of PED, before the use of more complex and expensive assessment tools, minimizes the occurrence of complications in patients.
Strengths and weaknesses
In this study, we conducted a comprehensive analysis of the incidence of PED in ICU patients who underwent orotracheal intubation across various subgroups, revealing a notable degree of heterogeneity among the included studies. In our study, we have expanded the search as much as possible and included a total of 30 papers after screening, half of which were published after 2020. There are several limitations that should be considered when interpreting the results of this meta-analysis. First, there was varied heterogeneity between methodological of the study and estimates of prevalence that may question the appropriateness of calculating pooled prevalence estimates. However, in order to address this heterogeneity, we addressed the heterogeneity with applying a random-effect model and conducting subgroup analysis and meta-regression to explore three sources of heterogeneity. Second, the overall quality of evidence for the incidence of PED was rated as low according to GRADE. Higher quality original studies on the incidence of PED should be performed in the future. As a result, the findings should be interpreted with caution in such cases.
Conclusion
In conclusion, our systematic review and meta-analysis revealed a high incidence of PED among ICU patients who underwent orotracheal intubation. It is also worth noting that the incidence of PED in the ICU may be underestimated. It is expected to increase awareness about the issue of PED among ICU patients. It will be important to develop guidelines or consensus on the most appropriate PED assessment time and assessment tools to accurately assess the incidence of PED.
Relevance to clinical practice
Each year, a substantial number of critically ill patients, ranging from 13 to 20 million, necessitate endotracheal intubation to sustain their lives. Patients undergoing orotracheal intubation are at heightened risk of developing PED. PED has been linked to prolonged hospital and ICU length of stay, increased rates of pneumonia, and all-cause mortality. Early identification of high-risk patients by clinical nurses is critical for reduce patient burden and adverse outcomes.
-
Early and multiple times assessment: Future investigations should early assess PED in clinical practice, especially within 6 h post-extubation. Furthermore, we suggest for follow-up assessments at multiple time to obtain a thorough comprehension of PED incidence and the recovery trajectory among ICU patients who have undergone orotracheal intubation.
-
Assessment tool: Considering the varying strengths and limitations of existing assessment tools, ICU professionals should carefully evaluate the characteristics of critically ill patients and select appropriate assessment tools, before the use of more complex and expensive assessment tools.
-
Routinely evaluate the need for orotracheal intubation: Healthcare professionals should routinely evaluate the need for orotracheal intubation, strive to minimize the duration of mechanical ventilation.
Availability of data and materials
All data related to the present systematic review and meta-analysis are available from the original study corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- ICU:
-
Intensive care unit
- PED:
-
Post-extubation dysphagia
- SSQ200:
-
Sydney Swallow Questionnaire 200
- WST:
-
Water swallowing test
- PEDS:
-
Post-Extubation Dysphagia Screening Tool
- BSE:
-
Bedside swallow evaluation
- YSP:
-
The Yale swallow protocol
- MASA:
-
Mann Assessment of Swallowing Ability
- ASHA:
-
American Speech-Language-Hearing Association
- VFSS:
-
Video Fluoroscopic Swallowing Study
- FEES:
-
Fiberoptic endoscopic evaluation of swallowing
- GUSS:
-
Gugging swallowing screen
- SSA:
-
Standardized Swallowing Assessment
- FOIS:
-
Functional Oral Intake Scale
- NPS-PED:
-
Nurse-performed screening for post-extubation dysphagia
- SLP:
-
Speech-language pathologists
- N :
-
Sample size
- n :
-
Events of PED
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
References
Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013;41(12):2712–9.
Brodsky MB, Akst LM, Jedlanek E, Pandian V, Blackford B, Price C, Cole G, Mendez-Tellez PA, Hillel AT, Best SR, et al. Laryngeal injury and upper airway symptoms after endotracheal intubation during surgery: a systematic review and meta-analysis. Anesth Analg. 2021;132(4):1023–32.
Brodsky MB, Chilukuri K, De I, Huang M, Needham DM. Coordination of pharyngeal and laryngeal swallowing events during single liquid swallows after oral endotracheal intubation. Am J Respir Crit Care Med. 2017;195:768–77.
McIntyre M, Doeltgen S, Dalton N, Koppa M, Chimunda T. Post-extubation dysphagia incidence in critically ill patients: a systematic review and meta-analysis. Aust Crit Care. 2021;34(1):67–75.
Tsai MH, Ku SC, Wang TG, Hsiao TY, Lee JJ, Chan DC, Huang GH, Chen C. Swallowing dysfunction following endotracheal intubation age matters. Medicine. 2016;95(24):e3871.
Leder SB, Warner HL, Suiter DM, Young NO, Bhattacharya B, Siner JM, Davis KA, Maerz LL, Rosenbaum SH, Marshall PS, et al. Evaluation of swallow function post-extubation: is it necessary to wait 24 hours? Ann Otol Rhinol Laryngol. 2019;128(7):619–24.
Zeng L, Song Y, Dong Y, Wu Q, Zhang L, Yu L, Gao L, Shi Y. Risk score for predicting dysphagia in patients after neurosurgery: a prospective observational trial. Front Neurol. 2021;12:605687.
Dan L, Yunfang C, Chengfen Y, Li T. Reliability and validity of the Chinese version of postextubation dysphagia screening tool for patients with mechanical ventilation. Tianjin J Nurs. 2022;30(2):161–5.
Troll C, Trapl-Grundschober M, Teuschl Y, Cerrito A, Compte MG, Siegemund M. A bedside swallowing screen for the identification of post-extubation dysphagia on the intensive care unit—validation of the Gugging Swallowing Screen (GUSS)—ICU. BMC Anesthesiol. 2023;23(1):122.
McInytre M, Doeltgen S, Shao C, Chimunda T. The incidence and clinical outcomes of postextubation dysphagia in a regional critical care setting. Aust Crit Care. 2022;35(2):107–12.
See KC, Peng SY, Phua J, Sum CL, Concepcion J. Nurse-performed screening for postextubation dysphagia: a retrospective cohort study in critically ill medical patients. Crit Care. 2016;20(1):326.
Schefold JC, Berger D, Zurcher P, Lensch M, Perren A, Jakob SM, Parviainen I, Takala J. Dysphagia in mechanically ventilated ICU patients (DYnAMICS): a prospective observational trial. Crit Care Med. 2017;45(12):2061–9.
Byun SE, Shon HC, Kim JW, Kim HK, Sim Y. Risk factors and prognostic implications of aspiration pneumonia in older hip fracture patients: a multicenter retrospective analysis. Geriatr Gerontol Int. 2019;19(2):119–23.
Jaillette E, Martin-Loeches I, Artigas A, Nseir S. Optimal care and design of the tracheal cuff in the critically ill patient. Ann Intensive Care. 2014;4(1):7.
Tang JY, Feng XQ, Huang XX, Zhang YP, Guo ZT, Chen L, Chen HT, Ying XX. Development and validation of a predictive model for patients with post-extubation dysphagia. World J Emerg Med. 2023;14(1):49–55.
Xia C, Ji J. The characteristics and predicators of post-extubation dysphagia in ICU patients with endotracheal intubation. Dysphagia. 2022;38:253.
Beduneau G, Souday V, Richard JC, Hamel JF, Carpentier D, Chretien JM, Bouchetemble P, Laccoureye L, Astier A, Tanguy V, et al. Persistent swallowing disorders after extubation in mechanically ventilated patients in ICU: a two-center prospective study. Ann Intensive Care. 2020;10(1):1–7.
Johnson KL, Speirs L, Mitchell A, Przybyl H, Anderson D, Manos B, Schaenzer AT, Winchester K. Validation of a postextubation dysphagia screening tool for patients after prolonged endotracheal intubation. Am J Crit Care. 2018;27(2):89–96.
Yılmaz D, Mengi T, Sarı S. Post-extubation dysphagia and COVID-2019. Turkish J Neurol. 2021;27:21–5.
Oliveira A, Friche A, Salomão MS, Bougo GC, Vicente L. Predictive factors for oropharyngeal dysphagia after prolonged orotracheal intubation. Brazil J Otorhinolaryngol. 2018;84(6):722–8.
Yamada T, Ochiai R, Kotake Y. Changes in maximum tongue pressure and postoperative dysphagia in mechanically ventilated patients after cardiovascular surgery. Indian J Crit Care Med. 2022;26(12):1253–8.
Brodsky MB, Huang M, Shanholtz C, Mendez-Tellez PA, Palmer JB, Colantuoni E, Needham DM. Recovery from dysphagia symptoms after oral endotracheal intubation in acute respiratory distress syndrome survivors. A 5-year longitudinal study. Ann Am Thorac Soc. 2017;14(3):376–83.
Skoretz SA, Yau TM, Ivanov J, Granton JT, Martino R. Dysphagia and associated risk factors following extubation in cardiovascular surgical patients. Dysphagia. 2014;29(6):647–54.
Park HS, Koo JH, Song SH. Association of post-extubation dysphagia with tongue weakness and somatosensory disturbance in non-neurologic critically ill patients. Ann Rehabil Med Arm. 2017;41(6):961–8.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74(9):790–9.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ Br Med J. 2011;343: d5928.
Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ-Br Med J. 2008;336(7650):924–6.
El SA, Okada M, Bhat A, Pietrantoni C. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med. 2003;29(9):1451–5.
Yang WJ, Park E, Min YS, Huh JW, Kim AR, Oh HM, Nam TW, Jung TD. Association between clinical risk factors and severity of dysphagia after extubation based on a videofluoroscopic swallowing study. Korean J Intern Med. 2020;35(1):79.
Megarbane B, Hong TB, Kania R, Herman P, Baud FJ. Early laryngeal injury and complications because of endotracheal intubation in acutely poisoned patients: a prospective observational study. Clin Toxicol. 2010;48(4):331–6.
Scheel R, Pisegna JM, McNally E, Noordzij JP, Langmore SE. Endoscopic assessment of swallowing after prolonged intubation in the ICU setting. Ann Otol Rhinol Laryngol. 2016;125(1):43–52.
Fan GUO, Mingming WANG, Shengqiang ZOU. Analysis of risk factors and establishment of prediction model for post-extubation swallowing dysfunction in ICU patients with endotracheal intubation. Chin Nurs Res. 2020;34(19):3424–8.
Yaqian W: Localization and evaluation of reliability and validity of GuSS-ICU bedside swallowing screening tool. Master: Huzhou University; 2020.
Yun D, Yuan Z, Yanli Y. Risk factors and nursing strategies of the occurrences of acquired swallowing disorders after ICU patients treated with oral tracheal intubation and extubation. Med Equip. 2021;34(1):20–2.
JinTian Y. Study on the recovery of swallowing function and the real experience of patients with acquired swallowing disorder after cardiac surgery. Master: Nanjing University; 2020.
de Medeiros GC, Sassi FC, Mangilli LD, Zilberstein B, de Andrade C. Clinical dysphagia risk predictors after prolonged orotracheal intubation. Clinics. 2014;69(1):8–14.
Kwok AM, Davis JW, Cagle KM, Sue LP, Kaups KL. Post-extubation dysphagia in trauma patients: it’s hard to swallow. Am J Surg. 2013;206(6):924–7 (927–928).
Barker J, Martino R, Reichardt B, Hickey EJ, Ralph-Edwards A. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can J Surg. 2009;52(2):119–24.
Bordon A, Bokhari R, Sperry J, Testa D, Feinstein A, Ghaemmaghami V. Swallowing dysfunction after prolonged intubation: analysis of risk factors in trauma patients. Am J Surg. 2011;202(6):679–82.
Limin Z. The application of gugging swallowing screenin post-extubation swallowing dysfunction assessment after long-term intubation. Master. Tianjin Medical University; 2016.
Omura K, Komine A, Yanagigawa M, Chiba N, Osada M. Frequency and outcome of post-extubation dysphagia using nurse-performed swallowing screening protocol. Nurs Crit Care. 2019;24(2):70–5.
Regala M, Marvin S, Ehlenbach WJ. Association between postextubation dysphagia and long-term mortality among critically ill older adults. J Am Geriatr Soc. 2019;67(9):1895–901.
Meng PP, Zhang SC, Han C, Wang Q, Bai GT, Yue SW. The occurrence rate of swallowing disorders after stroke patients in Asia: a PRISMA-compliant systematic review and meta-analysis. J Stroke Cerebrovasc Dis Off J Nat Stroke Assoc. 2020;29(10): 105113.
Yingli H, Mengxin C, Donglei S. Incidence and influencing factors of post-extubation dysphagia among patients with mechanical ventilation: a meta-analysis. Chin J Modern Nurs. 2019;25(17):2158–63.
Spronk PE, Spronk LEJ, Egerod I, McGaughey J, McRae J, Rose L, Brodsky MB, Brodsky MB, Rose L, Lut J, et al. Dysphagia in intensive care evaluation (DICE): an international cross-sectional survey. Dysphagia. 2022;37(6):1451–60.
Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14–7.
Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press J Orthod. 2014;19(4):27–9.
Zuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care. 2019;23(1):103.
Malandraki GA, Markaki V, Georgopoulos VC, Psychogios L, Nanas S. Postextubation dysphagia in critical patients: a first report from the largest step-down intensive care unit in Greece. Am J Speech Lang Pathol. 2016;25(2):150–6.
Ambika RS, Datta B, Manjula BV, Warawantkar UV, Thomas AM. Fiberoptic endoscopic evaluation of swallow (FEES) in intensive care unit patients post extubation. Indian J Otolaryngol Head Neck Surg. 2019;71(2):266–70.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Weixia Yu, Limi Dan, Jianzheng Cai, and Yuyu Wang developed the original concept of this systematic review and meta-analysis. Weixia Yu, Limi Dan, Jianzheng Cai and Yuyu Wang contributed to the screening of eligible studies, data extraction, and data synthesis. Weixia Yu, Limi Dan, Jianzheng Cai, Yuyu Wang and Qingling Wang drafted the first version of the manuscript. Yingying Zhang, Qingling Wang and Xin Wang prepared the tables and figures. All the authors have edited and contributed for intellectual content. All the authors read and approved the final manuscript and take public responsibility for it.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
40001_2024_2024_MOESM1_ESM.docx
Additional file 1: Table S1. PRISMA 2020 Checklist. Table S2. Search strategy. Table S3. Quality evaluation results of the cohort studies. Table S4. Quality evaluation results of the cross-sectional study. Fig. S1. Subgroup analysis of the incidence of PED by assessment time. Fig. S2. Subgroup analysis of the incidence of PED by sample size. Fig. S3. Incidence of PED by assessment tool. Fig. S4. Incidence of PED by mean intubation time. Fig. S5 Incidence of PED by mean age. Fig. S6. Incidence of PED by ICU type. Fig. S7. Incidence of PED by evaluator. Fig. S8. Incidence of PED by year of publication. Fig. S9. Incidence of PED by study design. Fig. S10. Incidence of PED by quality of cohort study. Fig. S11. Incidence of PED by quality of Cross-sectional study. Fig. S12. Bubble plot of meta-regression result for evaluate time as a covariate. Fig. S13. Bubble plot of meta-regression result for sample size as a covariate. Fig. S14. Bubble plot of meta-regression result for assessment tool as a covariate. Fig. S15. Bubble plot of meta-regression result for mean intubation time as a covariate. Fig. S16. Bubble plot of meta-regression result for mean age as a covariate. Fig. S17. Bubble plot of meta-regression result for ICU type as a covariate. Fig. S18. Bubble plot of meta-regression result for evaluator as a covariate. Fig. S19. Bubble plot of meta-regression result for year of publication as a covariate. Fig. S20. Bubble plot of meta-regression result for study design as a covariate. Fig. S21. Bubble plot of meta-regression result for quality of cohort study as a covariate. Fig. S22. Bubble plot of meta-regression result for quality of cross-sectional study as a covariate. Fig. S23. Sensitivity analysis of PED. Fig. S24. Publication bias assessment plot. Fig. S25. Publication bias assessment plot. “Trim and Full test” method.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, W., Dan, L., Cai, J. et al. Incidence of post-extubation dysphagia among critical care patients undergoing orotracheal intubation: a systematic review and meta-analysis. Eur J Med Res 29, 444 (2024). https://doi.org/10.1186/s40001-024-02024-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-02024-x