Abstract
Background
Colistin is regarded as a last-resort antimicrobial against multi-drug resistant Gram-negative bacteria (GNB), therefore the dissemination of colistin resistance in the environment is of great concern. Horizontal transfer of mobile colistin resistance (mcr) genes to potential pathogens poses a serious problem. This study aimed to describe the presence of colistin resistant GNB and mcr genes in river and storm water in regions of the Western Cape.
Methods
Water samples were collected from three rivers during May 2019 and January 2020 and two storm water samples were collected in November 2019. Colistin resistant GNB were cultured on MacConkey agar containing colistin and identified by MALDI-TOF. Colistin resistance was confirmed using broth microdilution (BMD). mcr-1-5 genes were detected by PCR performed directly on the water samples and on the colistin resistant isolates. mcr functionality was assessed by BMD after cloning the mcr genes into pET-48b(+) and expression in SHuffle T7 E. coli.
Results
mcr-5.1 and various mcr-3 gene variants were detected in the Plankenburg-, Eerste- and Berg rivers and in storm water from Muizenberg, and only mcr-5.1 was detected in storm water from Fish Hoek. Colistin resistant GNB were isolated from all of the water sources. Aeromonas spp. were the most common colistin resistant organisms detected in the water sources; 25% (6/24) of colistin resistant Aeromonas spp. isolated from the Berg river contained novel mcr-3 variants; mcr-3.33 (n = 1), mcr-3.34 (n = 1) mcr-3.35 (n = 1) mcr-3.36 (n = 2) and mcr-3.37 (n = 1), which were confirmed to confer colistin resistance.
Conclusions
The mcr-5.1 and mcr-3 colistin resistance gene variants were present in widely dispersed water sources in regions of the Western Cape. The mcr genes were only detected in water sampled downstream of and alongside communities, suggesting that their presence is driven by human influence/contamination. This is the first documentation of mcr-3 and mcr-5 gene variants in any setting in South Africa. Spill-over of these genes to communities could result in horizontal gene transfer to pathogenic bacteria, exacerbating the challenge of controlling multidrug resistant GNB infections.
Similar content being viewed by others
Introduction
The emergence and spread of mobile colistin resistance (mcr) genes threaten the efficacy of colistin, a last resort antibiotic for treating infections caused by multidrug-resistant Gram-negative bacteria (GNB). Colistin resistance can also arise from chromosomal mutations, including those in genes encoding the two-component systems PmrAB and PhoPQ in Enterobacterales. However, in contrast to the mcr genes, these are not horizontally transferable.
Ten mcr genes, mcr-1 (30 variants), mcr-2 (7 variants), mcr-3 (40 variants), mcr-4 (6 variants), mcr-5 (4 variants), mcr-6, mcr-7, mcr-8 (3 variants), mcr-9 (3 variants) and mcr-10 [1,2,3,4,5,6,7,8,9,10], have been described in various GNB, including Acinetobacter spp., Aeromonas spp., Citrobacter spp., Enterobacter spp., Escherichia coli, Klebsiella spp., Kluyvera spp., Proteus spp., Providencia spp., Pseudomonas spp., Raoultella spp., and Salmonella spp., isolates [11]. However, some of these mcr variants do not cause colistin resistance [12, 13].
The mcr genes are harboured by different conjugative and non-conjugative plasmids and have been found to be integrated into the chromosome in some isolates [14]. In addition to clinical isolates, mcr genes have been detected in various isolates from environmental sources including water, soil, livestock, vegetables, flies, wildlife, companion animals and birds [1, 2, 15,16,17,18,19,20,21,22,23,24].
The mcr-1 gene has been identified in clinical isolates in multiple hospitals across South Africa, [19, 25, 26] however data regarding the distribution of the mcr genes in the environment in South Africa is limited. mcr-1 has been detected in E. coli isolated from broiler chickens, a pig and final effluents from wastewater treatment plants in South Africa [27,28,29,30]. The presence of mcr genes in the environment could be a reservoir for colistin resistance in clinical settings.
This study investigated the presence of colistin resistant GNB and mcr genes in samples from three surface water sources, the Plankenburg-, Eerste- and Berg rivers, as well as storm water from Muizenberg and Fish Hoek in the Western Cape province, South Africa, and characterised the role of the mcr variants in colistin resistance. Informal housing schemes are situated on the banks of these rivers, which are highly polluted by sewage, industrial and agricultural run-off [31], while storm water systems are often used for the illegal disposal of human waste. As such, the presence of colistin resistance mechanisms in these water sources provides insight into the dissemination of colistin resistance in communities and poses a risk for further dissemination of resistance genes.
Methods
Isolate collection
The Plankenburg, Eerste and Berg rivers and tributaries were sampled between May 2019 and January 2020 (Fig. 1). Sample sites P1 in the Plankenburg river and E1 in the Eerste river are situated above two separate Stellenbosch communities, while sample sites P2 and E2 are situated below the respective communities. Upstream and downstream community samples were sampled on the same day, approximately 1 h apart. The samples were taken at 30 cm depth, except if the water depth was less than 30 cm, the samples were taken at the halfway point between the water surface and the bottom of the water body. Special care was taken to open the sample bottle only at the correct sample depth to avoid capturing floating contamination. Rivers were only sampled once, except the Plankenburg river which was sampled twice, in May 2019 (P2a) and January 2020 (P2b). No upstream sample was obtained in January 2020 as the river was dry upstream of the community. We thus also used the 2019 samples in this study, as they had been collected both upstream and downstream. Sampling sites B1, B2 and B3 in the Berg river are situated in three different communities: Paarl (Boulevard), Paarl (Mbekweni) and Wellington, respectively. Storm water from Muizenberg (M) and Fish Hoek (FH) was sampled in November 2019 (Fig. 1 and Additional file 1: Table 1). Sampling sites were chosen based on representativity and accessibility. Sampling took place during dry weather conditions in order to get an accurate representation of the water in an undiluted state. The water temperature, turbidity, river flow speed and other visible problems were documented in conjunction with recent weather patterns and are included in Additional file 1: Table 1. Water was collected in sterile bottles, kept on ice during transport and subsequently refrigerated at 4 °C.
Overview of the sampling region in the Western Cape of South Africa. Sampling sites were situated in the Plankenburg river (P1-before a community and P2-after a community) and Eerste river (E1-before a community and E2-after a community) in Stellenbosch and three sampling sites (B1–B3) from three communities from the Berg river in Paarl and Wellington and two storm water sampling sites (M and FH) in Muizenberg and Fish Hoek respectively
Bacterial enumeration and culture-based screening
E. coli and total coliform counts were performed on the water samples using the Colilert-18 water test as per manufacturer’s instructions (IDEXX, South Africa). Colilert-18 is a semi-automated quantitative method which uses the metabolism of nutrient indicators o-nitrophenyl (ONPG) and 4-methyl-umbelliferyl (MUG) to detect coliforms and E. coli, respectively, using colorimetric changes and fluorescence. The South African national guidelines for water quality guidelines for full recreational contact, indicates a threshold of > 130 cfu/100 ml of definite bacterial growth in the water system as a significant and increased risk of infectious disease transmission [32].
Serial dilutions (10−1–10−6) of each water sample were plated out (0.1 ml) in triplicate on MacConkey agar (MCC, Sigma-Aldrich, South Africa) and MacConkey agar containing 10 mg/L vancomycin and 2 mg/L colistin (Mac-Col2-Van10, Sigma-Aldrich), using the spread plate method. Vancomycin was included to eliminate the growth of most Gram-positive bacteria and colistin was used to select for colistin resistant organisms. Plates were incubated aerobically at 37 °C for 18–22 h. Colonies were also enumerated on the MCC plates in order to compare the growth on plates with and without antibiotics and to determine the proportion of colistin resistant GNB isolates in the water.
Selection of colistin resistant bacteria
Distinct bacterial colonies were selected based on their morphological appearance on Mac-Col2-Van10 agar. The colonies were inoculated onto UriSelect agar (NHLS Media Laboratory, Green Point, South Africa) for preliminary identification after incubation aerobically at 37 °C for 18–22 h. Colonies were excluded if they were any colour other than pink (indicative of E. coli) or blue (indicative of Klebsiella/Enterobacter/Serratia/Citrobacter spp.) on the UriSelect agar plate, e.g. white, cream or brown. Matrix assisted laser desorption ionization-time of flight (MALDI-TOF, Bruker) was performed to confirm the identification of all isolates. Intrinsically colistin resistant organisms, specifically Serratia spp. and Providencia rettgerii, were excluded from further analysis.
Susceptibility testing
Colistin resistance was confirmed by broth microdilution (BMD) following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines and breakpoints version 10 (ISO-standard broth microdilution method 20776-1) [33]. Isolates with a minimum inhibitory concentration (MIC) of ≤ 2 mg/L were classified as colistin susceptible and > 2 mg/L as colistin resistant. E. coli ATCC® 25922 and NCTC® 13846 were used as controls.
Molecular detection of the mcr genes
DNA was extracted directly from each water sample using the DNeasy PowerWater Kit (Qiagen, South Africa) on the day of sampling and from the isolates using a crude DNA extraction method (boil-freeze) [34].
Multiplex PCR detection of the mcr-1, -2, -3, -4 and -5 genes was performed on all isolates, as well as directly on the water samples, using previously described protocols and controls [35] (Table 1). Singleplex PCR detection of the mcr-6, -7 and -8 genes was performed using previously designed primers (Table 1) and mcr-9 was detected using MCR-9YF and MCR-9YR (Table 1), however no positive controls were available for these genes. Sanger sequencing was performed on all mcr amplicons to determine the mcr variants present.
The entire mcr-3 and mcr-5 genes were amplified and sequenced using primers MCR-3YF and MCR3-YR2, and previously described mcr-5 gene primers MCR5_FW and MCR5_REV [5], respectively (Table 1). The PCR reactions were performed using the KAPA2G Fast Multiplex PCR Kit (Kapa Biosystems) with 0.4 μM of each primer in a 25 μL reaction volume, with an annealing temperature of 60 °C for 30 cycles. Amplicons were separated on a 1.5% w/v agarose gel and detected using the Alliance 2.7 imaging system (UVITec). Additional internal primers (MCR-3YF2 and MCR-3YR3, Table 1) were designed to complete the sequencing of the mcr-3 gene.
mcr-3 variant functionality assay
Novel mcr-3 gene variants with unknown colistin susceptibility functionality were cloned and expressed to determine their functionality. The entire 1623 bp mcr-3 coding sequence was amplified using primers MCR-3 YF3 and MCR-3 YR4 (IDT, USA, Table 1) using the HIFI HotStart ReadyMixPCR kit (Kapa Biosystems, South Africa) with 0.2 μM of each primer and an annealing temperature of 60ºC. The mcr-1 gene was amplified from NCTC® 13846 as a control using primers MCR-1YF, MCR-1YR (Table 1) with the same PCR conditions and an annealing temperature of 58 °C.
The mcr genes were cloned into the XhoI and NdeI restriction sites of the pET-48b(+) expression vector via the CloneJET PCR Cloning Kit (Thermo Fisher Scientific, Difco Laboratories, US) [37] and transformed into a colistin susceptible SHuffle T7 Competent E. coli strain (Inqaba Biotechnical Industries (Pty) Ltd, South Africa). Colonies were selected on 15 mg/L kanamycin Luria–Bertani (LB) agar plates and the presence of the mcr gene was confirmed using PCR. Colistin susceptibility of the recombinant E. coli was determined in triplicate, using BMD.
Results
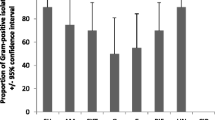
The river and storm water samples were heavily contaminated with bacteria, as they contained an abundance of E. coli and coliforms (Additional file 1: Table 2). The total E. coli count from all water sources ranged between 50 cfu/100 mL–450000 cfu/100 mL and the total coliform counts ranged between 550 cfu/100 mL–2400000 cfu/100 mL. These counts considerably exceeded the South African guidelines of < 130 cfu/100 ml for full recreational contact. The coliform count was higher in the water samples collected downstream as compared to samples taken upstream of communities (~ 1000-fold in the Plankenburg river, 42-fold in the Eerste river). Water samples from the Berg river and storm water samples from Muizenberg and Fish Hoek, in which sampling sites were situated alongside communities, also contained high coliform counts (2600–2,400,000 cfu/mL, 45,000 cfu/mL and 12,700 cfu/mL, respectively) (Additional file 1: Table 2). Two sites closest to the informal settlements of Kayamandi in Stellenbosch (Plankenburg River, site P2) and Mbekweni in Paarl (Berg river, site B2) contained the most coliforms and E. coli, based on the Colilert 18® results (Fig. 1 and Additional file 1: Table 2). The proportion of colistin resistant colonies based on the colony counts on MCC vs. Mac-Col2-Van10 (Additional file 1: Table 2), ranged between 5–80% and are given in Table 2.
Following exclusion of intrinsically colistin resistant bacteria and BMD confirmation of colistin resistance, a total of 98 isolates were screened for mcr genes, including 39 from the Plankenburg river, 14 from the Eerste river, 30 from the Berg river, 13 from Muizenberg storm water and two from Fish Hoek storm water (Table 1 and Additional file 1: Table 2). Aeromonas spp. (70/98, 71%) was the most common colistin resistant GNB isolated from the river and storm water samples (Table 2). No colistin resistant E. coli isolates were detected and only two colistin resistant Klebsiella oxytoca isolates were isolated from river water at the P2a and E2 sites.
mcr-3 was detected in six isolates, including five Aeromonas jandei isolates (BB, BL, BT, BCC and BMM) and one Aeromonas veronii isolate (S2K), all from the Berg river samples (Table 2). The Sanger sequences of the mcr-3 genes were uploaded to GenBank as new allele variants and allele curation was requested. Five novel mcr-3 gene variants were detected; mcr-3.33 (BB: MT791039, n = 1), mcr-3.34 (BL: MT791040, n = 1), mcr-3.35 (BT: MT809044, n = 1), mcr-3.36 (BCC: MT809046 and BMM: MT809045, n = 2) and mcr-3.37 (S2K: MT809047, n = 1) (Table 2). Recombinant expression of mcr-3.33, mcr-3.34, mcr-3.35, mcr-3.36 and mcr-3.37 in E. coli showed that all of the variants confer resistance to colistin, resulting in an eightfold increase in colistin MIC (Table 3). No mcr genes were detected in the remaining colistin resistant isolates.
Based on the DNA extracted directly from the water, the mcr-3 and mcr-5.1 genes were detected in the Plankenburg river (sites P2a and P2b), the Eerste river (site E2) and the Berg river (site B3), while another site in the Berg river (site B1) contained only mcr-3. The mcr genes were only detected in river water samples taken downstream of, or alongside communities, and not upstream of the communities. The mcr-5.1 gene was detected in both storm water samples from Muizenberg and Fish Hoek and the Muizenberg storm water sample also contained mcr-3 (Table 2). All of the mcr-5 genes shared 100% identity with the mcr-5.1 variant found in Salmonella enterica subsp. enterica serovar Paratyphi B (GenBank: KY807921).
Discussion
The river and storm water investigated in this study were heavily contaminated with faecal bacteria as evidenced by elevated E. coli and coliform levels (Additional file 1: Table 2). E. coli counts are a definitive indication of faecal contamination. High counts pose a public health risk and are associated with transmission of infectious diseases. Many South African rivers have been found to be unsuitable as a raw source for purification for drinking water, or for irrigation and other recreational purposes [31] and our findings support previous reports on the high level of bacterial contamination in water sources [31, 38].
Comparison of the samples collected up- and downstream of communities on the Plankenburg and Eerste rivers, provides an indication of the contamination introduced by runoff from the communities, and can be used as an indication of the carriage of resistance in the communities. The coliform and E. coli enumerations showed that there was substantial faecal contamination of the water between the two collection sites as well as in the Berg river and Fish Hoek and Muizenberg storm water systems (Additional file 1: Table 2). These water sources are highly polluted with various genera of bacteria, especially where these water sources are used for irrigational and/or recreational purposes (sites P2, E2, B2 and B3). This poses a significant public health risk via numerous pathways: direct exposure, irrigation of edible food crops, livestock watering and exposure to raw water usage by industry.
Aeromonas spp. was the most prevalent colistin resistant organism detected in this study. Aeromonas hydrophila plays a key role in degrading carcinogenic, teratogenic and mutagenic polycyclic aromatic hydrocarbons (PAHs) in water, especially acenaphthene and fluorine which are used to bioremediate PAH contaminated river systems [39]. However, Aeromonas spp. are opportunistic pathogens and have been found to be responsible for a variety of infectious complications such as gastroenteritis, sepsis, meningitis, respiratory and genitourinary infections, wound infections and infections of skin and soft tissue [40].
mcr-3 and mcr-5.1 gene variants were found to be widely dispersed in water sources in the study area, based on direct screening for these genes in the water samples. These genes were detected in water which is contaminated by communities, and not at upstream sites from communities. Although, the prevalence of mcr genes in the colistin resistant isolates was low, novel mcr-3 variants conferring colistin resistance were detected in 6 Aeromonas spp. isolates from the Berg river. The mcr-3 gene has been previously detected in Aeromonas spp. from fish and turkey in 2005, 2006, 2008 and 2012 in Germany [41] and from humans, retail meat, and environmental water samples in 2016 and 2017 in China [14, 42,43,44]. Two mcr-5 positive A. hydrophila were previously isolated from a pig faecal sample and hospital sewage in China in 2017 and 2014, respectively [45, 46]. No mcr-1-5 genes were detected in any of the other 92 colistin resistant isolates, suggesting that the mechanism of colistin resistance could be chromosomal in nature or conferred by additional recently (mcr-10) or undescribed mcr genes. However, there is limited knowledge on the mechanisms of colistin resistance in the genera of bacteria isolated in this study. The mcr genes could also be present in bacteria that are viable but non culturable (VBNC) or in the isolates that were excluded during the selection process and therefore can only be found when performing PCR screening directly on the water samples. Intrinsically colistin resistant organisms have shown to be potential traffickers of mcr genes [21, 47]. In our study we excluded a Providencia rettgerii (n = 1) and Serratia spp. (n = 8) (Additional file 1: Table 2), however these intrinsically colistin resistant could be carrying mcr genes and should be further investigated in the future.
The mcr-3 and mcr-5 genes described in this study have not previously been described in any sample type in South Africa, however, this study suggests that the mcr-3 and mcr-5.1 genes are commonly carried in the environment. Only mcr-1 genes have been described in clinical E. coli and Klebsiella spp isolates [19, 25], in contrast with these findings. This suggests that the environment carries different colistin resistance genes to those present in hospitalised patients. However, patients could present with infections associated with these unique colistin resistance genes in the near future. Active surveillance is therefore recommended to determine the prevalence of mcr genes in clinical isolates.
The plasmid transmissibility of the mcr genes means they can be easily transported to a variety of organisms, including those that are clinically significant. Although none of the E. coli were colistin resistant, they can possibly become colistin resistant via horizontal gene transmission of the mcr genes. The presence of colistin resistant isolates and genes conferring this resistance in the water may be linked to high-density industrial farming practises, involving routine use of antibiotics therapeutically, prophylactically and for growth promotion.
In many areas of South Africa, and worldwide, informal housing schemes are often established along the banks of river systems. Due to a lack of adequate sanitation and waste removal facilities in the informal settlements, as well as poor management and disposal of sewage, the storm water drainage pipes leading directly to the rivers are often used as a means of disposal of human and animal waste. Contamination of colistin resistant bacteria in river and storm water catchments is a cause of great concern, especially to surrounding communities, farms and industries, as in South Africa, river water is used for irrigation and domestic purposes, often without treatment. Controlling and preventing the spread of antimicrobial resistance requires a holistic approach, inclusive of environmental reservoirs of resistant organisms.
Conclusions
Several colistin resistant GNB, mainly Aeromonas spp. isolates, were isolated from three different rivers and two storm water samples in the Western Cape province of South Africa, suggesting that environmental water sources could serve as a reservoir and/or distribution network for colistin resistance. Mobile colistin resistance genes (mcr-3 and mcr-5) in opportunistically pathogenic bacteria (e.g. Aeromonas spp.) from these water sources can be horizontally transferred to other human pathogens which could present a potential human health risk. The high levels of colistin resistance in the mcr negative bacterial isolates suggests that there are alternative mechanisms of colistin resistance in these environmental bacteria. The presence of colistin resistance and mobile colistin resistance genes in these water sources raises major health concerns as human population densities along these water sources are high, thus resulting in increased human exposure to these organisms which are resistant to a last-resort antibiotic. The need to strengthen and monitor water treatment systems, proper sanitation and waste removal in order to improve the health of the communities living along various water sources is well understood. Our findings highlight the potential role of contaminated water sources in the transmission of resistant organisms. Improving water quality has the potential to both reduce infections in the community as well as to reduce the transmission of resistant organisms.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- BMD:
-
Broth microdilution
- cfu:
-
Colony forming unit
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- GNB:
-
Gram-negative bacteria
- LB:
-
Luria–Bertani (LB)
- MALDI-TOF:
-
Matrix assisted laser desorption ionization-time of flight
- Mac-Col2-Van10:
-
MacConkey agar containing 10 mg/L vancomycin and 2 mg/L colistin
- MCC:
-
MacConkey agar
- mcr :
-
Mobile colistin resistance
- VBNC:
-
Viable but non culturable
References
Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–8. https://doi.org/10.1016/S1473-3099(15)00424-7.
Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016;21(27):6–11.
Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio. 2017;8(3):4–9.
Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013 , Spain and Belgium , 2015 to 2016. 2017;1–5. https://www.ncbi.nlm.nih.gov/pubmed/28797329
Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother. 2017;72(12):3317–24.
Yang Y-Q, Li Y-X, Lei C-W, Zhang A-Y, Wang H-N. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73(7):1791–5.
AbuOun M, Stubberfield EJ, Duggett NA, Kirchner M, Dormer L, Nunez-Garcia J, et al. mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J Antimicrob Chemother. 2017;72(10):2745–9.
Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, et al. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect. 2018;7(1):122.
Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmann M. Identification of Novel Mobilized Colistin Resistance Gene mcr-9 in a Multidrug-Resistant, Colistin-Susceptible Salmonella enterica Serotype Typhimurium Isolate. Turner MS, editor. MBio. 2019;10(3):1–6. https://doi.org/10.1128/mBio.00853-19
Wang C, Feng Y, Liu L, Wei L, Kang M, Zong Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg Microbes Infect. 2020;9(1):508–16.
Anyanwu MU, Jaja IF, Nwobi OC. Occurrence and characteristics of mobile colistin resistance (Mcr) gene-containing isolates from the environment: a review. Int J Environ Res Public Health. 2020;17(3):1–38.
Osei Sekyere J, Maningi NE, Modipane L, Mbelle NM. Emergence of mcr-9.1 in extended-spectrum-β-lactamase-producing clinical enterobacteriaceae in Pretoria, South Africa: global evolutionary phylogenomics, resistome, and mobilome. mSystems. 2020;5(3):1–23.
Teo JWP, Kalisvar M, Venkatachalam I, Ng OT, Lin RTP, Octavia S. mcr-3 and mcr-4 variants in carbapenemase-producing clinical enterobacteriaceae do not confer phenotypic polymyxin resistance. J Clin Microbiol. 2018;56(3):1–8.
Ling Z, Yin W, Li H, Zhang Q, Wang X, Wang Z, et al. Chromosome-mediated mcr-3 variants in aeromonas veronii from chicken meat. Antimicrob Agents Chemother. 2017;61(11):1–5.
Ruzauskas M, Vaskeviciute L. Detection of the mcr-1 gene in Escherichia coli prevalent in the migratory bird species Larus argentatus. J Antimicrob Chemother. 2016;71(8):2333–4.
Liu B-T, Song F-J, Zou M, Hao Z-H, Shan H. Emergence of colistin resistance gene mcr-1 in cronobacter sakazakii producing NDM-9 and in Escherichia coli from the same animal. Antimicrob Agents Chemother. 2017;61(2):1–6.
Hasman H, Hammerum AM, Hansen F, Hendriksen RS, Olesen B, Agersø Y, et al. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill. 2015;20(49):30085.
Zurfluh K, Tasara T, Poirel L, Nordmann P, Stephan R. Draft genome sequence of Escherichia coli S51, a chicken isolate harboring a chromosomally encoded mcr-1 gene. Genome Announc. 2016;4(4):4–5.
Poirel L, Kieffer N, Brink A, Coetze J, Jayol A, Nordmann P. Genetic features of MCR-1-producing colistin-resistant Escherichia coli isolates in South Africa. Antimicrob Agents Chemother. 2016;60(7):4394–7.
Doumith M, Godbole G, Ashton P, Larkin L, Dallman T, Day M, et al. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J Antimicrob Chemother. 2016;71(8):2300–5.
Zhang J, Wang J, Chen L, Yassin AK, Kelly P, Butaye P, et al. Housefly (Musca domestica) and blow fly (Protophormia terraenovae) as vectors of bacteria carrying colistin resistance genes. Appl Environ Microbiol. 2018;84(1):1–8.
Wang R, van Dorp L, Shaw LP, Bradley P, Wang Q, Wang X, et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat Commun. 2018;9(1):1179.
Zhao F, Zong Z. Kluyvera ascorbata strain from hospital sewage carrying the mcr-1 colistin resistance gene. Antimicrob Agents Chemother. 2016;60(12):7498–501.
Luo J, Yao X, Lv L, Doi Y, Huang X, Huang S, et al. Emergence of mcr-1 in Raoultella ornithinolytica and Escherichia coli Isolates from retail vegetables in China. Antimicrob Agents Chemother. 2017;61(10):1–4.
Newton-Foot M, Snyman Y, Maloba MRB, Whitelaw AC. Plasmid-mediated mcr-1 colistin resistance in Escherichia coli and Klebsiella spp. clinical isolates from the Western Cape region of South Africa. Antimicrob Resist Infect Control. 2017;6:78.
Coetzee J, Corcoran C, Prentice E, Moodley M, Mendelson M, Poirel L, et al. Emergence of plasmid-mediated colistin resistance (MCR-1) among Escherichia coli isolated from South African patients. S Afr Med J. 2016;106(5):35–6.
Mendelson M, Brink A, Gouws J, Mbelle N, Naidoo V, Pople T, et al. The One Health stewardship of colistin as an antibiotic of last resort for human health in South Africa. Lancet Infect Dis. 2018;18(9):e288–94.
Igwaran A, Iweriebor BC, Okoh AI. Molecular characterization and antimicrobial resistance pattern of Escherichia coli recovered from wastewater treatment plants in Eastern Cape South Africa. Int J Environ Res Public Health. 2018;15(6):1237.
Perreten V, Strauss C, Collaud A, Gerber D. Colistin Resistance Gene mcr-1 in Avian-Pathogenic Escherichia coli in South Africa. Antimicrob Agents Chemother. 2016;60(7):4414–5.
Founou LL, Founou RC, Allam M, Ismail A, Essack SY. Extended-spectrum beta-lactamase-producing Escherichia coli harbouring mcr-1 gene isolated from pigs in South Africa. S Afr Med J. 2018;108(10):796–7.
Paulse AN, Jackson VA, Khan W. Comparison of microbial contamination at various sites along the Plankenburg-and Diep Rivers, Western Cape, South Africa. Water SA. 2009;35(4):469–78.
Department of Water Affairs and Forestry. South African Water Quality Guidelines. Volume 2: Recreational Water Use. Second Edition. Vol. 2, Agricultural Water Use: Aquaculture. 1996. 85 p. http://www.dwa.gov.za/iwqs/wq_guide/Pol_saWQguideFRESHAquaticecosystemsvol7.pdf.
EUCAST. Recommendations for MIC determination of colistin ( polymyxin E ) As recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group. EUCAST [Internet]. 2016;(March, 22). http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf.
Ndlovu T, Le Roux M, Khan W, Khan S. Co-detection of virulent Escherichia coli genes in surface water sources. PLoS ONE. 2015;10(2):e0116808.
Snyman Y, Whitelaw AC, Reuter S, Dramowski A, Maloba MRB, Newton-Foot M. Clonal expansion of colistin-resistant Acinetobacter baumannii isolates in Cape Town, South Africa. Int J Infect Dis. 2020;91:94–100https://doi.org/10.1016/j.ijid.2019.11.021
Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017;22(31):1–5.
Michelsen BK. Transformation of Escherichia coli Increases 260-Fold upon Inactivation of T4 DNA Ligase. Anal Biochem. 1995;225(1):172–4.
Paulse AN, Jackson VA, Khan W. Comparison of enumeration techniques for the investigation of bacterial pollution in the Berg River, Western Cape, South Africa. Water SA. 2007;33(2):165–73.
Alegbeleye OO, Opeolu BO, Jackson V. Bioremediation of polycyclic aromatic hydrocarbon (PAH) compounds: (acenaphthene and fluorene) in water using indigenous bacterial species isolated from the Diep and Plankenburg rivers, Western Cape, South Africa. Braz J Microbiol. 2017;48(2):314–25. https://doi.org/10.1016/j.bjm.2016.07.027.
Piotrowska M, Popowska M. Insight into the mobilome of Aeromonas strains. Front Microbiol. 2015;6(MAY).
Eichhorn I, Feudi C, Wang Y, Kaspar H, Feßler AT, Lübke-Becker A, et al. Identification of novel variants of the colistin resistance gene mcr-3 in Aeromonas spp. from the national resistance monitoring programme GERM-Vet and from diagnostic submissions. J Antimicrob Chemother. 2018;73(5):1217–21.
Sun Q, Hu Y, Zhou H, Shu L, Wang H, Huang Z, et al. Alkaline peptone water-based enrichment method for mcr-3 from acute diarrheic outpatient gut samples. Front Med. 2018;5(MAY):99.
Shen Y, Xu C, Sun Q, Schwarz S, Ou Y, Yang L, et al. Prevalence and genetic analysis of mcr-3-positive aeromonas species from humans, retail meat, and environmental water samples. Antimicrob Agents Chemother. 2018;62(9):7–9.
Xu Y, Zhong L-L, Srinivas S, Sun J, Huang M, Paterson DL, et al. Spread of mcr-3 colistin resistance in china: an epidemiological, genomic mechanistic study. EBioMedicine. 2018;34:139–57. https://doi.org/10.1016/j.ebiom.2018.07.027.
Ma S, Sun C, Hulth A, Li J, Nilsson LE, Zhou Y, et al. Mobile colistin resistance gene mcr-5 in porcine Aeromonas hydrophila. J Antimicrob Chemother. 2018;73(7):1777–80.
Zhang H, Zong Z, Lei S, Srinivas S, Sun J, Feng Y, et al. A genomic, evolutionary, and mechanistic study of mcr-5 action suggests functional unification across the mcr family of colistin resistance. Adv Sci. 2019;6(11).
Khedher MB, Baron SA, Riziki T, Ruimy R, Raoult D, Diene SM, et al. Massive analysis of 64,628 bacterial genomes to decipher water reservoir and origin of mobile colistin resistance genes: is there another role for these enzymes? Sci Rep. 2020;10(1):5970.
Acknowledgements
The authors would like to thank Alanah Hartzer for collecting and analysing the Plankenburg river samples (P1 and P2a).
Funding
This work was supported by the NHLS Research Trust and the Harry Crossley Foundation, and personal funding was obtained by the National Research Foundation Innovation Masters Scholarship. The funders had no role in study design, data collection, analysis and interpretation, decision to submit the work for publication, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
YS performed all experiments, interpreted and organised the project and drafted the manuscript. MNF, AW and MRBM designed the study, supervised, interpreted the results, and edited the manuscript. JB assisted in the collection of environmental river water samples and analysis of the results. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
River water sampling and characterisation in this study was approved by the Health Research Ethics Committee at Stellenbosch University (S18/10/259).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary Table 1. Field notes on water samples. Supplementary Table 2. Colistin resistant isolates collected in river and storm water in the Western Cape of South Africa during 2019–2020.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Snyman, Y., Whitelaw, A.C., Barnes, J.M. et al. Characterisation of mobile colistin resistance genes (mcr-3 and mcr-5) in river and storm water in regions of the Western Cape of South Africa. Antimicrob Resist Infect Control 10, 96 (2021). https://doi.org/10.1186/s13756-021-00963-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-021-00963-2