Abstract
Background
The metabolic score for insulin resistance (METS-IR) has been validated as a novel, simple, and reliable surrogate marker for insulin resistance; however, its utility for evaluating the prognosis of heart failure with preserved ejection fraction (HFpEF) remains to be elucidated. Therefore, we aimed to analyze the association between METS-IR and the long-term prognosis of HFpEF.
Methods
We enrolled a total of 4,702 participants with HFpEF in this study. The participants were divided into three groups according to METS-IR tertiles: (Ln [2 × fasting plasma glucose + fasting triglycerides] × body mass index) / (Ln [high-density lipoprotein cholesterol]). The occurrence of primary endpoints, including all-cause mortality and cardiovascular (CV) death, was documented.
Results
There were 3,248 participants with HFpEF (mean age, 65.7 ± 13.8 years; male, 59.0%) in total who were included in the final analysis. The incidence of primary outcomes from the lowest to the highest METS-IR tertiles were 46.92, 86.01, and 124.04 per 1000 person-years for all-cause death and 26.75, 49.01, and 64.62 per 1000 person-years for CV death. The multivariate Cox hazards regression analysis revealed hazard ratios for all-cause and CV deaths of 2.48 (95% CI 2.10–2.93; P < 0.001) and 2.29 (95% CI 1.83–2.87; P < 0.001) when the highest and lowest METS-IR tertiles were compared, respectively. In addition, the predictive efficacy of METS-IR remained significant across various comorbidity subgroups (all P < 0.05). Further, adding the METS-IR to the baseline risk model for all-cause death improved the C-statistic value (0.690 for the baseline model vs. 0.729 for the baseline model + METS-IR, P < 0.01), the integrated discrimination improvement value (0.061, P < 0.01), the net reclassification improvement value (0.491, P < 0.01), and the clinical net benefit.
Conclusions
An elevated METS-IR, which is associated with an increased mortality risk, is a potential valuable prognostic marker for individuals with HFpEF.
Similar content being viewed by others
Introduction
Heart failure (HF) is a major disease that affects the health and quality of life of approximately 64 million people worldwide [1]. Heart failure with preserved ejection fraction (HFpEF) is a distinct phenotype of HF, and it accounts for approximately 50% of all HF cases [2]. The proportion of individuals hospitalized due to HFpEF is rapidly increasing; these individuals have a poor prognosis, with a 5-year mortality rate of 53–74% [3]. Currently, therapeutic strategies that effectively improve adverse outcomes in HFpEF are extremely limited. Although existing clinical data support the ability of SGLT2 inhibitors to improve clinical outcomes in HFpEF [4], the underlying pathophysiological mechanisms of HFpEF remain poorly understood, and effective treatment options remain scarce [2]. Therefore, an in-depth exploration of prognostic factors and identification of populations with poor prognosis are of profound importance for enhancing risk management and improving disease outcomes.
HFpEF is frequently accompanied by multiple systemic abnormalities, and the burden of its comorbidities increases over time [2, 5]. Metabolic disturbance and inflammatory burden are common and significant comorbidities of HFpEF, and they are closely associated with its onset and progression [6]. Insulin resistance (IR), a central alteration in metabolic syndrome, is closely associated with various cardiovascular diseases (CVDs) [7]. Research has confirmed that IR also plays a significant role in the pathogenesis of HFpEF [8].
The current gold standard for assessing IR is the hyper insulinemic-euglycemic clamp (HEC); however, its clinical application is limited because of the time-consuming, expensive, and complicated nature of the procedure [9]. The metabolic score for insulin resistance (METS-IR) is easily calculated and reflects the interplay between glucose, lipid metabolism and body weight, which are the primary components of metabolic disorders [10, 11]. Further, METS-IR demonstrates a high degree of consistency with the HEC, and it can serve as an effective alternative marker for IR in clinical research [11]. Prior studies have found that METS-IR is associated with various CVDs and their risk factors, such as ischemic heart disease (IHD), diabetes, and hypertension [10,11,12,13].
There are currently limited data on the relationship between METS-IR and the prognosis of HFpEF. Despite the high prevalence of comorbidities in HFpEF, it remains unclear whether different comorbid conditions influence the prognostic relationship between METS-IR and HFpEF. Therefore, this study investigated the potential prognostic value of METS-IR in HFpEF and conducted further exploratory analyses in subjects with different comorbidities.
Methods
Study design and population
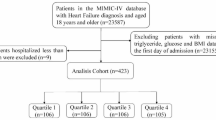
This was a retrospective, multicenter cohort study of participants with HFpEF who were hospitalized at The First Affiliated Hospital of Henan University of Science and Technology from January 1, 2016, to December 31, 2020, at the Second Affiliated Hospital from January 1, 2016, to December 31, 2019, and at the Sixth Medical Center of PLA General Hospital from January 1, 2016, to December 31, 2018. In reference to the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure [14], the diagnosis of HFpEF required meeting the following three criteria: (1) symptoms and signs of HF; (2) LVEF ≥ 50%; and (3) left ventricular hypertrophy, left atrial enlargement, or diastolic dysfunction reported on echocardiography, accompanied by elevated NT-proBNP levels (> 125 pg/ml for sinus rhythm or > 365 pg/ml for atrial fibrillation). From the initial cohort of 4,702 participants, 1,454 were excluded according to the following criteria: (1) age < 18 years or pregnancy; (2) severe hepatic or renal dysfunction; (3) advanced cancer or connective tissue diseases; (4) lacking data on body mass index (BMI), fasting blood glucose (FBG), triglyceride (TG), or high-density lipoprotein cholesterol (HDL-C) at admission; and (5) in-hospital mortality or loss to follow-up. There were 3,248 subjects in total who were enrolled (1,916 men, 1,332 women; average age 65.7 ± 13.8 years). Further, subjects were categorized into three groups according to METS-IR tertiles, namely, T1 (METS-IR < 36.49, n = 1083), T2 (36.49 ≤ METS-IR < 43.96, n = 1082), and T3 (METS-IR ≥ 43.96, n = 1083) (Fig. 1). Finally, we evaluated the severity of comorbidities using the age-adjusted Charlson Comorbidity Index (ACCI) [15] and conducted subgroup stratifications based on these scores: ≤ 3 points, 4–6 points, and ≥ 7 points.
Flow diagram of participants selection. HFpEF heart failure with preserved ejection fraction, CTD connective tissue diseases, BMI body mass Index, FBG fasting blood glucose, TG triglyceride, HDL-C high density lipoprotein cholesterol, CV death cardiovascular death, METS-IR the metabolic score for insulin resistance
Ethics statement
This retrospective study was conducted in accordance with the principles of the Declaration of Helsinki, and it was approved by the ethics committees of the Affiliated Hospital of Henan University of Science and Technology (2023-03-K0026) and the PLA General Hospital (S2023-065-02). Owing to the retrospective design, the institutional review board waived the requirement for informed consent and ensured that all of the patient-related information was anonymized.
Data collection and definitions
Clinical data, including vital signs, laboratory tests, echocardiographic data, comorbidities, and medication details, were collected from an electronic medical records system. The METS-IR was calculated as Ln [2 × FBG (mg/dL) + fasting TG (mg/dL)] × BMI (kg/m2) / Ln [HDL-C (mg/dL)] [10]. The mean arterial pressure (MAP) was calculated using the following formula: (systolic blood pressure + 2 × diastolic blood pressure)/3. BMI was determined using the following formula: body weight (kg) / [height (m)]2, expressed as kg/m2. Chronic kidney disease was determined through medical history or identified by an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [16]. A clinical diagnosis of diabetes was further confirmed through the following criteria: a prior diagnosis of diabetes and/or FBG ≥ 7.0 mmol/L and/or random blood glucose ≥ 11.1 mmol/L and/or the use of hypoglycemic agents. Hypoglycemic medications included those prescribed at discharge as well as oral hypoglycemic drugs used during hospitalization (excluding SGLT2 inhibitors, as these were not exclusively used for participants with diabetes).
Follow-up and outcomes
Prognostic data were acquired through telephone follow-ups or by examining relevant electronic medical records over a median follow-up period of 4.2 years (interquartile range (IQR), 3.0–5.1 years). The primary outcomes were all-cause mortality and cardiovascular (CV) death.
Statistical analysis
The characteristics of the participants were delineated according to the tertiles of METS-IR. Continuous variables are reported as the mean ± standard deviation or median with IQR, depending on the normality of their distribution. For continuous data, comparisons were made using one-way ANOVA for normally distributed data or the Kruskal–Wallis test for non-normally distributed data. Categorical variables are presented as frequencies and percentages, with group differences evaluated using the chi-squared test or Fisher’s exact test, as appropriate.
The cumulative incidence of the primary endpoints was estimated using the Kaplan‒Meier method, and group differences were evaluated using the log-rank test. The association between METS-IR and the incidence of primary outcomes was investigated using Cox proportional hazards models. Predictors that were significant in univariate Cox analyses (Table S1) or deemed clinically important were included as covariates in the multivariate Cox model. Furthermore, the multivariate analysis accounted for collinearity among the variables. METS-IR was analyzed as both a categorical variable (with the lowest tertile as the reference) and a continuous variable (per standard deviation (SD) increase). The linear trends across METS-IR tertiles were evaluated using the within-tertile median as a continuous variable. Two additional models were fitted (in addition to the unadjusted model): Model 1 controlled for age, gender, heart rate, New York Heart Association classification and left ventricular ejection fraction (LVEF), and Model 2 included all of the variables from Model 1 with additional adjustments for NT-proBNP, hemoglobin, creatinine, low-density lipoprotein cholesterol (LDL), atrial fibrillation, hypertension, stroke, peripheral arterial disease, ischemic etiology, statins, ACEI/ARB/ARNI, beta-blocker, calcium channel blockers, mineralocorticoid antagonists, diuretics, insulin, and SGLT2 inhibitors. Multiple imputations using chained equations were employed to handle missing covariates. The proportional hazards assumption was assessed utilizing Schoenfeld residuals, which revealed no potential violations. Propensity score matching (PSM) was used to adjust for covariates, thus guaranteeing comparability among groups when analyzing baseline characteristics. In addition, restricted cubic spline (RCS) regression model with three assumed knots was conducted to delineate the relationship between METS-IR and the hazard ratio (HR) [17], adjusted for the variables in Model 2. We carried out exploratory analyses across various subgroups, categorized by different comorbidities and their severity as indicated by ACCI scores. We used the likelihood ratio test to evaluate interactions between these subgroups.
Finally, the incremental effect of METS-IR on risk stratification was further evaluated using the C-statistic, net reclassification index (NRI), and integrated discrimination improvement (IDI), with the baseline model (MAGGIC score + NT-proBNP) serving as the reference. NRI measures the extent of improvement in correctly reclassifying individuals into appropriate risk categories, while IDI quantifies the overall enhancement in the model’s discrimination ability across all risk levels. Additionally, we performed exploratory analyses to evaluate the incremental effect of METS-IR among different sex subgroups (male and female) and age subgroups (under 65 years and 65 years or older). Statistical analyses were performed using R software (version 4.4.0; R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P value < 0.05 was considered to be significant.
Results
Participant characteristics
A total of 3,248 eligible participants were included in the analysis, with a mean age of 65.7 years; 59.0% were male. Table 1 details the baseline population characteristics, categorized by METS-IR tertiles. Participants with higher baseline METS-IR were older and had a greater prevalence of comorbidities, including hypertension, diabetes, chronic kidney disease, stroke, and ischemic etiology. This group also had a higher incidence of using antiplatelet agents, ACEI/ARB/ARNI, beta-blockers, calcium channel blockers, statins, diuretics, SGLT2 inhibitors, and other hypoglycemic drugs, but a lower incidence of digoxin use. In addition, this group exhibited higher BMI, MAP, white blood cell count, platelet count, creatinine, FBG, TG, total cholesterol, LDL, and NT-proBNP levels, but lower eGFR, HDL-C, and LVEF (all P < 0.05).
Correlations between METS-IR and adverse outcomes
Over a median follow-up of 4.15 years, the incidence of primary events was 83.08 per 1000 person-years for all-cause death and 45.52 per 1000 person-years for CV death. The incidence of primary events from the lowest to the highest METS-IR tertiles were 46.92, 86.01, and 124.04 per 1000 person-years for all-cause death and 26.75, 49.01, and 64.62 per 1000 person-years for CV death. The cumulative incidence of both all-cause death and CV death increased with higher METS-IR tertiles (Fig. 2, log-rank test, both P < 0.001). The RCS regression model also revealed that higher levels of METS-IR was associated with an increased risk of all-cause death (Model 2: HR per SD increase = 1.26, 95% CI 1.21–1.33) and CV death (Model 2: HR per SD increase = 1.23, 95% CI 1.16–1.32) (both P < 0.001) (Fig. S1).
Table 2 displays the three Cox regression models utilized to evaluate the associations between METS-IR and outcomes. In all three of the models, the highest METS-IR tertile was linked to a higher incidence of all-cause mortality (unadjusted Model: HR 2.61, 95% CI 2.23–3.06, P < 0.001; Model 1: HR 2.46, 95% CI 2.09–2.89, P < 0.001; Model 2: HR 2.48, 95% CI 2.10–2.93, P < 0.001). As a continuous variable, METS-IR was also significantly associated with all-cause death (Model 2: HR 1. 26, 95% CI 1.21–1.33, P < 0.001). Similar results were observed in the multivariate Cox proportional hazards analysis for METS-IR and CV death (Model 2: HR 2.29, 95% CI 1.83–2.87, P < 0.001 for the categorical variable with T1 vs. T3; and HR 1.23, 95% CI 1.16–1.32, P < 0.001 for the continuous variable).
In addition, to assess the consistency of our findings, PSM was conducted to adjust for key confounding variables such as age, sex, comorbidities, and treatments across the three groups (Table S2). The results remained unchanged even after the PSM analysis: the highest METS-IR tertile was also linked to higher incidences of all-cause mortality (Model 2: HR 2.27, 95% CI 1.86–2.77, P < 0.001) and CV death (Model 2: HR 2.13, 95% CI 1.63–2.77, P < 0.001) (Table S3).
Implications of METS-IR on survival outcomes in subgroups categorized by comorbidities
Further exploratory analyses were conducted across subgroups categorized by different comorbidities and ACCI scores. The Kaplan–Meier analysis revealed significant differences in the risk of primary endpoints among the three METS-IR tertiles across different comorbidity subgroups, including hypertension, diabetes, ischemic etiology, atrial fibrillation, CKD, obesity, stroke, and dyslipidemia (all log-rank test, P < 0.001) (Figs. S2, S3).
The results of the multivariate Cox proportional hazards models for the relationship between the METS-IR and all-cause death among different comorbidities are displayed in Fig. 3. The association between METS-IR and all-cause mortality remained consistently strong across comorbidities, even after adjusting for multiple factors, thus indicating its persistent link to poor prognosis in all of the subgroups (all P < 0.05). The relationship between METS-IR and CV death exhibited similar outcomes across all of the groups (Table S4).
We conducted further exploratory analyses to investigate the prognostic value of METS-IR across different ACCI scores, which reflected the severity of comorbidities. After excluding individuals with missing data necessary for ACCI scoring, a total of 2,622 subjects (from the original population of n = 3,248) were ultimately included in the ACCI scores analysis. Participants were divided into three groups based upon ACCI scores, namely, ≤ 3 points, 4–6 points, and ≥ 7 points. The previously discovered associations between METS-IR and adverse outcomes remained unchanged in the ACCI analysis population, as well as across the three designated subgroups (all P < 0.05). In addition, we found that although the interaction of the prognostic efficacy of METS-IR across the three subgroups did not reach statistical significance (P > 0.05), the trend suggested seemingly opposite patterns for all-cause mortality and CV death. Specifically, the predictive efficacy of METS-IR for all-cause mortality, based on Model 2, was more pronounced in the group with the highest ACCI scores: HR 2.71, 95% CI 2.04–3.61, for the ≥ 7 scores group, comparing the lowest (T1) to the highest tertile (T3). However, this trend was not observed for CV death. Instead, the predictive efficacy of METS-IR for CV death appeared to be stronger in the subgroup with the lowest ACCI scores (Table S5).
Incremental impact of METS-IR on risk stratification in HFpEF
Finally, we assessed the incremental value of METS-IR for enhancing the baseline risk model, including NT-proBNP and the MAGGIC score [18] (Fig. 4; Table 3). The cut-off value for METS-IR in predicting mortality was calculated to be 40.50, with a sensitivity of 63.30% and a specificity of 57.34%. The addition of METS-IR significantly improved risk prediction beyond the baseline risk model, with the C-statistic increasing from 0.690 to 0.729 for 3-year mortality (P < 0.01). Analysis of NRI and IDI demonstrated statistically significant enhancements in predictive value: continuous NRI (95% CI: 0.491 [0.412–0.569], P < 0.01) and IDI (95% CI: 0.061 [0.052–0.070], P < 0.01). The incremental impact of METS-IR persisted even across different subgroups stratified by sex and age (Table 3). Decision curve analysis revealed the net benefit of the new model (baseline risk model + METS-IR) was superior to the baseline risk model alone (Fig. S4).
ROC curves evaluating the incremental effect of METS-IR beyond the baseline risk model in HFpEF. ROC curve receiver operator characteristic curve, METS-IR the metabolic score for insulin resistance, HFpEF heart failure with preserved ejection fraction, MAGGIC score Meta-analysis Global Group in Chronic Heart Failure score. The baseline risk model includes the MAGGIC score and NT-proBNP
Discussion
To our knowledge, this study represents the first investigation into the association between METS-IR and the long-term outcomes in subjects with HFpEF. The principal findings of our study were as follows: (1) METS-IR was closely associated with adverse outcomes, and this association remained consistent across various comorbidities; (2) the predictive power of METS-IR for all-cause death and CV death appeared to follow different trends for individuals with higher or lower ACCI scores; and (3) adding METS-IR to the baseline risk model significantly enhanced the predictive efficacy and clinical net benefit. In summary, our research substantiated the potential use of METS-IR as an independent and valuable prognostic marker for the prognosis of HFpEF.
HFpEF is a common, complex, and heterogeneous syndrome. With advances in modern medical technology and enhanced understanding of health management, the incidence of HF, particularly heart failure with reduced ejection fraction (HFrEF), is decreasing; the incidence of HFpEF, however, is gradually increasing [19]. A survey of national hospitalizations in the United States revealed that the number of HFpEF hospitalizations more than doubled over a decade, increasing from 189,260 in 2008 to 495,095 in 2018 [20, 21]. Although the high prevalence and extensive impact of HFpEF are well recognized, the lack of effective treatment options has resulted in poor survival rates. In the Get With The Guidelines—HF registry, linked with Medicare data for longitudinal follow-up, the 5-year mortality rate was 75.7% for those with HFpEF and 75.3% for those with HFrEF [22]. Studies on the pathogenesis of HFpEF are still in an exploratory stage, and the underlying mechanisms of this condition have not been fully elucidated. Studies currently suggest that HFpEF is associated not only with hypertension but also with obesity, diabetes, dysregulated lipid metabolism, and other conditions [23,24,25].
The modern prevalence of metabolic syndrome is closely linked to contemporary lifestyles marked by high-calorie diets, sedentariness, and reduced physical activity [26].
Insulin resistance (IR) is a central feature of this syndrome, clinically characterized by a decrease in the biological efficacy of insulin and a reduced ability of tissues to absorb glucose. Research has confirmed that hyperglycemia induced by IR can lead to CVDs through multiple pathways, including dyslipidemia, atherosclerosis, energy metabolism disorders, and endothelial dysfunction [27, 28]. Furthermore, IR may precipitate or intensify the progression of HF, especially HFpEF [8]. One cohort study of 22,681 participants from four communities utilized the Homeostatic Model Assessment of Insulin Resistance to evaluate levels of IR. The incidence rates of HFrEF and HFpEF were analyzed over a median follow-up duration of 12 years. They found that IR was associated with HFpEF (HR: 1.20 per 1-SD increase; 95% CI: 1.05–1.37), but not with HFrEF (HR: 0.99; 95% CI: 0.88–1.11), with a statistically significant difference in the comparison between HFpEF and HFrEF (P < 0.05) [29]. This further substantiated the close association between IR and HFpEF. Importantly, a vicious cycle can form between IR and HR; IR is a significant risk factor for the onset and progression of HF, while HF can in turn exacerbate the degree of IR [7, 30].
METS-IR is a novel non-insulin-dependent score used to assess the degree of IR that shows high concordance with HEC, the gold standard for measuring IR [11]. A large (n = 116,855) cohort study from China assessed the relationship between METS-IR and the incidence of diabetes by stratifying participants into quartiles based on their METS-IR scores. The results revealed a significant association between METS-IR and the development of diabetes after multivariable adjustment (HR: 1.08; 95% CI: 1.07–1.08, P < 0.05). Further, individuals in Quartile 4 had a 6.26-fold higher risk of developing diabetes than those in Quartile 1 [31]. In a study involving 17,943 non-diabetic Korean participants, 332 developed IHD over the follow-up period; the study observed an increase in the incidence of IHD corresponding to higher METS-IR. The HRs of IHD for METS-IR quartiles 1–4 were as follows (after adjusting for potential confounders): 1.00, 1.62 (95% CI 1.04–2.53), 1.87 (95% CI 1.20–2.91), and 2.11 (95% CI 1.35–3.30), thus indicating a clear trend of increased risk with elevated METS-IR levels [12]. Several studies have demonstrated an association between METS-IR and adverse outcomes, particularly mortality. For instance, data from a prospective cohort study in China involving 14,234 individuals with hypertension revealed that, after adjusting for multiple factors, METS-IR was significantly and positively associated with both all-cause mortality and CV death (both P for trend < 0.05) [32]. Similarly, another study explored the relationship between METS-IR and adverse cardiovascular events, including cardiac death, in individuals with ischemic cardiomyopathy and type 2 diabetes mellitus. Multivariate Cox proportional hazards regression showed that the HR for the highest versus the lowest METS-IR tertiles was 1.89 (95% CI: 1.61–2.20), indicating an increased incidence of adverse events with higher METS-IR tertiles [33]. Moreover, METS-IR has also been found to be a reliable prognostic predictor for adverse outcomes, including all-cause mortality, in individuals with premature coronary artery disease [34].
However, current research on the relationship between METS-IR and the prognosis of HFpEF is very limited. Our study found a positive correlation between METS-IR and the incidence of adverse outcomes in HFpEF (HR: 1.26 per 1-SD increase; 95% CI: 1.21–1.33 for all-cause death and HR: 1.23 per 1-SD increase; 95% CI: 1.16–1.32 for CV death), and this association persisted across various comorbidities (all P < 0.05). We hypothesize that the primary mechanisms underlying this relationship are the various adverse effects of IR on HF: First, the state of IR alters the metabolic environment through various complex signaling pathways, leading to maladaptive responses in the myocardium and inducing myocardial damage. These adverse effects include impaired mitochondrial oxidative capacity and dysfunction, oxidative stress, inflammation, and myocardial fibrosis [35]; Second, hyperinsulinemia, a hallmark of IR, activates and enhances sympathetic nervous system activity, resulting in cardiac sympathetic dysfunction, which is closely associated with diastolic dysfunction in HFpEF [36, 37]; Third, IR can impact HF by impairing cardiac metabolic flexibility and disrupting various energy metabolism pathways [38]; Finally, IR leads to significant left ventricular dysfunction and promotes adverse myocardial remodeling, including increased left ventricular mass index and relative wall thickness [39]. Additionally, the substantial correlation between METS-IR and visceral fat levels also plays a critical role [11]. Visceral fat or abdominal obesity is not only associated with the incidence of HFpEF but also significantly linked to its poor prognosis [40, 41]. In a longitudinal, multicenter cohort study, VAT measured by CT was an effective predictor of HFpEF-related hospitalization even after multivariable adjustment; however, it did not predict HFrEF [42]. VAT can provide additional risk stratification for HFpEF even in individuals with overweight or obesity. Recent studies increasingly recognize cardiac adipose tissue within VAT as a critical factor in cardiovascular risk [43]. In HFpEF, epicardial adipose tissue may contribute to adverse clinical outcomes through several mechanisms, including lipid infiltration resulting in myocardial fatty degeneration, the promotion of local inflammation and fibrosis, as well as mechanical compression [44].
A key characteristic of HFpEF is its propensity to be associated with multiple extracardiac comorbidities. A large-scale multicenter heart failure cohort study from China demonstrated that HFpEF accounted for approximately 43.8% of the total heart failure population. Compared to other heart failure phenotypes, HFpEF was associated with a higher prevalence of comorbidities, including hypertension, stroke, pulmonary diseases, and atrial fibrillation [45]. We further conducted analyses across multiple subgroups based on different comorbidities and found that the predictive performance of METS-IR was consistently significant across all subgroups (all P < 0.05). Additionally, we found that the predictive efficacy of METS-IR appeared to be more pronounced in individuals without hypertension or obesity. This may be due to the fact that individuals with hypertension or obesity are more likely to have other coexisting metabolic abnormalities or confounding factors, which could obscure or weaken the association between IR and outcomes. However, the specific mechanisms and underlying causes of this phenomenon require further investigation in future clinical and fundamental research. Furthermore, we performed an exploratory analysis to evaluate whether the predictive ability of METS-IR is influenced by the severity of comorbidities, as indicated by the ACCI scores. The results showed that the ability of METS-IR to predict all-cause mortality is relatively more pronounced at the highest ACCI scores, whereas its ability to predict CV death appears to be stronger at the lowest ACCI scores, although the interaction test has not yet reached statistical significance. This may be because, although IR can exacerbate both cardiac diseases and noncardiogenic comorbidities [46,47,48], in individuals with a greater comorbidity burden, noncardiogenic diseases may have a more pronounced impact on prognosis. Conversely, in individuals with fewer or less severe comorbidities, cardiogenic factors may play a greater role in determining prognosis. It is important to note that this part of the findings primarily serves as an exploratory extension of the overall study and may be influenced by random errors or other confounding factors, warranting cautious interpretation and requiring confirmation in larger-scale future studies. Nonetheless, it still suggests the potential need for more nuanced risk assessments and management strategies tailored to the varying comorbidity burdens in individuals with HFpEF to improve their prognosis. Finally, another major finding of this study was that adding METS-IR to the baseline predictive model significantly improved its efficacy in predicting mortality risk.
Strengths and limitations
Our study has several advantages. First, it was based on a multicenter cohort, thus enhancing its representativeness. Second, it was the first to explore the prognostic value of METS-IR in HFpEF, and it conducted exploratory analyses under various comorbid conditions. Additionally, we included a wide range of baseline characteristics in our multivariate analysis to minimize confounding from these factors and conducted propensity score matching analysis.
Several limitations that should also be noted. First, due to the retrospective nature of the study, comprehensive control over clinical data changes during the follow-up period was unattainable. Second, the absence of baseline insulin measurements prevented the calculation of HOMA-IR values and subsequent comparison with METS-IR. Third, the follow-up phase could be influenced by a degree of recall or reporting bias. Fourth, unmeasured confounding factors may affect the outcomes, requiring careful interpretation of the results. Ultimately, while our research indicated that METS-IR possessed prognostic relevance for HFpEF, its actual value in clinical practice still requires validation via future prospective studies.
Conclusions
METS-IR has significant predictive value for adverse outcomes in individuals with HFpEF. Furthermore, as a simple, readily available, and reliable surrogate marker of IR, it can effectively assist in the risk assessment and clinical management of HFpEF.
Data availability
The datasets utilized and analyzed in this study are accessible from the corresponding author upon reasonable request.
Abbreviations
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- CVD:
-
Cardiovascular disease
- IR:
-
Insulin resistance
- HEC:
-
Hyperinsulinemic-euglycemic clamp
- METS-IR:
-
Metabolic score for insulin resistance
- IHD:
-
Ischemic heart disease
- HTN:
-
Hypertension
- VAT:
-
Visceral adipose tissue
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- IQR:
-
interquartile range
- CV death:
-
Cardiovascular death
- BMI:
-
Body mass index
- MAP:
-
Mean arterial pressure
- HR:
-
Heart rate
- NYHA:
-
New York Heart Association
- AF:
-
Atrial fibrillation
- CKD:
-
Chronic kidney disease
- PAD:
-
Peripheral arterial disease
- WBC:
-
White blood cell
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- eGFR:
-
Estimated glomerular filtration rate
- FBG:
-
Fasting blood glucose
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- LVEF:
-
Left ventricular ejection fraction
- ACEI/ARB/ARNI:
-
Angiotensin converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitors
- CCB:
-
Calcium channel blockers
- hospita inhibitors:
-
Sodium-glucose co-transporter-2 inhibitors
- MAGGIC score:
-
Meta-analysis Global Group in Chronic Heart Failure score
- ACCI:
-
Age-adjusted Charlson comorbidity index
- PSM:
-
Propensity score matching
- RCS:
-
Restricted cubic spline
- NRI:
-
Net reclassification index
- IDI:
-
Integrated discrimination improvement
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- SD:
-
Standard deviation
- ROC curve:
-
Receiver operator characteristic curve
- DCA:
-
Decision curve analysis
References
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovascular Res. 2023;118(17):3272–87.
Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329(10):827–38.
Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Reviews Cardiol. 2017;14(10):591–602.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in Heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
Pandey A, Vaduganathan M, Arora S, Qamar A, Mentz RJ, Shah SJ, Chang PP, Russell SD, Rosamond WD, Caughey MC. Temporal trends in Prevalence and Prognostic implications of comorbidities among patients with Acute Decompensated Heart failure: the ARIC Study Community Surveillance. Circulation. 2020;142(3):230–43.
Schiattarella GG, Rodolico D, Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovascular Res. 2021;117(2):423–34.
Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res 2023, 51(3).
Fazio S, Mercurio V, Fazio V, Ruvolo A, Affuso F. Insulin Resistance/Hyperinsulinemia, neglected risk factor for the development and worsening of Heart failure with preserved ejection fraction. Biomedicines 2024, 12(4).
Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26.
Wu Z, Cui H, Zhang Y, Liu L, Zhang W, Xiong W, Lu F, Peng J, Yang J. The impact of the metabolic score for insulin resistance on cardiovascular disease: a 10-year follow-up cohort study. J Endocrinol Investig. 2023;46(3):523–33.
Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–44.
Yoon J, Jung D, Lee Y, Park B. The metabolic score for insulin resistance (METS-IR) as a predictor of Incident Ischemic Heart Disease: a longitudinal study among Korean without diabetes. J Pers Med 2021, 11(8).
Liu XZ, Fan J, Pan SJ. METS-IR, a novel simple insulin resistance indexes, is associated with hypertension in normal-weight Chinese adults. J Clin Hypertens (Greenwich). 2019;21(8):1075–81.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, Donat SM, Herr HW, Bochner BH. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112(11):2384–92.
AS. L, LA. S, CH. S, YL. Z, 3rd. CA, HI. F, JW. K, P. E, F. VL, T. G et al. A New equation to Estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57.
Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34(19):1404–13.
Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal trends in the incidence of and Mortality Associated with Heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6(8):678–85.
Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC Scientific Statement. J Am Coll Cardiol. 2023;81(18):1810–34.
Clark KAA, Reinhardt SW, Chouairi F, Miller PE, Kay B, Fuery M, Guha A, Ahmad T, Desai NR. Trends in Heart failure hospitalizations in the US from 2008 to 2018. J Card Fail. 2022;28(2):171–80.
Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, Borderline, and reduced ejection fraction: 5-Year outcomes. J Am Coll Cardiol. 2017;70(20):2476–86.
Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovascular Res. 2023;118(18):3434–50.
Ng ACT, Delgado V, Borlaug BA, Bax JJ. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Reviews Cardiol. 2020;18(4):291–304.
Leggat J, Bidault G, Vidal-Puig A. Lipotoxicity: a driver of heart failure with preserved ejection fraction? Clin Sci (Lond). 2021;135(19):2265–83.
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122.
Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8(4):609–17.
Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, et al. The Association of Obesity and cardiometabolic traits with Incident HFpEF and HFrEF. JACC Heart Fail. 2018;6(8):701–9.
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac Energy Metabolism in Heart failure. Circul Res. 2021;128(10):1487–513.
Chen Z, Huang C, Zhou Z, Zhang Y, Xu M, Tang Y, Fan L, Feng K. A nonlinear associations of metabolic score for insulin resistance index with incident diabetes: a retrospective Chinese cohort study. Front Clin Diabetes Healthc. 2022;3:1101276.
Zhang L, Yu C, Wang T, Zhou W, Bao H, Cheng X. Association of the metabolic score for insulin resistance with cardiovascular diseases, cardiovascular and all-cause mortality in Chinese hypertensive population. Front Endocrinol 2024, 14.
Zhang X, Liu F, Li W, Zhang J, Zhang T, Yu X, Luo J, Zhao Q, Zhang J, Fang B, et al. Metabolic score for Insulin Resistance (METS-IR) predicts adverse Cardiovascular events in patients with type 2 diabetes and ischemic cardiomyopathy. Diabetes Metabolic Syndrome Obes. 2023;16:1283–95.
Guo D, Zhang C, Zhang M, Wu Z, Liu X, Zhang Y, Liu L, Sun M, Yang J. Metabolic score for insulin resistance predicts major adverse cardiovascular event in premature coronary artery disease. Aging. 2024;16(7):6364–83.
Riehle C, Abel ED. Insulin signaling and heart failure. Circul Res. 2016;118(7):1151–69.
Paolillo S, Rengo G, Pellegrino T, Formisano R, Pagano G, Gargiulo P, Savarese G, Carotenuto R, Petraglia L, Rapacciuolo A, et al. Insulin resistance is associated with impaired cardiac sympathetic innervation in patients with heart failure. Eur Heart J Cardiovasc Imaging. 2015;16(10):1148–53.
Aikawa T, Naya M, Obara M, Manabe O, Tomiyama Y, Magota K, Yamada S, Katoh C, Tamaki N, Tsutsui H. Impaired myocardial sympathetic innervation is Associated with Diastolic Dysfunction in Heart failure with preserved ejection fraction: 11 C-Hydroxyephedrine PET study. J Nucl Med. 2017;58(5):784–90.
Wang X, Ni J, Guo R, Li L, Su J, He F, Fan G. SGLT2 inhibitors break the vicious circle between heart failure and insulin resistance: targeting energy metabolism. Heart Fail Rev. 2021;27(3):961–80.
Huang R, Lin Y, Ye X, Zhong X, Xie P, Li M, Zhuang X, Liao X. Triglyceride–glucose index in the development of heart failure and left ventricular dysfunction: analysis of the ARIC study. Eur J Prev Cardiol. 2022;29(11):1531–41.
Rao VN, Fudim M, Mentz RJ, Michos ED, Felker GM. Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(9):1540–50.
Tsujimoto T, Kajio H. Abdominal obesity is Associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70(22):2739–49.
Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS, Michos ED. Adiposity and Incident Heart failure and its subtypes: MESA (multi-ethnic study of atherosclerosis). JACC Heart Fail. 2018;6(12):999–1007.
Iacobellis G, Sharma AM. Epicardial adipose tissue as New Cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Design. 2007;13(21):2180–4.
van Woerden G, van Veldhuisen DJ, Manintveld OC, van Empel VPM, Willems TP, de Boer RA, Rienstra M, Westenbrink BD, Gorter TM. Epicardial adipose tissue and outcome in heart failure with mid-range and preserved ejection fraction. Circulation Heart Fail 2021:Circheartfailure121009238.
Wang H, Li Y, Chai K, Long Z, Yang Z, Du M, Wang S, Zhan S, Liu Y, Wan Y, et al. Mortality in patients admitted to hospital with heart failure in China: a nationwide Cardiovascular Association Database-Heart Failure Centre Registry cohort study. Lancet Global Health. 2024;12(4):e611–22.
Wells CE, Polkey MI, Baker EH. Insulin resistance is associated with skeletal muscle weakness in COPD. Respirology. 2016;21(4):689–96.
Jing J, Pan Y, Zhao X, Zheng H, Jia Q, Mi D, Chen W, Li H, Liu L, Wang C, et al. Insulin Resistance and prognosis of nondiabetic patients with ischemic stroke: the ACROSS-China Study (abnormal glucose regulation in patients with Acute Stroke across China). Stroke. 2017;48(4):887–93.
Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Ren Physiol. 2016;311(6):F1087–108.
Acknowledgements
There is no acknowledgement.
Funding
This work was supported by the National Engineering Research Center of Medical Big Data of Chinese PLA General Hospital.
Author information
Authors and Affiliations
Contributions
All authors have made significant contributions. YZ performed the implementation of the study and manuscript preparation. YZ, YX and LD were responsible for performing the data collection and statistical analysis. YX and JD engaged in the discussion and revision of the manuscript. KH designed and supervised the experiments, serving as the corresponding author. All authors have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study followed the principles of the Declaration of Helsinki and was approved by the ethics committees of The Affiliated Hospital of Henan University of Science and Technology and the PLA General Hospital. Due to the retrospective nature of this study, the institutional review board waived the requirement for informed consent, and all patient-identifying information was anonymized.
Consent for publication
NA.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1: Fig. S1
. HRs for all-cause death and CV death in HFpEF using spline analyses adjusted for Model 2.
Additional file 2: Fig. S2
. Kaplan–Meier estimation of all-cause death across tertiles of METS-IR among different subgroups defined by comorbidities.
Additional file 3: Fig. S3
. Kaplan–Meier estimation of CV death across tertiles of METS-IR among different subgroups defined by comorbidities.
Additional file 4: Fig. S4.
Decision curve analysis comparing the baseline risk model with its integration of the METS-IR.
Additional file 5: Table S1
. Univariable analysis of the relationship between each predictor and mortality. Table S2. Baseline characteristics of the study population according to METS-IR tertiles after PSM analysis. Table S3. HRs of primary outcomes according to METS-IR tertiles after PSM analysis. Table S4. HRs of primary outcomes according to METS-IR tertiles among different subgroups based on comorbidities. Table S5. HRs of primary outcomes according to METS-IR tertiles based on ACCI scores.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Y., Xie, Y., Du, L. et al. Metabolic score for insulin resistance as a predictor of mortality in heart failure with preserved ejection fraction: results from a multicenter cohort study. Diabetol Metab Syndr 16, 220 (2024). https://doi.org/10.1186/s13098-024-01463-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01463-0