Abstract
Background
Viral pathogens causing significant economic losses in lilies (Lilium spp. and hybrids) include Lily symptomless virus (LSV), Lily mottle virus (LMoV), Cucumber mosaic virus (CMV), and Plantago asiatica mosaic virus (PlAMV). Rapid and efficient virus detection methods are pivotal to prevent the spread of these viruses.
Results
In this study, four specific primer pairs designed from conserved regions of genomic sequences of each virus were used to amplify a 116 bp product for LSV, a 247 bp product for LMoV, a 359 bp product for CMV, and a 525 bp product for PlAMV in a multiplex reverse transcription-polymerase chain reaction (multiplex RT-PCR). The amplified products were clearly separated by 2% agarose gel electrophoresis. The optimal reaction annealing temperature and cycle number were 53.8 °C and 35, respectively. The developed multiplex RT-PCR method was then used to test virus infections from lily samples collected from different regions of China.
Conclusions
An effective multiplex RT-PCR assay was established for the simultaneous detection and differentiation of LSV, LMoV, CMV, and PlAMV in lilies, which offers a useful tool for routine molecular diagnosis and epidemiological studies of these viruses.
Similar content being viewed by others
Background
Lilies are one the most important flower bulb plants, and widely used as cut flowers, flowering potted plants, and landscape plants. Besides, some lily species are cultivated as vegetables and medicines [1]. Viral diseases are one of main limiting factors in lily cultivation. More than 10 different viruses have been reported to infect lilies [2]. Among them, Lily symptomless virus (LSV; genus Carlavirus), Cucumber mosaic virus (CMV; genus Cucumovirus), Lily mottle virus (LMoV; genus Potyvirus), and Plantago asiatica mosaic virus (PlAMV; genus Potexvirus) are four of the most prevalent economically important viruses [3,4,5]. LSV-infected lilies exhibit symptoms of leaf mottle, vein clearing, and diminished growth [3]. LMoV often cause flower-color breaking, leaf mottle, leaf mosaic, chlorotic and yellow streaking, vein clearing, leaf curling, and narrowing [4]. CMV-infected lilies exhibit typical symptoms of brittleness of leaves and malformation of flowers and flower buds [4]. PlAMV-infected lily leaves show rust-brown necrotic spots and are often brittle [5].These viruses can be transmitted from generation to generation through clonal propagation, which is the major propagation method used for the commercial production of lily bulbs. Single and multiple viral infections with these viruses cause a significant reduction in bulb quality and yields and reduce the value of lily flowers. To prevent the spread of these viruses, a rapid, reliable, and specific virus detection method is urgently needed for the simultaneous detection of these viruses.
Currently, several techniques have been developed to detect viruses [6,7,8]. ELISA, simplex RT-PCR, uniplex real time RT-PCR, and LAMP have been developed for the detection of lily viruses [4, 9,10,11]. Multiplex RT-PCR amplifying multiple nucleic acid fragments in one reaction enables rapid and sensitive identification of several viruses simultaneously in a single assay, which greatly reduces cost and increases efficiency of viral surveys [12, 13]. To date, multiplex RT-PCR has been used widely to detect viruses in chrysanthemum, soybean, maize, pepper, apple, and tomato [14,15,16,17,18,19,20].
In this study, four specific primer pairs that can amplify DNA fragments of different sizes were designed according to genomic sequences of LSV, LMoV, CMV, and PlAMV. After optimizing RT-PCR conditions, an efficient multiplex RT-PCR assay was established and validated forthe simultaneous of LSV, LMoV, CMV, and PlAMV infecting lily plants.
Methods
Plant materials
Fresh leaves from Lilium ‘Tiny Padhye’ grown in greenhouses at the Chinese Academy of Agricultural Sciences (Beijing, China), which were confirmed to be co-infected with LSV, LMoV, CMV, and PlAMV by RT-PCR and sequencing were used to set up and optimize the multiplex RT-PCR assay.
RNA extraction and reverse transcription
Total RNA was isolated from the lily leaf samples as described before [21]. First strand cDNA was synthesized from 5 µg total RNA using a SuperScript III reverse transcription kit (Invitrogen, Carlsbad, CA, USA) in a 20 µL reaction mixture with random primers, according to the manufacturer’s protocol.
Design of virus-specific primers
The genomic sequences of LSV (accession numbers: KT923149.1, KJ627242.1, LC720794.1, LC704532.1, KC884548.1, KF958301.1, FJ456352.1, DQ531052.1, KJ627237.1, and LC649240.1), LMoV (accession numbers: MK368809.1, MK368792.1, MF983709.1, MT784663.1, KJ627230.1, JQ710690.1, AB054886.1, AJ310203.1, and LC733900.1), CMV (accession numbers: MN057671.1, HG316104.1, JN830618.1, EF158110.1, AY374328.1, AJ495841.1, LC733907.1, AM040193.1, and AJ131615.1), and PlAMV (accession numbers: KX245539.1, LN794199.1, KF601695.1, KU159091.1, LC422371.1, KU159089.1, LC592411.1, and KU159093.1) were obtained from GenBank. Nucleotide sequence alignment was performed using ClustalW implemented in MEGA X [22], and conserved regions of each virus were identified. Virus-specific primers were designed based on the identified conserved regions of each virus using Primer Premier 5.0 software (Premier Bio-soft International, Palo Alto, CA) (Additional file 1: Fig. S1–S4, Table 1).
Evaluation of primer specificity
Uniplex RT-PCR assays were performed to evaluate the specificity of primer pairs using a multi PCR Kit (Tiangen, Beijing, China). Each 50 μL reaction mixture contained 2.5 μL each primer (2 μM), 2 μL cDNA, 4 μL super pure dNTPs (2.5 mM each), 5 μL 10 × multi hotstart buffer, 1 μL multi hotstart DNA polymerase, and 33 μL double-distilled water. The thermal cycling conditions were as follows: 95 °C for 15 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 60 s, 72 °C for 90 s, and a final extension at 72 °C for 10 min. The PCR products were electrophoresed in a 2% agarose gel in 0.5 × TBE buffer.
To further confirm primer specificity, PCR products were cloned into the pEasy-T1 vector (Transgen, Beijing, China) for sequencing. Sequence alignment was performed using DNAMAN 5.0 Software (Lynnon Biosoft, San Ramon, CA, USA).
Optimization of multiplex RT-PCR assay
The multiplex RT-PCR was optimized by varying the cycling conditions (annealing temperature and the number of amplification cycles). Different annealing temperatures were set from 53.0 to 65.0 °C (53.0 °C; 53.8 °C; 55.3 °C; 57.6 °C; 60.3 °C; 62.6 °C; 64.1 °C; 65.0 °C). The number of amplification cycles ranged from 25 to 40 (25; 30; 35; 40). The PCR products were analyzed as described above.
Sensitivity of multiplex PCR assay
To compare the sensitivity of multiplex RT-PCR versus, 10-fold serial dilutions of cDNA from lily samples co-infected with LSV, LMoV, CMV, and PlAMV were used as templates. The PCR products were analyzed as described above.
Survey of lily viruses by multiplex RT-PCR assay
A total of 180 lily samples were collected from different regions of China for the detection of LSV, LMoV, CMV, and PlAMV using the developed multiplex RT-PCR assay. Of them, 30 samples were an edible lily (Lilium lancifolium Thunb.) collected from Xiangxi, Hunan province; 30 samples were an edible lily (Lilium davidii var. unicolor) collected from Lanzhou, Gansu province; and 120 samples were ornamental lily cultivars collected from other regions (Table 2).
Results
Specificity and compatibility of primer pairs
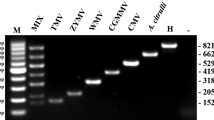
In the uniplex RT-PCR, fragments of the expected size for LSV, LMoV, CMV, and PlAMV were amplified specifically using virus-specific primer pairs (Fig. 1A–D). In multiplex RT-PCR, mixed primers could also amplify fragments of the expected size for the four viruses with no non-specific bands (Fig. 1E). To further confirm the specificity and compatibility of the designed primer pairs, the amplified fragments were cloned separately and sequenced. Sequencing analysis showed that the sequences of the amplified products were highly homologous to the corresponding sequences of LSV, LMoV, CMV, and PlAMV (data not shown). These results showed that the primers could be used to separate the four lily viruses in the developed multiplex RT-PCR assay.
Optimization of multiplex RT-PCR assay
The multiplex RT-PCR was optimized by varying the annealing temperature and the number of amplification cycles. According to the amplification efficiency and specificity of the virus-specific primers, the optimal annealing temperature and number of cycles were determined to be 53.8 °C and 35, respectively (Figs. 2, 3).
Sensitivities of uniplex RT-PCR and multiplex RT-PCR assays
The sensitivity of each assay was determined using 10-fold serial dilutions (100–10–7) of cDNA from lily samples co-infected with the four viruses. Both uniplex RT-PCR and multiplex RT-PCR could detect LSV and LMoV from cDNA diluted up to 10–4, and CMV from cDNA diluted up to 10–3. Uniplex RT-PCR and multiplex RT-PCR could detect PlAMV from cDNA diluted up to 10−3 and 10–2, respectively (Fig. 4).
Application of multiplex RT-PCR assay in survey of lily viruses
The results showed that 146 of the 180 samples were infected with at least one virus (Additional file 2: Table S1). LSV were detected in 70 samples (38.89%), LMoV were detected in 67 samples (37.22%), CMV were detected in 97 samples (53.89%), and PlAMV were detected in 16 (8.89%), respectively (Table 2). Single infection was detected in 70 (38.89%) of the samples, and mixed infection was detected in 76 (42.22%) of the samples (Additional file 2: Table S1). To validate the accuracy of the multiplex RT-PCR survey, uniplex RT-PCR assays were performed to detect LSV, LMoV, CMV, and PlAMV separately from all collected samples, and the results were consistent with those of the multiplex RT-PCR (data not shown).
Discussion
Designing and selecting proper primer combinations is of vital importance for establishing an efficient multiplex RT-PCR detection system [23]. In this study, virus-specific primers were designed based on the highly conserved regions of each virus. Using the final primer combinations, distinguishable amplicons with specific expected sizes for LSV (116 bp), LMoV (247 bp), CMV (359 bp, and PlAMV (525 bp) were obtained. The four products could be easily and clearly differentiated after electrophoresis on a 2% agarose gel since their size differences were 100 bp or more, and there were no non-specific products. Using a low concentration of agarose in the gel reduces the sensitivity of multiplex RT-PCR assays. Therefore, the agarose concentration in the gel was 2% in this study, which permitted the clear separation of the four amplicons.
Since the presence of more than one pair of primers in the same reaction mix may limit the sensitivity and compatibility of multiplex RT-PCR detection system, it is essential to optimize the PCR conditions to achieve an efficient multiplex detection. Here, annealing temperatures and cycling conditions were selected for optimization. Results showed that the intensity of the four target bands was the strongest at 53.8 °C and no further increase in intensity was achieved beyond 35 cycles (Fig. 2, 3), therefore, the optimal reaction annealing temperature and cycle number were set at 53.8 °C and 35, respectively. Sensitivity is another criterion to evaluate the multiplex system. The sensitivity analysis showed that the detection limits of the multiplex RT-PCR assay for the four viruses were similar to that of each uniplex RT-PCR assay, with only a slight reduction of 10-fold in the sensitivity for PlAMV detection in the multiplex assay (Fig. 4). The same results were also observed in other studies [23, 24]. This discrepancy may be partly due to competition in multiplex RT-PCR assay for key reactants, such as Taq polymerase and dNTPs.
Viral infection is one of the important diseases in lily cultivation [2]. In this study, 180 lily samples from different regions in China were tested using the developed multiplex RT-PCR assay. The results showed 146 samples were infected by at least one of the viruses, and the coinfection rate was 42.22% (Additional file 2: Table S1). Notably, 2/30 (6.67%) edible lily (L. lancifolium Thunb) samples were infected with PlAMV (Table 2). This is the first report of PlAMV in edible lily (Lilium lancifolium Thunb.) in China, which was only previously reported in ornamental lilies in China [25]. The high viral incidence could be due to the use of virus-infected bulbs for propagation and the lack of practical virus control and management measures. To manage these lily viral diseases, it is relevant to use resistant tolerant cultivars or virus-free propagation materials and use effective and sensitive detection methods to monitor the virus occurrence. The present developed multiplex RT-PCR assay will no doubt become an effective tool for solving these problems.
Conclusions
In this study, by selecting proper primer combinations, annealing temperature, and cycle number, an effective multiplex RT-PCR assay was established for simultaneous detection of these four common viruses in lilies in a single reaction system. This assay will be useful for routine molecular diagnosis and epidemiological studies of these viruses.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Liang ZX, Zhang JZ, Xin C, Li D, Sun MY, Shi L. Analysis of edible characteristics, antioxidant capacities, and phenolic pigment monomers in Lilium bulbs native to China. Food Res Int. 2022;151: 110854.
Asjes CJ. Control of aphid-borne Lily symptomless virus and Lily mottle virus in Lilium in the Netherlands. Virus Res. 2000;71(1–2):23–32.
Li YQ, Jia AN, Qiao Y, Xiang J, Zhang YJ, Wang WH. Virome analysis of lily plants reveals a new potyvirus. Arch Virol. 2018;163(4):1079–82.
Niimi Y, Han DS, Mori S, Kobayashi H. Detection of Cucumber mosaic virus, Lily symptomless virus and Lily mottle virus in Lilium species by RT-PCR technique. Sci Hortic. 2003;97(1):57–63.
Tanaka M, Verbeek M, Takehara M, Pham K, Lemmers M, Slootweg C, Arie T, Komatsu K. Differences in infectivity and pathogenicity of two Plantago asiatica mosaic virus isolates in lilies. Eur J Plant Pathol. 2019;153(3):813–23.
Boonham N, Kreuze J, Winter S, van der Vlugt R, Bergervoet J, Tomlinson J, Mumford R. Methods in virus diagnostics: from ELISA to next generation sequencing. Virus Res. 2014;186:20–31.
Santiago GA, Vazquez J, Courtney S, Matias KY, Andersen LE, Colon C, Butler AE, Roulo R, Bowzard J, Villanueva JM, et al. Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat Commun. 2018;9:1391.
Rubio L, Galipienso L, Ferriol I. Detection of plant viruses and disease management: relevance of genetic diversity and evolution. Front Plant Sci. 2020;11:1092.
Sharma A, Mahinghara BK, Singh AK, Kulshrestha S, Raikhy G, Singh L, Verma N, Hallan V, Ram R, Zaidi AA. Identification, detection and frequency of lily viruses in Northern India. Sci Hortic. 2005;106(2):213–27.
Zhang YB, Wang YJ, Xie ZK, Wang RY, Guo ZH, He YH. Rapid Detection of Lily mottle virus and Arabis mosaic virus infecting lily (Lilium spp.) using reverse transcription loop-mediated isothermal amplification. Plant Pathol J. 2020;36(2):170–8.
Zhao BN, Yang D, Zhang YC, Xu Y, Zhao X, Liang JL, Fan XR, Du YN, Zhu ZP, Shi B, et al. Rapid visual detection of Lily mottle virus using a loop-mediated isothermal amplification method. Arch Virol. 2018;163(2):545–8.
Pallas V, Sanchez-Navarro JA, James D. Recent advances on the multiplex molecular detection of plant viruses and viroids. Front Microbiol. 2018;9:2087.
Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE. Multiplex PCR. Optimization and application in diagnostic virology. Clin Microbiol Rev. 2000;13(4):559–70.
Song A, You Y, Chen F, Li P, Jiang J, Chen S. A multiplex RT-PCR for rapid and simultaneous detection of viruses and viroids in chrysanthemum. Lett Appl Microbiol. 2013;56(1):8–13.
Alvarez-Diaz JC, Ortiz-Echeverry BA, Velasquez N. Duplex RT-PCR assay for simultaneous detection of TSWV and CSVd in chrysanthemum. J Virol Methods. 2019;266:41–8.
Xue B, Shang J, Yang J, Zhang L, Du JB, Yu L, Yang WY, Naeem M. Development of a multiplex RT-PCR assay for the detection of Soybean mosaic virus, Bean common mosaic virus and Cucumber mosaic virus in field samples of soybean. J Virol Methods. 2021;298: 114278.
Li XQ, Li Y, Hu WL, Li YJ, Li Y, Chen SY, Wang JG. Simultaneous multiplex RT-PCR detection of four viruses associated with maize lethal necrosis disease. J Virol Methods. 2021;298: 114286.
Kwon SJ, Cho YE, Byun HS, Kwak HR, Seo JK. A multiplex RT-PCR assay for detection of emergent pepper Tsw resistance-breaking variants of tomato spotted wilt virus in South Korea. Mol Cell Probes. 2022;61: 101792.
Nabi SU, Madhu GS, Rao GP, Baranwal VK. Development of multiplex RT-PCR assay for simultaneous detection of four viruses infecting apple (Malus domestica). Lett Appl Microbiol. 2022;74(4):586–92.
Tu LQ, Wu SH, Gan SX, Zhao WH, Li S, Cheng ZB, Zhou YJ, Zhu YL, Ji YH. A simplified RT-PCR assay for the simultaneous detection of Tomato chlorosis virus and Tomato yellow leaf curl virus in tomato. J Virol Methods. 2022;299: 114282.
Xu LF, Xu H, Cao YW, Yang PP, Feng YY, Tang YC, Yuan SX, Ming J. Validation of reference genes for quantitative real-time PCR during bicolor tepal development in asiatic hybrid lilies (Lilium spp.). Front Plant Sci. 2017;8:669.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9.
Hao L, Xie JP, Chen SY, Wang SJ, Gong ZQ, Ling KS, Guo LY, Fan ZF, Zhou T. A multiple RT-PCR assay for simultaneous detection and differentiation of latent viruses and apscarviroids in apple trees. J Virol Methods. 2016;234:16–21.
Yao BY, Wang GP, Ma XF, Liu WB, Tang HH, Zhu H, Hong N. Simultaneous detection and differentiation of three viruses in pear plants by a multiplex RT-PCR. J Virol Methods. 2014;196:113–9.
Xu LF, Ming J, Yuan SX. First report of Plantago asiatica mosaic virus in lily hybrids in China. Plant Dis. 2017;101(1):263–363.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (32172624, and 31801899). This research was conducted at the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture, China.
Author information
Authors and Affiliations
Contributions
XL-F and MJ designed the experiments. XL-F performed the experiments. XL-F analyzed the data and wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to the publication of this manuscript.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Specific primers designed based on multiple sequences alignment for LSV. Figure S2. Specific primers designed based on multiple sequences alignment for LMoV. Figure S3. Specific primers designed based on multiple sequences alignment for CMV. Figure S4. Specific primers designed based on multiple sequences alignment for PlAMV
Additional file 2: Table S1.
Survery of viral infection in lily plants collected from different geographic regions of China using the multiplex RT-PCR assay.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, L., Ming, J. Development of a multiplex RT-PCR assay for simultaneous detection of Lily symptomless virus, Lily mottle virus, Cucumber mosaic virus, and Plantago asiatica mosaic virus in Lilies. Virol J 19, 219 (2022). https://doi.org/10.1186/s12985-022-01947-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-022-01947-3