Abstract
Background
Gut microbiota dysbiosis is closely associated with psychiatric disorders such as depression and anxiety (DA). In our preliminary study, fecal microbiota transplantation from volunteers with psychological stress and subclinical symptoms of depression (Vsd) induced DA-like behaviors in mice. Escherichia fergusonii (Esf) was found to be more abundant in the feces of Vsd compared to healthy volunteers. Therefore, we investigated the effect of Esf on DA-like behavior and neuroinflammation in mice with and without celiac vagotomy.
Methods and results
Orally gavaged Esf increased DA-like behaviors, tumor necrosis factor (TNF)-α, and toll-like receptor-4 (TLR4) expression, and NF-κB+Iba1+ and lipopolysaccharide (LPS)+Iba1+ cell populations, while decreasing serotonin, 5-HT1A receptor, and brain-derived neurotrophic factor (BDNF) expression in the hippocampus and prefrontal cortex. However, celiac vagotomy attenuated Esf-induced DA-like behavior and neuroinflammation. Orally gavaged extracellular vesicle (EV) from Vsd feces (vfEV) or Esf culture (esEV) induced DA-like behavior and inflammation in hippocampus, prefrontal cortex and colon. However, celiac vagotomy attenuated vfEV- or esEV-induced DA-like behaviors and inflammation in the brain alone, while vfEV- or esEV-induced blood LPS and TNF-α levels, colonic TNF-α expression and NF-κB-positive cell number, and fecal LPS level were not. Although orally gavaged fluorescence isothiocyanate-labeled esEV was translocated into the blood and hippocampus, celiac vagotomy decreased its translocation into the hippocampus alone.
Conclusions
esEVs may be translocated into the brain via the vagus nerve and bloodstream, subsequently inducing TNF-α expression and suppressing serotonin, its receptor, and BDNF expression through the activation of TLR4-mediated NF-κB signaling, thereby contributing to DA pathogenesis.
Similar content being viewed by others
Background
Depression and anxiety (DA) are the most common mental disorders [1]. They often co-occur: approximately 90% of individuals with anxiety disorders also exhibit symptoms of depression, and 85% of those with depression exhibit symptoms of anxiety [2, 3]. Excessive exposure to stressors, such as social defeat and pathogens, triggers the adrenal release of glucocorticoids, which can lead to anxiety and/or depression and subsequently induce systemic inflammation, including neuroinflammation [4, 5]. Stresses also cause gut inflammation-mediated gut dysbiosis including bacterial lipopolysaccharide (LPS) overproduction [6, 7]. Overproduced LPS causes toll-like receptor 4-involved NF-κB-mediated inflammation including neuroinflammation [8,9,10,11,12]. Neuroinflammation suppresses the release of serotonin, brain-derived neurotrophic factor (BDNF), and γ-aminobutyric acid (GABA) in the brain, resulting to psychiatric disorder [13,14,15,16]. Nevertheless, understanding the role of gut dysbiosis in the development of DA is still elusive.
The gut microbiota communicates with systemic organs, including the hippocampus, hypothalamus, amygdala, and prefrontal cortex of the brain, through intricate interactions involving the nervous, endocrine, and immune systems located within the intestinal environment. [17, 18]. Metabolites from gut microbiota such as short-chain fatty acids, lipopolysaccharides (LPS), and extracellular vesicle (EV) play crucial roles in maintaining host health [19,20,21]. Patients with inflammatory bowel disease (IBD) often exhibit the dysbiosis of gut microbiota along with comorbid DA [22, 23]. Fecal microbiota transplantation (FMT) from individuals with or without IBD into mice induces depressive-like symptoms and intestinal inflammation [24, 25]. Compared to healthy volunteers, patients with IBD and depression (IBD/D) show higher abundances of Enterococcaceae, Enterobacteriaceae, and Lactobacillaceae [25]. Escherichia coli isolated from gut microbiota of IBD/D patients causes DA and gut inflammation in conventional and germ-free mice [12, 26]. Therefore, gut dysbiosis is suggested to be closely connected with the occurrence of DA and gut inflammation. Nevertheless, understanding how gut bacteria cause depression is still a challenge.
In our preliminary study, FMT from volunteers with psychological stress and subclinical symptoms of DA (Vsd) caused DA-like behavior in mice. The population of Proteobacteria and Firmicutes including Escherichia fergusonii was higher in the feces of Vsd than in the feces of healthy volunteers (Vh). Therefore, we isolated E. fergusonii from the feces of Vsd and examined its effects on vagus nerve-involved DA-like symptom in mice.
Methods
Bacterial strains
E. fergusonii NK3001, a gut bacterium, was isolated from freshly collected feces of Vsd. These bacteria were cultured in anaerobic conditions at 37 °C in standard broth (GAM broth, Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) for 16–20 h until reaching an optical density (OD) of 0.7–0.9. Cultures were then centrifuged (4 °C, 4000 g, 10 min) and washed twice with distilled water. For in vitro experiments, collected bacteria were heat-treated (75 °C, 15 min) to tyndallize them and suspended in sterile distilled water. For in vivo experiments, collected bacteria were suspended in saline.
Preparation of extracellular vesicle (EV)
The EV was prepared from Vsd fecal suspensions or E. fergusonii culture supernatants as previously described [26]. Briefly, the Vsd fecal suspension or E. fergusonii culture was centrifuged at 10,000 g for 30 min at 4 °C. The supernatant was filtered sequentially through 0.45 μm and 0.22 μm microporous filters. The filtered supernatant was then centrifuged at 120,000 g for 3 h at 4 °C. The resulting pellet was resuspended in saline and purified using an iodixanol density gradient medium (Sigma) to isolate E. fergusonii EV (esEV).
The purified esEV was characterized by transmission electron microscopy (Hitachi), revealing a size range of 45–70 nm (spherical vesicle structures without intact E. fergusonii). The purified esEV contained 33.48% protein, 0.21% lipopolysaccharide (LPS), and 0.02% nucleic acids (comprising 42.7% DNA and 57.3% RNA). Protein concentration was determined using the Bradford protein assay kit. Nucleic acid content was quantified using DNA and RNA purification kits from Qiagen, with the total amount calculated as the sum of DNA and RNA. Protein analysis of esEV was performed by SDS-PAGE and LC-MS/MS using a Thermo Fisher Orbitrap XL with Easy nLC 1000.
For in vivo experiments, esEV (2 mg of protein) was labeled with fluorescence isothiocyanate (FITC) using the FITC antibody labeling kit (50 µg), following the manufacturer’s instructions [26]. The FITC-labeled esEV (FEV) solution (1.5 mL) was then used for subsequent experiments.
Volunteers
Vsd (age, 39.2 ± 12.1 [25–52] years: Stress Response Inventory (SRI) score; and Beck Depression Inventory-II (BDI-II) score, 30.0 ± 6.7: n = 6 [male, 2; female, 4]) and Vh (age, 32.3 ± 11.9 [20–46] years; Stress Response Inventory (SRI) score, < 15; and Beck Depression Inventory-II (BDI-II) score, < 5: n = 6 [male, 3; female, 3]) were enrolled in the sleep clinic of Seoul National University Bundang Hospital and Kyung Hee National University Medical, respectively. Participants were included if they had not received antibiotic treatment within the past 3 months and had no acute gastrointestinal symptoms such as diarrhea or abdominal pain within the week prior to fecal collection. The study protocol and informed consent for fecal sample collection were approved by the Medical School of Kyung Hee University Clinical Research Ethics Committee (IRB No.: KMC-202103102) and the Institutional Review Board of Seoul National University Bundang Hospital (IRB No.: B-1807/483-005). All experimental procedures were conducted in accordance with the principles of the Declaration of Helsinki and the Korean Good Clinical Practice guidelines.
Animals
Male C57BL/6 mice (7 weeks old, 18–21 g) were purchased from Koatech Inc. (Gyeonggi-do, Korea). The mice were housed in well-ventilated plastic cages under controlled conditions (temperature 20–22 °C, humidity 50% ± 10%, 12-hour light/dark cycle), as previously described [26]. All animal experiments were approved by the Kyung Hee University Institutional Animal Care and Use Committee (IACUC No.: KUASP(SE)-21451) and conducted in accordance with the university’s guidelines for the care and use of laboratory animals, adhering to ethical standards.
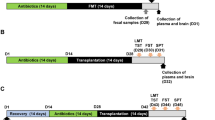
First, to investigate whether the gut microbiota from Vsd could induce DA-like behaviors in mice, the mice were divided into three groups: NC (normal control), Vsd, and Vh. Each group consisted of 7 mice. Mice in the Vsd and Vh groups were orally administered suspensions of Vsd or Vh fecal microbiota (1 × 109 CFU per mouse, suspended in 0.1 mL saline) daily for 5 consecutive days. The NC group received saline instead of Vsd or Vh suspensions.
Fecal microbiota suspensions from Vsd and Vh were prepared as follows. Fresh fecal samples from Vsd or Vh were collected and immediately suspended in saline within 2 h. The suspension was filtered through sterile gauze and centrifuged at 500 g for 5 min at 4 °C. The resulting supernatant was diluted with saline to a concentration of 1 × 10¹⁰ CFU/mL, forming the fecal microbiota suspension. The bacterial counts in the fecal suspensions were determined by culturing on GAM agar plates.
Second, to investigate the role of the vagus nerve pathway in the pathogenesis of depression induced by Vsd, mice were divided into five groups: NC (normal control), Sham (sham-operated mice treated with saline), vEv (sham-operated mice treated with Vsd extracellular vesicle (vfEV)), Vx (mice with vagotomy treated with saline), and VvEv (mice with vagotomy treated with vfEV). Each group consisted of seven mice. Mice in the vfEV treatment groups (vEv and VvEv) received oral administration of vfEV (20 µg per mouse, dissolved in 0.2 mL saline) daily for 5 consecutive days. The weight of EV was determined based on protein content using the Bradford protein assay.
Third, to elucidate the mechanisms by which gut bacteria induce depression-like symptoms, mice were divided into four groups: NC (normal control), Es (mice gavaged with E. fergusonii), Vx (vagotomized mice gavaged with saline), and VEs (vagotomized mice gavaged with E. fergusonii). Each group comprised seven mice. E. fergusonii (1 × 109 CFU in 0.2 mL saline) was orally gavaged in mice with with or without celiac vagotomy daily for 5 consecutive days.
Fourth, to investigate the role of the vagus nerve in the pathogenesis of esEV-induced depression, mice were divided into four groups: NC (normal control), eEv (mice treated with esEV), Vx (vagotomized mice treated with saline), and VeEv (vagotomized mice treated with esEV). Each group comprised seven mice. esEV (20 µg per mouse per day, dissolved in 0.2 mL saline) was orally gavaged in mice with or without celiac vagotomy daily for 5 consecutive days.
Fifth, to investigate whether esEV can translocate to the brain, mice were divided into four groups: NC (normal control), FEv (mice treated with FITC-labeled esEV), Vx (vagotomized mice treated with saline), and VFEv (vagotomized mice treated with FITC-labeled esEV). Each group consisted of seven mice. Mice in the FEv and VFEv groups received oral gavage of FITC-labeled esEV (20 µg per mouse per day, dissolved in 0.2 mL saline) daily for 5 consecutive days.
Celiac vagotomy was performed, as previously described [27]. In brief, a midline abdominal incision was made to expose the anterior esophageal wall. The celiac branch of the vagus nerve was identified and transected subdiaphragmatically. Sham-operated mice underwent the same midline abdominal incision without nerve transection.
DA-like behaviors (open-field test [OFT], elevated plus maze test [EPMT], and tail suspension test [TST]) were measured after the final treatment with gut bacteria on the following day (between 1:00 and 5:00 pm). Mice were euthanized in a CO2 chamber 18 h after the final behavioral tasks, followed by the cervical dislocation. Blood was collected and sera were prepared, as previously reported [12]. Brain and colon were collected for the enzyme-linked immunosorbent assay (ELISA) and stored at -80 °C.
Behavioral tasks
The DA-like behavior tasks were conducted in a room equipped with a recording camera and analyzed using EthoVision XT software, as previously reported [8,9,10,11,12]. The OFT was conducted in a chamber measuring 40 × 40 cm, with a defined center zone of 20 × 20 cm. Each test lasted 10 min, during which the movements of the subjects were recorded and analyzed to assess their activity levels and anxiety-related behaviors.
The EPMT consists of a cross-shaped platform with two open arms and two enclosed arms (30 × 7 × 20 cm walls), elevated 40 cm above the floor. Mice were placed on the central platform facing one of the open arms. Behavior was recorded for 5 min. Entries into the open arms (OE) and closed arms (CE) were recorded. The time spent in the open arms (OT) and closed arms (CT) was measured. An entry was defined as all four paws entering an arm. The following calculations were performed to quantify behavior: OT (%) = 100 × [OT / (OT + CT)], OE (%) = 100 × [OE / (OE + CE)].
The TST was performed by suspending the mice by the tail from the edge of a table, 30 cm above the surface, for a duration of 5 min. Mice were considered immobile if they did not move and hung passively. The duration of immobility was recorded to assess depressive-like behavior.
Assay of cytokines and LPS
Hippocampal, prefrontal cortex, and colonic tissues were lysed and centrifuged in RIPA lysis buffer as previously described [27]. ELISA kits were utilized to measure levels of tumor necrosis factor (TNF)-α (R&D, DY410), IL-1β (R&D, DY401), IL-6 (R&D, DY406), IL-10 (R&D, DY417), corticosterone (Elabscience), serotonin (Eagle Biosciences), myeloperoxidase (R&D, DY3667), and brain-derived neurotropic factor (BDNF, R&D, DY248) in hippocampal and colonic supernatants and sera. LPS levels were determined using the Limulus Amebocyte Lysate (LAL) assay kit (Cape Cod Inc.) [27].
Immunofluorescence staining
Brain and colon tissues of mice transcardially perfused with paraformaldehyde were sectioned (thickness, 25 μm). [12]. The sections were then subjected to immunostaining. After washing with PBS, antigen retrieval was performed by incubating the sections in citrate buffer (pH 6.0) at 95 °C for 10 min. Subsequently, the sections were blocked in 10% normal donkey serum (Abcam) for 1 h and incubated overnight at 4 °C with the following primary antibodies: Iba1 (1:800, Abcam), NF-κB (1:800, Cell Signaling Technology), CD11c (1:250, Cell Signaling Technology), NeuN (1:1000, Abcam), and BDNF (1:800, Abcam). The sections were then incubated with secondary antibodies (Alexa-conjugated secondary antibodies, diluted 1:800, Jackson ImmunoResearch Laboratories) for 1 h at room temperature. Fluorescent sections were mounted with anti-fade mounting medium containing DAPI and analyzed using confocal microscopy.
Quantitative real-time polymerase chain reaction (qPCR)
Bacterial DNA was purified from the fresh feces of mice using QIAamp Fast DNA stool mini kit (Qiagen) and its cDNA was prepared using cDNA synthesis kit [26]. qPCR for E. fergusonii and 16 S rRNA were performed using SYBER premix Ex Taq II (TaKaRa).
mRNAs (2 µg) from the prefrontal cortex and thalamus (PT) were isolated using a RNeasy Mini kit (Qiagen) and their cDNAs were prepared using cDNA synthesis kit (TaKaRa) [28]. qPCR for N-methyl-D-aspartate receptor (NMDAR) R1/R2, serotonin 1 A receptor (5-HT1AR), toll-like receptor 4 (TLR4), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed using SYBER premix Ex Taq II (TaKaRa). Primers used for qPCR are listed in Supplementary Table S1.
Microbiota analysis
Microbial genomic DNA was extracted from mouse fecal samples using the QIAamp DNA Stool Mini Kit. Subsequently, amplification and sequencing of the 16 S rRNA gene were conducted according to previously described methods [12]. Sequencing data have been deposited in NCBI (PRJNA1122947).
Statistics
Data were presented as mean ± standard deviation (SD) using GraphPad Prism 9. For multi-group comparisons of parametric data, either one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons post hoc test or two-way ANOVA with Tukey’s post hoc test was employed. Non-parametric data were analyzed using the Kruskal-Wallis test with Dunn’s multiple comparisons test (p < 0.05).
Results
FMT from Vsd caused depression-like symptoms in mice
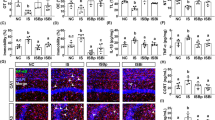
To understand whether gut microbiota dysbiosis is associated with the onset of depression, we transplanted fecal microbiota from Vsd or Vh into mice and measured DA-like symptoms (Fig. 1a-h, Supplement Figure S1). FMT from Vsd (FVsd) decreased OT in the EPMT to 20.3% (F2,18 = 12.32, p < 0.001) of NC and increased immobility time (IT) in the TST to 123.0% (F2,18=7.701, p = 0.003) of NC. However, FMT from Vh (FVh) did not significantly alter DA-like behaviors in EPMT and TST. FVsd resulted in decreased expression of serotonin, BDNF, and IL-10, along with a reduction in the BDNF+NeuN+ cell population in the hippocampus. Furthermore, FVsd led to increased expression of TNF-α, IL-1β, and IL-6 and an upregulation of the NF-κB+Iba1+ cell population. However, oral gavage of FVh did not significantly affect DA-related biomarker levels.
Fecal microbiota transplantation (FMT) from Vsd caused DA-like behavior and neuroinflammation in mice. Effects of FVsd and FVh on OT (a) and track path (b) in the EPMT and IT in the TST (c). Effects on serotonin (d), BDNF (e), TNF-α (f), and IL-10 (g) expression and BDNF+NeuN+ and NF-κB+Iba1+ cell populations (h) in the hippocampus. Effects on IL-6 (i) and corticosterone (CORT) levels (j) in the blood. Effects on TNF-α (k) and IL-10 expression (l) and NF-κB+CD11c+ cell populations (m) in the colon. NC, vehicle in normal control mice; Vsd, fecal microbiota of Vsd; Vh, the fecal microbiota of Vh. Data values were indicated as mean ± SD (n = 7). *p < 0.05. **p < 0.01. ***p < 0.001. ns, no significant
FVsd also increased the levels of IL-6 and corticosterone in the blood and the expression of myeloperoxidase, TNF-α, IL-1β, and IL-6, and the NF-κB+CD11c+ cell population in the colon, while decreasing IL-10 expression (Fig. 1i-m). In contrast, FVh had no significant impact on these measures.
Bacterial EVs exposed in the intestine are suggested to be able to cause psychiatric disorder through the vagus nerve [27]. Therefore, to understand the role of Vsd fecal EVs (vfEV) in the onset of depression, we investigated their effects on DA-like behavior and neuroinflammation in mice subjected to either sham or celiac vagotomy (Fig. 2, Supplement Figure S2). Oral gavage of vfEV decreased total travelled distance (TD), distance moved in the center area (DC), and time spent in the center area (TC) in mice in the OFT to 85.6% (F4,30=3.792, p = 0.013), 76.7% (F4,30=3.486, p = 0.018), and 73.0% (F4,30=3.737, p = 0.013) of NC, respectively. vfEV also decreased OT in the EPMT to 54.1% (F4,30=3.619, p = 0.016) of NC and increased IT in the TST to 126.0% (F4,30=3.746, p = 0.013) of NC. However, celiac vagotomy recovered vfEV-suppressed TD, DC, and TC in the OFT to 95.0% (F4,30=3.792, p = 0.013), 94.4% (F4,30=3.486, p = 0.018), and 88.7% (F4,30=3.737, p = 0.013) of NC, respectively. Furthermore, celiac vagotomy significantly recovered vfEV-suppressed OT in EPMT to 78.1% of NC and vfEV-induced IT in the TST to 107.6% of NC. Celiac vagotomy also decreased vfEV-induced TNF-α and IL-6 expression, as well as NF-κB+Iba1+ and LPS+Iba1+ cell populations in the hippocampus. Conversely, celiac vagotomy increased the expression of BDNF and IL-10 suppressed by vfEV, along with the BDNF+NeuN+ (BDNF-positive neuron) cell population.
EV from FVsd (vfEV) caused DA-like behavior and neuroinflammation in mice. Effect on IT in the TST (a), OT (b) and track path (c) in the EPMT, and total travelled distance (TD, d), distance travelled in the center (DC, e), and time spent in central area (TC, f), and track path (g) in OFT. Effect on serotonin (h), BDNF (i), TNF-α (j), and IL-10 expression (k), and BDNF+NeuN+, NF-κB+Iba1+, and LPS+Iba1+ cell populations (l) in the hippocampus. NC, vehicle in normal control mice; Sham, vehicle; vEv, vfEV in mice; Vx, vehicle in mice with vagotomy; VvEv, vfEV in mice with vagotomy. Data values were indicated as mean ± SD (n = 7). *p < 0.05. **p < 0.01. ***p < 0.001. ****p < 0.0001. ns, no significant
Oral administration of vfEV resulted in increased levels of TNF-α, IL-6, and LPS in the blood, and upregulated the expression of myeloperoxidase, TNF-α, IL-1β and IL-6 and an increased NF-κB+CD11c+ cell population in the colon. Additionally, vfEV elevated fecal LPS levels (Fig. 3, Supplement Figure S3). Celiac vagotomy did not significantly alter vfEV-induced biomarker levels in the blood, colon, and feces.
vfEV increased inflammation-related marker levels in the blood and colon of mice. Effect of efEV on IL-6 (a), corticosterone (CORT, b), and LPS levels (c) in the blood. Effect on myeloperoxidase (MPO, d), TNF-α (e), IL-6 (f), and IL-10 expression (g) and NF-κB+CD11c+ cell populations (h) in the colon. (i) Effect on fecal LPS level. NC, vehicle in normal control mice; Sham, vehicle; vEv, vfEV in mice; Vx, vehicle in mice with vagotomy; VvEv, vfEV in mice with vagotomy. Data values were indicated as mean ± SD (n = 7). *p < 0.05. **p < 0.01. ***p < 0.001. ****p < 0.0001. ns, no significant
To investigate the influence of gut microbiota on the occurrence of DA, we analyzed the fecal microbiota composition of Vsd and Vh (Fig. 4, Supplement Tables S2 and S3). Alpha-diversity, measured by estimated operational taxonomic units (OTUs), was not significantly different between the two groups, whereas β-diversity showed the significant difference. The populations of Proteobacteria and Firmicutes including Lachnospiraceae, Veillonellaceae, and Enterobacteriaceae were higher in the Vsd than in the Vh, while the populations of Actinobacteria and Verrucomicrobia including Coriobacteriaceae, Rikenellaceae, and Barnesiellaceae were lower in the Vsd. Culturing on selective DHL agar plates, as well as qPCR analysis, revealed the higher abundance of E. fergusonii in Vsd feces compared to those of Vh feces. In particular, exposure to E. fergusonii significantly induced TNF-α expression in Caco-2 cells and suppressed BDNF expression in SH-SY5Y cells (Supplement Figure S4).
The fecal microbiota composition of Vsd and Vh. (a) The composition at the phylum level (a) and family level (b). The α-diversity (OTU richness, c) and β-diversity (principal coordinate analysis plot based on Jensen-Shannon analysis, d). The number of gut bacteria Enterobacteriaceae and Enterococacceae grown in DHL (e) agar plates (inoculating Vsd and Vh feces). The levels of E. fergusonii (f), assessed Vsd and Vh feces by qPCR. Fmci, the feces of volunteers with MCI; Fhv, the feces of healthy volunteers. Vsd, n = 6; Vh, n = 6. #p < 0.05 vs. Vh
Effect of E. Fergusonii on the occurrence of DA and colitis in mice with or without celiac vagotomy
To understand whether E. fergusonii, which was significantly more abundant in the feces of Vsd than in those of Vh, might contribute to the occurrence of depression, we assessed their impact on DA-like behavior and neuroinflammation in mice (Fig. 5, Supplement Figure S5). Oral gavage of E. fergusonii caused DA-like behaviors. In particular, E. fergusonii decreased OT in EPMT to 55.3% (F3,24=10.43, p < 0.001) of NC and TD, DC, and TC in the OFT to 86.7% (F3,24=6.861, p = 0.002), 61.3% (F3,24=5.746, p = 0.004), and 68.1% (F3,24=3.133, p = 0.044) of NC. They increased IT in the TST to 123.8% (F3,24=15.56, p < 0.001) of NC. Oral gavage of E. fergusonii upregulated the expression of TNF-α, IL-1β, and IL-6 and the activation of NF-κB in the hippocampus, along with NF-κB+Iba1+ and LPS+Iba1+ cell populations, while reducing the expression of BDNF, serotonin, and IL-10, as well as the BDNF+NeuN+ cell population. Furthermore, E. fergusonii decreased BDNF, serotonin, and 5-HT1AR expression in the prefrontal cortex, while TNF-α, TLR-4, and NF-κB expression increased. E. fergusonii did not significantly affect NMDAR R1 and R2 expression.
Effect of E. fergusonii on DA-like behavior and neuroinflammation in mice with or without celiac vagotomy. Effect on IT in the TST (a), OT (b) and track path (c) in the EPMT, and TD (d), DC (e), TC (f), and track path (g) in OFT. Effect on serotonin (h), BDNF (i), TNF-α (j), and IL-10 expression (k) and BDNF+NeuN+, NF-κB+Iba1+, and LPS+Iba1+ cell populations (l) in the hippocampus. Effect on BDNF (m), serotonin (n), TNF-α (o), 5-HT1AR (p), and NF-κB (p) in the prefrontal cortex. NC, vehicle in normal control mice; sham, vehicle; Es, E. fergusonii in mice; Vx, vehicle in mice with vagotomy; VEs, E. fergusonii in mice with vagtomy. Data values were indicated as mean ± SD (n = 7). *p < 0.05. **p < 0.01. ***p < 0.001. ****p < 0.0001. ns, no significant
However, celiac vagotomy significantly attenuated E. fergusonii-induced DA-like behaviors: it recovered TD, DC, and TC in the OFT to 96.4%, 90.7%, and 89.1% of NC, respectively, OT in the EMPT to 66.7% of NC, and IT in the TST to 98.2% of NC. Celiac vagotomy also reduced E. fergusonii-induced expression of TNF-α, IL-1β and IL-6 and activation of NF-κB, as well as NF-κB+Iba1+ and LPS+Iba1+ cell populations in the hippocampus. It also recovered E. fergusonii-suppressed IL-10 expression and BDNF+NeuN+ cell population. Furthermore, celiac vagotomy increased E. fergusonii-suppressed decreased BDNF, serotonin, and 5-HT1AR expression in the prefrontal cortex, while E. fergusonii-induced TNF-α, TLR4, and NF-κB expression decreased. Celiac vagotomy did not alter the NMDAR R1 and R2 expression in the prefrontal cortex.
Next, we examined the effect of E. fergusonii on the occurrence of gut inflammation in mice with or without vagotomy (Fig. 6, Supplement Figure S6). Orally gavaged E. fergusonii increased levels of IL-6, corticosterone, and LPS in the blood, as well as myeloperoxidase, TNF-α, and IL-6 expression and NF-κB+Iba1+ cell population in the colon. Additionally, it elevated LPS levels in the feces. However, celiac vagotomy did not significantly alter these biomarker levels in the blood, colon, and feces.
Effects of E. fergusonii on DA-related biomarker levels in the blood and colon of mice with or without celiac vagotomy. Effects on IL-6 (a), corticosterone (CORT, b), and LPS levels (c) in the blood. Effects on myeloperoxidase (MPO, d), TNF-α (e), IL-6 (f), and IL-10 expression (g) and NF-κB+CD11c+ cell populations (h) in the colon. (i) Effects on fecal LPS level. NC, vehicle in normal control mice; sham, vehicle; Es, E. fergusonii in mice; Vx, vehicle in mice with vagotomy; VEs, E. fergusonii in mice with vagtomy. Data values were indicated as mean ± SD (n = 7). *p < 0.05. **p < 0.01. ****p < 0.0001. ns, no significant
Effect of E. Fergusonii EV (esEV) on the occurrence of depression and colitis in mice with or without celiac vagotomy
esEV isolated from the culture of E. fergusonii in GAM broth was round, vesicular structures of 30–70 nm, which consisted of proteins, lipopolysaccharide, and nucleic acid (Supplement Figure S7a-c). Of proteins, ompA, ompC, and LpoB (outer membrane lipoprotein B) were main components. The esEV significantly induced the expression of TNF-α in Caco-2 cells and decreased the expression of BDNF in SH-SY5Y cells (Supplement Figure S7d, e).
Next, we examined the effects of esEV on DA-like behavior and neuroinflammation in mice (Fig. 7, Supplement Figure S8). Orally gavaged esEV decreased it decreased OT in EPMT to 61.8% (F3,24 = 4.998, p = 0.007) of NC and TD, DC, and TC in the OFT to 85.2% (F3,24=3.639, p = 0.027), 73.4% (F3,24=4.481, p = 0.012) and 70.0% (F3,24 = 5.062, p = 0.007) of NC, respectively, and increased IT in the TST to 129.9% (F3,24 = 4.272, p = 0.015) of NC. esEV treatment upregulated the expression of TNF-α, IL-1β, and IL-6 and the activation of NF-κB in the hippocampus, as well as the NF-κB+Iba1+ and LPS+Iba1+ cell populations, while reducing the expression of BDNF and IL-10, as well as the BDNF+NeuN+ cell population. Furthermore, esEV decreased BDNF, serotonin, and 5-HT1AR expression in the prefrontal cortex, while increasing the expression of TNF-α, TLR-4, and NF-κB expression. The expression of NMDAR R1 and R2 was not affected by exposure to esEV. Celiac vagotomy partially recovered esEV-induced DA-like behaviors: it increased OT in the EMPT to 86.5% of NC and TD, DC, and TC in the OFT to 93.3%, 89.0%, and 87.7% of NC, respectively, and decreased IT in the TST to 109.4% of NC. Furthermore, celiac vagotomy decreased esEV-induced hippocampal TNF-α, IL-1β and IL-6 expression and NF-κB+Iba1+ and LPS+Iba1+ cell populations. The vagotomy also attenuated esEV-mediated suppression of hippocampal IL-10 expression and BDNF+NeuN+ cell population. Celiac vagotomy partially recovered esEV-suppressed BDNF, serotonin, and 5-HT1AR expression in the prefrontal cortex and expression, while decreasing esEV-induced TNF-α, TLR-4, and NF-κB expression. Celiac vagotomy did not alter the NMDAR R1 and R2 expression in the prefrontal cortex.
Effect of esEV on DA-like behavior and neuroinflammation in mice with or without celiac vagotomy. Effects on IT in the TST (a), OT (b) and track path (b) in the EPMT, and TD (d), DC (e), TC (f), and track path (g) in OFT. Effects on serotonin (h), BDNF (i), TNF-α (j), and IL-10 expression (k) and BDNF+NeuN+, NF-κB+Iba1+, and LPS+Iba1+ cell populations (l). Effect on BDNF (m), serotonin (n), TNF-α (o), 5-HT1AR (p), and NF-κB (p) in the prefrontal cortex. NC, vehicle; eEv, esEV in mice; Vx, vehicle in mice with vagotomy; VeEv, esEV in mice with vagotomy. Data values were indicated as mean ± SD (n = 7). *p < 0.05. **p < 0.01. ***p < 0.001. ****p < 0.0001. ns, no significant
Oral gavage of esEV increased IL-6, corticosterone, and LPS levels in the blood, myeloperoxidase, TNF-α, IL-1β, and IL-6 expression and the NF-κB+Iba1+ cell population in the colon, and LPS level in the feces (Fig. 8, Supplement S9). Celiac vagotomy did not significantly affect these biomarker levels in the blood, colon, and feces.
Effect of esEV on inflammation-related marker levels in the blood and colon mice. Effects on IL-6 (a), corticosterone (CORT, b), and LPS levels (c) in the blood. Effects on myeloperoxidase (MPO, d), TNF-α (e), IL-6 (f), and IL-10 expression (g) and NF-κB+CD11c+ cell populations (h) in the colon. (i) Effect on fecal LPS level. NC, vehicle; eEv, esEV in mice; Vx, vehicle in mice with vagotomy; VeEv, esEV in mice with vagotomy. Data values were indicated as mean ± SD (n = 7). *p < 0.05. **p < 0.01. ***p < 0.001. ****p < 0.0001. ns, no significant
esEV caused depression-like behavior and neuroinflammation through the vagus nerve. Therefore, to confirm whether orally gavaged esEV could be translocated into the brain, we prepared FITC-labelled esEV (FEV), and orally gavaged it in mice with or without celiac vagotomy (Fig. 9). When FEV was gavaged in mice (without vagotomy), it was detectable in the hippocampal pyramidal region and blood. However, when orally administered to mice that had undergone celiac vagotomy, FEV was undetectable in the hippocampus although it remained detectable in the blood.
Discussion
The gut microbiota communicates bidirectionally with the brain via the immune, endocrine, and nervous systems, which control the expression and turnover of neurotransmitters and cytokines [13, 17, 29, 30]. Exposure to stressors such as social defeat disrupts the expression of neurotransmitters and cytokines through the hypothalamic-pituitary-adrenal axis, leading to DA [31, 32]. Oral exposure to pathogens such as Klebsiella oxytoca and Escherichia coli alters gut microbiota and its byproducts, which induces proinflammatory cytokine expression in gut immune cells, leading to gut dysbiosis and inflammation. [26, 33]. Gut dysbiosis-mediated intestinal inflammation induces NF-κB activation and suppresses BDNF expression in the brain, thereby contributing to the development of DA [12, 25]. However, understanding the pathogenesis of gut pathogen-mediated depression is still elusive.
In the present study, the fecal microbiota from Vsd induced DA-like behaviors in mice. Vsd feces exhibited the higher abundance of Proteobacteria population compared to Vh feces, as previously reported [25]. Of Vsd feces-rich bacteria, we isolated gram-negative E. fergusonii, which induced the expression of TNF-α in Caco2 cells and suppressed the expression of BDNF in SH-SY5Y cells. When E. fergusonii was orally gavaged in mice, TD, DC, and TC in the OFT were decreased. Of these, DC the most strongly decreased, followed by TC. E. fergusonii also strongly increased IT in the TST and decreased OT in the EPMT. These suggest that E. fergusonii infection can cause DA-like behavior. Orally gavaged E. fergusonii increased TNF-α, IL-6, and TLR4 expression levels, and NF-κB+ and LPS+ microglial cell number in the hippocampus and prefrontal cortex, while decreasing the expression of BDNF, serotonin, and its receptor 5-HT1AR, but not NMDAR. Oral gave of E. fergusonii also increased TNF-α and LPS levels in the blood and elevated TNF-α and IL-6 expression and NF-κB-positive immune cell numbers in the colon and bacterial LPS production levels in the feces. These findings suggest that E. fergusonii overgrown in Vsd can induce gut inflammation through excessive production of pro-inflammatory bacterial metabolites (e.g., LPS) and facilitate their translocation via the bloodstream to the brain, thereby triggering DA-like behaviors and neuroinflammation by suppressing BDNF and serotonin/5-HT receptor (5-HTR) expression and inducing TNF-α expression through the activation of TLR4-mediated NF-κB signaling.
However, gram-negative E. fergusonii-induced DA-like behaviors, hippocampal TNF-α, IL-1β, IL-6 expression levels, and NF-κB+ and LPS+ microglial cell numbers were attenuated by celiac vagotomy. The vagotomy also attenuated the suppression of serotonin, BDNF, and 5-HTR expression by oral exposure to E. fergusonii. Nevertheless, celiac vagotomy did not affect E. fergusonii-induced TNF-α and LPS levels in the blood, expression levels of TNF-α and IL-6, number of NF-κB-positive immune cells in the colon, and LPS production levels in the feces. These findings suggest that gram-negative gut bacteria such as E. fergusonii may produce transmittable metabolites that can reach the brain via the vagus nerve, potentially inducing neuroinflammation and contributing to DA-like behavior by upregulating TNF-α expression and downregulating BDNF and serotonin/5-HTR expression through the activation of TLR4-mediated NF-κB signaling.
EV was easily isolated from the culture of gram-negative E. fergusonii. However, EV could not be isolated from the culture of gram-positive E. faecium, which was isolated from human gut microbiota, under various extraction conditions. Although no presentative, these results suggest that gram-negative bacteria may produce EVs in abundance compared to gram-positive bacteria. Oral gavage of esEVs isolated from the culture of E. fergusonii were induced DA-like behavior, hippocampal TNF-α and IL-6 expression levels, and NF-κB+ and LPS+ microglial cell numbers and suppressed hippocampal BDNF and serotonin levels in mice. Interestingly, celiac vagotomy significantly attenuated esEVs-induced DA-like behavior, TNF-α, IL-6, TLR4, and NMDAR expression levels and increased esEVs-suppressed BDNF, serotonin, and 5HT1AR expression levels in the hippocampus and prefrontal cortex. Furthermore, esEV, which consisted of LPS, proteins containing outer membrane proteins (omps), and nucleic acid, induced the expression of TNF-α in Caco-2 cells and the expression of BDNF in SH-SY5Y neuronal cells. LPS and omps induce inflammatory responses in vivo [12, 34]. In particular, omps such as ompA contribute to invasion of intestinal epithelial cells and brain microvascular endothelial cells (the brain-blood-barrier) [34,35,36]. Moreover, orally gavaged FITC-labeled esEV was translocated into the blood and the brain. However, it was hardly detected in the hippocampus of mice with celiac vagotomy. Celiac vagotomy attenuates gram-negative Paenalcaligenes hominis- or its EV-induced cognitive impairment in mice [27]. Gram-negative Porphyromonas gingivalis- and its EV-induced cognitive impairment are attenuated by trigeminal neurectomy [37]. These results suggest that gram-negative E. fergusonii can produce EV, which may be translocated into the brain from the gut through the vagus nerve and blood, and may cause neuroinflammation by inducing NF-κB activation, which can suppress BDNF, serotonin, and 5-HTR expression and induce TNF-α expression in the brain, resulting in DA (Supplementary Graphic abstract).
Conclusions
E. fergusonii overpopulated in Vsd can cause DA and neuroinflammation by overproducing inflammation-inducible gut bacteria byproducts such as LPS and EV, inducing gut inflammation, and accelerating their translocation into the brain, resulting in DA. Gram-negative E. fergusonii may produce inflammation-inducible EV, which may be translocated into the brain through the vagus nerve and blood and then suppress BDNF, serotonin, and 5-HTR expression and induce TNF-α expression in the brain through the NF-κB activation, resulting in depression and neuroinflammation. Finally, gram-negative E. fergusonii and its EV may be the risk factor for DA and neuroinflammation.
Data availability
The data that support the findings of this study are openly available in figures, table and NCBI under accession number PRJNA913477. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Abbreviations
- DA:
-
Depression and anxiety
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DC:
-
Distance moved in the center area
- EC:
-
Number of entries in the center area
- ELISA:
-
Enzyme-linked immunosorbent assay
- EMPT:
-
Elevated plus maze test
- esEV:
-
EV of Esf culture
- Esf:
-
Escherichia fergusonii
- EV:
-
Extracellular vesicle
- FEV:
-
FITC-labelled esEV
- FITC:
-
Fluorescence isothiocyanate
- FMT:
-
Fecal microbiota transplantation
- GABA:
-
γ-aminobutyric acid
- IBD:
-
Inflammatory bowel disease
- IT:
-
Immobility time
- LAL:
-
Limulus Amebocyte Lysate
- LPS:
-
Lipopolysaccharide
- OE:
-
Number of open arm entries
- OFT:
-
Open-field test
- OT:
-
Time spent in open arm
- PBS:
-
Phosphate-buffered saline
- SD:
-
Standard deviation
- SRI:
-
Stress Response Inventory
- TNF:
-
Tumor necrosis factor
- vfEV:
-
EV of Vsd feces
- Vh:
-
Healthy volunteers
- Vsd:
-
Volunteers with psychological stress and subclinical symptoms of depression
- TST:
-
Tail suspension test
- TD:
-
Total travelled distance
- TC:
-
Time spent in the center area
References
Irving P, Barrett K, Nijher M, de Lusignan S. Prevalence of depression and anxiety in people with inflammatory bowel disease and associated healthcare use: population-based cohort study. Evid Based Ment Health. 2021;24(3):102–9. https://doi.org/10.1136/ebmental-2020-300223.
Pollack MH. Comorbid anxiety and depression. J Clin Psychiatry. 2005;66(Suppl 8):22–9.
Tiller JW. Depression and anxiety. Med J Aust. 2013;199(S6):S28–31. https://doi.org/10.5694/mja12.10628.
Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63(9):847–51. https://doi.org/10.1016/j.biopsych.2007.10.008.
Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–71. https://doi.org/10.1016/s0022-3999(02)00429-4.
Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2023. https://doi.org/10.1007/s11739-023-03374-w.
Jang HM, Lee KE, Lee HJ, Kim DH. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci Rep. 2018;8(1):13897. https://doi.org/10.1038/s41598-018-31764-0.
Cavaillon JM. Exotoxins and endotoxins: inducers of inflammatory cytokines. Toxicon. 2018;149:45–53. https://doi.org/10.1016/j.toxicon.2017.10.016.
Kim HS, Kim S, Shin SJ, Park YH, Nam Y, Kim CW, et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: pathologic roles and therapeutic implications. Transl Neurodegener. 2021;10(1):49. https://doi.org/10.1186/s40035-021-00273-y.
Page MJ, Kell DB, Pretorius E. The role of Lipopolysaccharide-Induced cell signalling in chronic inflammation. Chronic Stress (Thousand Oaks). 2022;6:24705470221076390. https://doi.org/10.1177/24705470221076390.
Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy. 2002;1(2):181–91. https://doi.org/10.2174/1568010023344698.
Kim JK, Lee KE, Lee SA, Jang HM, Kim DH. Interplay between human gut Bacteria Escherichia Coli and Lactobacillus mucosae in the occurrence of Neuropsychiatric disorders in mice. Front Immunol. 2020;11:273. https://doi.org/10.3389/fimmu.2020.00273.
Maqsood R, Stone TW. The gut-brain Axis, BDNF, NMDA and CNS disorders. Neurochem Res. 2016;41(11):2819–35. https://doi.org/10.1007/s11064-016-2039-1.
Pourhamzeh M, Moravej FG, Arabi M, Shahriari E, Mehrabi S, Ward R, et al. The roles of Serotonin in Neuropsychiatric disorders. Cell Mol Neurobiol. 2022;42(6):1671–92. https://doi.org/10.1007/s10571-021-01064-9.
Finnell JE, Wood SK. Neuroinflammation at the interface of depression and cardiovascular disease: evidence from rodent models of social stress. Neurobiol Stress. 2016;4:1–14. https://doi.org/10.1016/j.ynstr.2016.04.001.
Crowley T, Cryan JF, Downer EJ, O’Leary OF. Inhibiting neuroinflammation: the role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav Immun. 2016;54:260–77. https://doi.org/10.1016/j.bbi.2016.02.001.
Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. https://doi.org/10.3389/fphys.2011.00094.
Liu P, Peng G, Zhang N, Wang B, Luo B. Crosstalk between the gut microbiota and the brain: an update on neuroimaging findings. Front Neurol. 2019;10:883. https://doi.org/10.3389/fneur.2019.00883.
Dogra N, Mani RJ, Katare DP. The gut-brain Axis: two Ways Signaling in Parkinson’s Disease. Cell Mol Neurobiol. 2022;42(2):315–32. https://doi.org/10.1007/s10571-021-01066-7.
Samra MS, Lim DH, Han MY, Jee HM, Kim YK, Kim JH. Bacterial microbiota-derived Extracellular vesicles in children with allergic Airway diseases: compositional and functional features. Allergy Asthma Immunol Res. 2021;13(1):56–74. https://doi.org/10.4168/aair.2021.13.1.56.
Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, et al. Short-chain fatty acids and Lipopolysaccharide as mediators between gut dysbiosis and amyloid Pathology in Alzheimer’s Disease. J Alzheimers Dis. 2020;78(2):683–97. https://doi.org/10.3233/jad-200306.
Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19(11):717–26. https://doi.org/10.1038/s41575-022-00634-6.
Bernstein CN. Psychological stress and depression: risk factors for IBD? Dig Dis. 2016;34(1–2):58–63. https://doi.org/10.1159/000442929.
Hashimoto K. Neuroinflammation through the vagus nerve-dependent gut-microbiota-brain axis in treatment-resistant depression. Prog Brain Res. 2023;278:61–77. https://doi.org/10.1016/bs.pbr.2023.01.003.
Jang HM, Kim JK, Joo MK, Shin YJ, Lee CK, Kim HJ, et al. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci Rep. 2021;11(1):20406. https://doi.org/10.1038/s41598-021-00088-x.
Jang HM, Kim JK, Joo MK, Shin YJ, Lee KE, Lee CK, et al. Enterococcus faecium and Pediococcus acidilactici deteriorate Enterobacteriaceae-induced depression and colitis in mice. Sci Rep. 2022;12(1):9389. https://doi.org/10.1038/s41598-022-13629-9.
Lee KE, Kim JK, Han SK, Lee DY, Lee HJ, Yim SV, et al. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8(1):107. https://doi.org/10.1186/s40168-020-00881-2.
Joo MK, Ma X, Yoo JW, Shin YJ, Kim HJ, Kim DH. Patient-derived Enterococcus mundtii and its capsular polysaccharides cause depression through the downregulation of NF-κB-involved serotonin and BDNF expression. Microbes Infect. 2023;25(6):105116. https://doi.org/10.1016/j.micinf.2023.105116.
Basiji K, Sendani AA, Ghavami SB, Farmani M, Kazemifard N, Sadeghi A, et al. The critical role of gut-brain axis microbiome in mental disorders. Metab Brain Dis. 2023;38(8):2547–61. https://doi.org/10.1007/s11011-023-01248-w.
Dicks LMT. Gut Bacteria and neurotransmitters. Microorganisms. 2022;10(9). https://doi.org/10.3390/microorganisms10091838.
de Kloet AD, Krause EG, Solomon MB, Flak JN, Scott KA, Kim DH, et al. Adipocyte glucocorticoid receptors mediate fat-to-brain signaling. Psychoneuroendocrinology. 2015;56:110–9. https://doi.org/10.1016/j.psyneuen.2015.03.008.
Juruena MF, Eror F, Cleare AJ, Young AH. The role of early life stress in HPA Axis and anxiety. Adv Exp Med Biol. 2020;1191:141–53. https://doi.org/10.1007/978-981-32-9705-0_9.
Jang HM, Lee HJ, Jang SE, Han MJ, Kim DH. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018;11(5):1386–97. https://doi.org/10.1038/s41385-018-0042-3.
Xie Y, Kim KJ, Kim KS. Current concepts on Escherichia coli K1 translocation of the blood-brain barrier. FEMS Immunol Med Microbiol. 2004;42(3):271–9. https://doi.org/10.1016/j.femsim.2004.09.001.
Kim KS. Strategy of Escherichia coli for crossing the blood-brain barrier. J Infect Dis. 2002;186(Suppl 2):S220–4. https://doi.org/10.1086/344284.
Prasadarao NV, Wass CA, Weiser JN, Stins MF, Huang SH, Kim KS. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64(1):146–53. https://doi.org/10.1128/iai.64.1.146-153.1996.
Ma X, Shin YJ, Yoo JW, Park HS, Kim DH. Extracellular vesicles derived from Porphyromonas gingivalis induce vagus nerve-mediated cognitive impairment. J Adv Res. 2023. https://doi.org/10.1016/j.jare.2023.02.006.
Acknowledgements
We thank Sung-Vin Yim, who is professor in Department of Clinical Pharmacology and Therapeutics, College of Medicine, Kyung Hee University (Seoul, Korea), for the assistance of healthy stool collection.
Funding
This research was supported by a grant of the Korea Dementia Research Project through the Korea Dementia Research Center (KDRC), funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (grant number: RS-2024-00344277) and a grant of the Medical Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT, Republic of Korea (NRF- 2017R1A5A2014768).
Author information
Authors and Affiliations
Contributions
XM, IYY, and DHK conceived and designed experiments and wrote the main manuscript test. MX, HSP, YJS, JKK, JKH, SWH, IYY prepared Figs. 1, 2, 3 and 4. MX and HSP prepared Figs. 5, 6, 7, 8 and 9. All authors contributed to reviewing the paper and agreed on the present version for submission.
Corresponding author
Ethics declarations
Ethical approval
The study protocol and consent forms for the collection of stool samples were approved by the Committee for the Care and Use of Clinical Study in the Medical School of Kyung Hee University (IRB No., KMC-202103102) and the Institutional Review Board of Seoul National University Bundang Hospital (IRB No., B-1807/483–005). All animal experiments were approved by the Institutional Animal Care and Use Committee of Kyung Hee University (IACUC No., KUASP(SE)-21451) and ethically performed according to the University Guide for Laboratory Animals Care and Usage.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, X., Park, HS., Shin, YJ. et al. The extracellular vesicle of depressive patient-derived Escherichia fergusonii induces vagus nerve-mediated neuroinflammation in mice. J Neuroinflammation 21, 224 (2024). https://doi.org/10.1186/s12974-024-03211-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-024-03211-7