Abstract
Parkinson’s disease (PD) is a chronic, progressive and second most prevalent neurological disorder affecting the motor system. Cardinal motor impairment and α-synucleinopathy are the characteristic features of PD. Recently, it has been identified that the gut-brain axis is substantially regulated by the gut microbiome (GM) through an immunological, neuroendocrine, and neural mechanism. However, disturbance in the gut-microbiome-brain axis in PD might proceed to gastrointestinal manifestations intermittently leading to the motor system and the PD pathogenesis itself. The gut microbial toxins may induce the production of α-synuclein (α-syn) aggregates in the enteric nervous system (ENS), which may proliferate and propagate in a prion-like-manner through the vagus nerve to the central nervous system (CNS); supporting the hypothesis that, GM might play a pivotal role in PD pathogenesis. Overstimulated innate immune system due to intestinal bacterial overgrowth or gut dysbiosis and the enhanced intestinal permeability may persuade systemic inflammation, while the activation of enteric glial cells and enteric neurons may contribute to α-synucleinopathy. Gut microbiota can bear a significant impact on neurological outcomes such as learning, memory and cognition. In this review paper, we summarize how the alterations in gut microbiota and ENS inflammation are associated with PD pathogenesis. The evidence supporting the causative role played by gut-associated dysbiosis and microbial byproducts, in the onset of PD is also discussed. We have highlighted the landmark discoveries in the field of PD particularly focusing on the gut-brain axis. A better comprehension of the interaction between the gut-brain axis, gut microbiota, and PD can usher in novel therapeutic and diagnostic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
PD is the second most prevalent neurodegenerative disorder, which is expected to affect more than 7–10 million people worldwide by 2030 (Burgueño and Abreu 2020; Marras et al. 2018). The characteristic features of PD start to appear when 55% of the dopaminergic neurons are already damaged in substantia nigra pars compacta. PD is identified by α-synucleinopathy, i.e. formation of round cytoplasmic inclusions having dense core-like structure surrounded by fibrils, called Lewy bodies. These unfolded proteins are liable for neuronal cell death. In PD the very first Lewy body appears outside the basal ganglia in non-dopaminergic neurons of the coeruleus-subcoerules complex, giganto cellular reticular nucleus, glossopharyngeal-vagal complex, olfactory pathways, and caudal raphe nuclei. In addition to dopamine (DA), acetylcholine (Ach, a neurotransmitter) plays a substantial role in controlling the voluntary movements exerted by the striatum (Nair et al. 2018). The constant stimulation of ACh without any inhibition leads to tremors, postural instability, bradykinesia and muscular fatigue. Beside motor impairment, PD patients also manifest metabolic imbalance with half of them experiencing constipation before the onset of other clinical features. This suggests a potential connection between early gastrointestinal (GI) problems and later developing stages of PD (Lionnet et al. 2018; König et al. 2015; Yang et al. 2019). In the course of the last half-decade, a predetermined number of studies were conducted, to investigate the effect of the gut microflora in the prodromal and initial stages of PD (Scheperjans 2018; Mertsalmi et al. 2017). Since, GI problems such as constipation considerably contribute toward the morbidity in PD; a recent clinical study has found that consistent intakes of Lactobacillus casei strain Shirota could decrease such disturbances and bowel movement disorders in PD (Cassani et al. 2011). It has been demonstrated the role of Vitamin D3 in the prevention of deterioration of Hoehn and Yahr stage in PD patients, and also explained the beneficial effects of vitamin D both in vivo and in vitro against 6-hydroxydopamine (Suzuki et al. 2013; Smith et al. 2006). Another finding identified a rise in the motor ability in PD patients following a six months administration of riboflavin by normalizing pyridoxine (vitamin B6) status and after all red meat was eliminated. Symptomatology did not reappear regardless of whether the treatment was interrupted for a few days; this suggests persistent low levels of vitamin B6 may promote motor impairment (Coimbra and Junqueira 2003). Heintz-Buschart et al. suggest that GM modification most likely precedes the advancement of motor impairment in PD (Heintz-Buschart et al. 2018a, b). Also, low levels of fecal SCFAs (short chain fatty acids) were reported in PD patients with deteriorating ENS (Unger et al. 2016a, b). Barichella et al. evaluated atypical parkinsonism, more specifically, the gut microbial composition in Steele–Richardson–Olszewski syndrome and multiple system atrophy, wherein some of the bacterial taxa have gone through changes like PD, while drug-naïve individuals showed a low concentration of Lachnospiraceae, nearly 43% reduction identified in comparison to Bifidobacterium (Barichella et al. 2019; Lin et al. 2018a, b). The gram-negative Prevotella abundance was found to be reduced to about 78% compared to healthy controls with 38.9% specificity in PD (Scheperjans et al. 2015a, b). Besides, cellulose-degrading bacterial concentration is lower, while putative pathobionts are significantly increased (Li et al. 2017a, b).
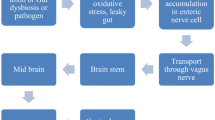
Animal models have demonstrated that the α-syn protein aggregates proliferate and transmit in a prion-like manner through the vagus nerve (Steiner et al. 2018). According to prion hypothesis of PD, the amyloidogenic α-syn induces significant changes through permissive templating in the anatomy of endogenous protein structure and converting it into a resemblance of itself and retrogradely proliferates via the vagus nerve from the ENS (Stopschinski and Diamond 2017). Although conclusive evidence of prion hypothesis of PD is still not found and also the probability of intestinal α-syn protein aggregate have a brain origin is controversial. It has been observed that vagotomy decreases the risk of PD in humans (Liu et al. 2017a, b). It is reported that in PD patient’s grafted neurons develops α-syn aggregate pathology and also these protein aggregates from PD patients can lead to substantia nigra degeneration in non-human primates and mice (Kordower et al. 2008; Recasens et al. 2014). It was observed that exogenous fibrils of α-syn derived either from PD patients or formed in E.coli, were competent in developing Lewy body-like structures which travel from the ENS to the CNS through the vagus nerve (Holmqvist et al. 2014). Here, we have highlighted the recent studies on the relationship between GI disorders and PD and analyzed the possible mechanisms of gut inflammation triggering PD.
Gut-Microbiome-Brain Axis and Parkinson’s Disease
A complex communication system exists between gut and brain, which does not only ensures the maintenance of GI homeostasis but also has multiple effects on cognitive functions; this integration is termed as the gut-brain axis. Its precise role is to coordinate the gut functions and also to integrate cognitive and emotional centers of the brain with peripheral GI functions and mechanisms like an enteric reflex, immune activation, entero-endocrine signaling and intestinal permeability. The mechanisms underlying gut-brain communication involves neuro-immuno-endocrine mediators. This bidirectional neural communication includes the ENS, the autonomic nervous system (ANS), the CNS; brain and spinal cord, and the hypothalamic–pituitary–adrenal (HPA) axis. The ANS, with the sympathetic and parasympathetic limbs, drives both afferent signals, emerging from the lumen and transmitted via enteric, spinal and vagal pathways to the CNS, similarly, efferent signals from CNS to the intestinal wall. The HPA axis coordinates the adaptive responses of the organisms to any kind of stressors and hence considered to be the core stress efferent axis (Tsigos et al. 2016; Carabotti et al. 2015). HPA axis is a part of the limbic system, an important zone of the brain primarily involved with memory and emotional responses.
Environmental stress, as well as the increased levels of systemic pro-inflammatory cytokines, stimulates this system that, via secretion of the corticotropin-releasing factor from the hypothalamus, further activates the adrenocorticotropic hormone secretion from the pituitary gland, which in turn, leads to the release of cortisol from the adrenal glands (Ilie et al. 2020). Hence, both hormonal and neural lines of communication combine to permit the brain to influence functions of intestinal effector cells, such as epithelial cells, enteric neurons, enterochromaffin cells, smooth muscle cells, immune cells and interstitial cells of Cajal. On the other these same cells, are under the influence of GM (Ilie et al. 2020; Mayer et al. 2014) whose contributing role in gut-brain communication has recently been evaluated.
The human gut microbiota evolves along with its host and therefore, keeps a narrow lead on innate competitive behavior of the microorganisms consisting of microbiota via the immune and nervous system (Cryan and Dinan 2012; Moeller et al. 2016; Bailey et al. 2011; Galley et al. 2014). This symbiotic relationship maximizes the advantages that the host can gain from this arrangement, apart from providing protection and stability from the pathogens, impaired mental health, nutrition, and GI tract (Foster et al. 2017). Enteroendocrine cells are the subtype of intestinal epithelial cells; mediate a biochemical signaling pathway facilitating communication between GM and the CNS through the vagus nerve (Hoffman and Lumpkin 2018). These Enteroendocrine cells release a range of peptides and hormones which acts as signaling molecules by hitting at specific receptors, both local and distant areas, and simultaneously a few chemoreceptors react to varied luminal stimuli (Feher 2017; Latorre et al. 2016).
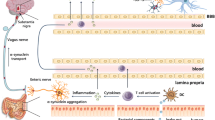
A relationship between the gut microflora and enteric neurons has been attributed to the novel discovery around toll-like receptors (TLR), proteins with a major role in innate immune system (Bogunovic et al. 2007; Caputi and Giron 2018; Liddle 2019; Burgueño and Abreu 2020) and their regulation potential upon the HPA axis (Sudo 2012) which gets followed by further chemical products that are involved in optimal functioning of the brain (De Vadder et al. 2014). New evidence has been reported indicating the role of TLR4 in the regulation of neuroinflammation, which results in the disruption of intestinal microflora (Perez-Pardo et al. 2019) (Fig. 1). Few rotenone models reported deregulation of HPA induced by chronic stress that culminates in dysbacteriosis and is signified by remarkable loss in the species of genus Bifidobacterium, to the detriment of Escherichia coli. Prolonged exposure often leads to increased gut permeability which further results in colitis, leaky gut and dysomia by induction of some particular neurochemical and neuroanatomical changes (Dodiya et al. 2018; Montojo et al. 2010; Perez-Pardo et al. 2018; Forsyth et al. 2011). A dysregulation in the host’s GM abundance (dysbacteriosis) can manifest in the development of cellular degeneration, low-grade enteric inflammation and dysregulation in cellular energy followed by an enhanced oxidative stress state (Noble et al. 2017). An overactivity of clusters of differentiation (CD) 1,4,17 (Kamada et al. 2013) will constrain the peripheral immune cells responses (Berer and Krishnamoorthy 2012) which eventually disturbs the blood–brain barrier integrity and functionality and also prevents the immune reactions against bacterial lipopolysaccharides (LPS) and microbial toxins (Stolp et al. 2005; Mulak and Bonaz 2015). Dysbiosis can cause several disorders, and one of these conditions is PD (Mulak and Bonaz 2015). The significance of the impact of microflora on the BBB is demonstrated by the species Eggerthella lenta and Enterococcus faecalis, both of which can metabolize levodopa, which is the gold standard drug recommended for PD. It was revealed that L-dopa could not cross the BBB to release dopamine and therefore, resulting in a considerably shorter route was chosen because of these gut microflora (Maini et al. 2019). Following literature studies, a bacterial amino acid named (TDC) has the ability to restrict the dopamine release thus repressing the effects of levodopa (Van Kessel et al. 2019).
Pictorial representation of bidirectional signaling between the gut and brain, interceded by neural (via the vagus nerve) and humoral pathway through blood and the lymphatic tissue stream (non-vagus nerve gateways). A single layer of intestinal epithelial cells splits the lumel and the GM from the beneath enteric nervous tissue. Members from the gut microbial community and the intrinsic molecules may elicit responses in the vagus nerve via enteroendoccrine cells, which are connected to vagus nerve via neuropods. Microbial antigens and its byproducts can cross the gut epithelial layers via the misfolded cells and hence resulting in localized inflammation. TLR helps in sensing the microbial proteins which are present in the intestine epithelial layer and hence mediating the identification of commensal bacteria amidst the harmful ones
Evidence Supporting the Transmission of α-Synuclein From Gut to Brain
The idea that the GI system is involved in PD is widely recognized and supported by clinical and empirical observations. Indications like early involvement of the vagus nerve, the presence of constipation, the strong connection between the gut and the dopamine-releasing system of the brain, are all therefore; consistent with the assumption that PD may originate from the gut (Fitzgerald et al. 2019; Perez-Pardo et al. 2017). In the year 2003, Heiko Braak conducted a study and looked into the patient’s brain with autopsy-confirmed PD. They demonstrated that in addition to the damage to particular subnuclei of the substantia nigra of the midbrain, a hallmark finding of PD pathology, the damage was likewise consistently found in the vagal nerves (Braak et al. 2003). This was a significant observation as before this; α-syn pathology was accepted to be limited to the brain. From that point forward, the hypothesis that PD may begin outside the CNS (or co-manifest in the periphery) has gained researchers interest. A recent study (Rietdijk et al. 2017), isolated three types of α-syn (monomeric, aggregated and oligomeric) from the brain tissue lysate of a PD and infused them into the submucosal layer of the ENS in mice to validate the theory that α-syn could travel from the ENS to the CNS via vagal nerve. In addition to the fact that they observed that α-syn deposited in a retrograde manner from the ENS to the vagus nerve and afterwards to the brain, however, it did as such in a time-dependent way that differed relying upon the type of α-syn injected (Rietdijk et al. 2017). Thereby confirming that retrogradely propagated α-syn can, indeed, lead to the spread of α-syn from the periphery to the midbrain (Rietdijk et al. 2017). Importantly, this pattern of α-syn deposition mirrors the rostral to a caudal pattern of destruction of dopaminergic neurons seen in the brains of PD patients. These findings support Braak’s proposal that PD could be the aftereffect of a GM like a virus, bacteria, pathogenic proteins or microbial toxins that may have passed through the submucosal layer of the GI tract, and entered into the CNS through unmyelinated preganglionic fibers of the vagus nerve (Braak et al. 2003). The finding that neurons in the ENS may develop a similar type of misfolded α-syn that aggregates in the CNS of PD patients suggests that α-syn might be the pathogen Braak was looking for. The disclosure that neurons in the ENS build up a similar type of misfolded α-syn that aggregates in the CNS of patients with PD proposes that α-syn might be the microbe Braak was searching for.
The Gut Microbiome and Regulation of Brain Function in Parkinson’s Disease
GMBA can influence the neurochemistry, and behavior (like dysregulated levels of neurotransmitters) and numerous neurotrophic factors (Martin et al. 2018). Many pieces of evidence have suggested an association between CNS and microbial dysfunction—related co-morbidities, like Alzheimer's disease, depression, anxiety, PD and autism spectrum disorders (Dinan and Cryan 2017; Hsiao et al. 2013; Kelly et al. 2017). This association is most likely due to the natural forces working to help the microorganisms to adapt themselves as that of the host environment and vice-versa (Johnson and Foster 2018). The proximate inflammatory and neuroendocrinology mechanisms like homeostasis, metabolism, drinking behavior, regulating reproduction and energy utilization explaining the nature of host manipulation are essentially shared by the host and the microbe (Freestone 2013; Mazzoli and Pessione 2016). The proportionate levels of neurotransmitters which are required for several social behavior expressions, like glutamate, serotonin, γ-aminobutyric, norepinephrine and dopamine are also regulated by the bacteria (Liu et al. 2017a, b; Strandwitz et al. 2019; Elfil et al. 2020). Substantially, a large amount of body’s serotonin is released by enterochromaffin cells in the gut under the significant influence of microbiome (Yano et al. 2015). The protein aggregates of α-syn are also observed in ENS, particularly in the appendix in ageing subjects and also in PD patients (Böttner et al. 2012; Gray et al. 2014; Barrenschee et al. 2017). Recently many in vivo models have shown that the modulations in the GM may result in the accumulation of aggregates of α-syn in the ENS (Chen et al. 2016). Sampson et al. (2016) explained the role of the presence of GM in promoting the motor deficits and pathological features resembling PD in genetically modified mice by overexpressing α-syn. It was also reported, that fecal transplants from the same strain of mice to healthy individual resulted in impairment in normal motor functions and hence strongly supporting the vital role of GM in an establishment of α-synucleinopathies like that of PD (Sampson et al. 2016). These studies thus suggest that microbial amyloids must be discharged in the extracellular space via certain families of GM from where they may be internalized into the neighboring cells and neurons and thus participates in developing the PD features like presence of aggregates of α-syn via permissive templating (Friedland and Chapman 2017; Soto and Pritzkow 2018). Furthermore, the failure of the body’s clearance mechanisms like the ubiquitin–proteasome system which itself a characteristic feature of both idiopathic and familial PD, to degrade the misfolded proteins may assist the seeding process (Naught et al. 2001). In addition to this, a direct connection was observed amid the amount of Enterobacteriaceae and the severity of gait difficulty, inflammation, and postural instability in PD (Santos et al. 2019). Another study reported that the severity in PD increases due to exposure to endotoxin produced by the coliform bacteria which also increases the mucosal permeability (Tan et al. 2014). Also, the count of Lactobacillus gasseri, Clostridium coccoides group and Lachnospiraceae group of a bacterial family were found to be high in the gut of PD patients as compared to the gut of the normal group (Shen 2020). The concept of gut microbial dysbiosis comprises bacteriophage components present in the GM (Manrique et al. 2017). Recently, a study had demonstrated the interference of phages in the normal processes of dopamine production and gut permeability, two vital factors associated with early stage symptoms of PD, via enlightening the significant difference in the composition of Lactococcus bacteria between a healthy individual and PD patient (Tetz et al. 2018; Darby et al. 2019; Houser and Tansey 2017). Phage therapies can be used as an alternative anti-microbial strategy which eventually contributes to combating PD via targeted approaches towards GM manipulations (Tetz et al. 2018).
Etiology of Gastrointestinal Dysfunction in Parkinson’s Disease
Clinical research has revealed that over 80% of patients suffering from PD may experience numerous severe GI symptoms such as vomiting, nausea and constipation, as well as increased intestinal permeability, also known as gut leakiness. These symptoms may reflect GM disorder in PD patients (Chen et al. 2019) and are well-associated with low-grade inflammation, oxidative stress, ENS neurodegeneration and the intestinal α-syn pathology, observed in PD patients (Devos et al. 2013; Glass et al. 2010; Quigley and Quera 2006). Numerous studies have been reported based on focusing on the changes in GM composition and modifying the GM in patients suffering from PD. One of the most widespread studies demonstrated a relationship of PD with the bacterium Helicobacter pylori. Among PD patients, a high infection by Helicobacter pylori is reported that hinders the levodopa absorption, a primary management drug for motor impairments in PD (Çamci and Oguz 2016). Likewise, a strong association between PD and small intestinal bacterial overgrowth (SIBO) has already been reported. SIBO nearly affects more than one-quarter of PD patients, a rate enormously higher than that found in healthy control. SIBO is associated with motor impairments; its elimination leads to improvement in motor symptoms, which further can be attributed to several other peripheral factors, including SIBO-induced malabsorption due to changes in the chyme composition and abnormal absorption of the drugs in the GI tract (Alfonso et al. 2013; Tan et al. 2014; Scheperjans et al. 2015a, b), due to associated intestinal mucosal inflammation or via altered metabolism by intraluminal bacteria (Alfonso et al. 2013). In one of the recent finding, Scheperjan et al. found a significant decline in Prevotellaceae in fecal samples of PD patients when compared with the control group, which resulted in GM dysbiosis. On further study, a positive correlation was observed between the relative abundance of Enterobacteriaceae with the severity of postural impairment and gait instability in PD patients (Scheperjans et al. 2015a, b). Further studies supporting gut dysbiosis in PD pathogenesis involves the observation that PD patients depict lower levels of Peptostreptococcus, Prevotella, Butyricicoccus and Lactobacillus spp. and enhanced levels of Enterobacter and Proteus spp. Compared to healthy controls (Sampson et al. 2016). PD patients display alterations in GM compositions at different stages of the pathogenesis. Increased levels of Clostridium coccoides and Lactobacillus gasseri were reported to be associated with the onset of PD and advanced-stage PD, respectively (Yang et al. 2019). A recent study conducted on 197 cases of PD with 130 healthy controls demonstrated that comparative abundances of Verrucomicrobiaceae, Christensenellaceae, Lactobacillaceae, Bifidobacteriaceae, Lachnospiraceae and Tissierellaceae were significantly altered in PD patients (Hill-Burns et al. 2017a, b, c). Similarly, Arumugam et al. also reported that decreased levels of Prevotellaceae reduce the levels of health-supporting neuroactive SCFAs, as well as folate and thiamine biosynthesis ability, an observation consistent with the reduced levels of these vitamins found in PD patients (Arumugam et al. 2011). The decreased abundance of Prevotella may be related to a reduction in mucin biosynthesis, which is further associated with increased gastrointestinal permeability and therefore, may increase the translocation of bacterial antigens (Andrews et al. 2009). Furthermore, PD patients with a reduced level of Prevotella exhibit an enhanced abundance of Lactobacilliceae. These alterations may be associated with reduced levels of ghrelin (gut hormone) whose secretion is impeded in PD patients and which may be involved in the maintenance and protection of the regular function of nigrostriatal dopamine (Andrews et al. 2009; Unger et al. 2011). Keshavarzian et al. also investigated the differences between microbial communities of the fecal and mucosal samples of PD patients with the healthy subjects which were reported to be similar, with certain clinical phenomena notable in PD patients due to enhanced mucosal permeability and systemic endotoxin exposure from coliform bacteria (Keshavarzian et al. 2015). They identified lower abundances of Roseburia, Blautia and Coprococcus in PD fecal samples; these SCFA butyrate-producing bacteria are associated with anti-inflammatory properties and their reduction may lead to a decline in SCFA levels and, eventually, gut leakiness. Furthermore, genes associated with type III bacterial secretion systems and lipopolysaccharide (LPS) biosynthesis were observed to be higher the fecal samples of PD patients compared with healthy controls. Type III bacterial secretion systems are reported to be associated with translocation of proteins and pathogenicity, which could intensify the bacterial-induced inflammation (Hueck 1998; Galan and Collmer 1999). In one of the recent study, Unger et al. demonstrated that both fecal SCFA and gut microbiota concentrations were considerably reduced in PD patients when compared to those from the same aged healthy controls (Unger et al. 2016a, b). They also reported a significant reduction in butyrate, acetate and propionate in fecal samples of PD patients; according to previously established studies, stimulation of SCFA receptor, resulting in anti-microbial activity, anti-inflammatory effects, and decreased gut barrier leakiness. Similarly, A germ-free transgenic model of a mouse having over-expressed α-syn receiving GM from PD patients reported a significant altered SCFA (butyrate, acetate and propionate) profile with enhanced motor impairment in comparison with animals colonized with microbiome isolated from the healthy gut (Sampson et al. 2016). Where depletion of GM reduced the α-syn pathology and also diminished the microglial activation and hence supporting the hypothesis of the role of GM in microglia activation. However, the introduction of SCFA contra-intuitively promoted α-syn pathology and motor impairment deprived of gut microbial colonization (Sampson et al. 2016). Thus, deviations in SFCA abundance may lead to changes in the ENS and therefore, contribute to gastrointestinal dysmotility in PD (Moro et al. 2019; Ganapathy et al. 2013; Singh et al. 2014; Maslowski and Mackay 2011).
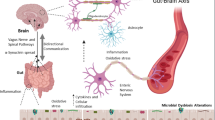
In another report, bacterium belongs to genus Faecalibacterium were observed to be significantly reduced in the mucosal samples of PD patients, while the abundance of bacteria from the genus Ralstonia were considerably increased. However, no such change was found in the abundance of Bifidobacteria in PD patients (Klann et al. 2020; Derkinderen et al. 2014). According to the Rating Scale scoring system of the Movement Disorder Society-United Parkinson's Disease, low counts of Bifidobacterium and B. fragilis were found to be directly related with worsening of hallucinations and initiative/motivation, respectively (Minato et al. 2017). It has been hypothesized by few studies that the enzymes involved in dopamine synthesis in the brain are regulated by the GM through the GM-brain axis and hence, supported the gut microbial interventions for PD (Zhu et al. 2020; Bested et al. 2013; Qian et al. 2018; Hopfner et al. 2017). Furthermore, GM such as Bacillus spp was reported to produce dopamine, and approximately half of its body’s production is controlled by GM (Zhu et al. 2020). Hence, PD pathogenesis might be caused or exacerbated by GM disorders and microbiota-persuaded inflammatory reactions. This may support in promoting α-syn pathology from the intestine to the brain or via a rostral-to-caudal route of transfer from cell to cell caused by enhanced oxidative stress, which might be due to the increase in abundance of pro-inflammatory bacteria (Wall et al. 2014; Eisenhofer et al. 1997). It is currently challenging to conclude if the observed gut microbial changes are a cause or an effect of PD. However, GM alterations may play a crucial role in neuronal loss via the promotion of oxidative stress and inflammatory cascades in the brain through SCFA-production or an LPS-mediated mechanism (Fig. 2).
The Links Between Quantitative Changes in the Gut Microbial Composition and ENS Inflammation in the Context of Parkinson’s Disease
The gut membrane acts as an obstruction against intrusion by pathogens (Clairembault et al. 2015; Sachdev and Pimentel 2013). Interruption of GI barriers can prompt a progression of positive feedback loops that significantly disturb the GM to support intestinal inflammation, inflammophiles, and reactive oxygen species in the gut lumen (Levy et al. 2017). In this manner, destabilized GI barriers can be an effective means utilized by the GM to modify the GMBA and for the translocation of bacterial byproducts. This results in enhanced oxidative stress, mucosal permeability, and inflammatory reactions, along with the accumulation of α-syn in the ENS (Houser and Tansey 2017; Houser et al. 2018). GI permeability has frequently been observed to be increased in PD patients compared with healthy control and also in animal models of PD (Kelly et al. 2014) and is accompanied by increased accumulation of α-syn in the ENS, along with tissue oxidative stress. However, morphological alterations of the intestinal epithelial obstructions have been reported by a few studies but no changes have been observed in the gut permeability. Furthermore, numerous studies demonstrated that patients suffering from inflammatory bowel disease, which is known to increase GI permeability, have enhanced the risk of PD development, which additionally suggests a role of intestinal inflammation in the progression of PD (Clairembault et al. 2015; Ilie et al. 2020; Mukherjee et al. 2016).
Studies have reported, that inflammation caused by particular microbial cell structures and pattern-inflammatory recognition receptor signaling pathways might be closely associated with PD. Several reports on enteric inflammation proposed that increased abundance of Escherichia coli (Ilie et al. 2020) may cause high-grade intestinal inflammation and also reflect high exposure to endotoxins, including LPS-binding protein, along with enhanced levels of pro-inflammatory cytokines, for example, IFN-gamma, IL-1β, IL-6, TNF-α and activation of EGCs (Hasegawa et al. 2015; Keshavarzian et al. 2015; Devos et al. 2013). By exploring inflammation in the CNS in PD patients, evidence of increased pro-inflammatory cytokines, such as IL-1β and IL-6 have been reported by the researchers. These findings support the assumption of the existence of the gut-brain axis in PD. At the genetic level, a single nucleotide polymorphism in the CARD15 gene, which is related to inflammatory bowel disease, was found to be over-expressed in PD patients (Ilie et al. 2020; Bialecka et al. 2007). Similarly, it has been reported that methylation of α-syn promoter region affects the expression of α-syn and also the possibility of PD. Hence, the role of enteric microbiome as an epigenetic factor is speculated due to DNA methylation (Mosley et al. 2006; Miranda-Morales et al. 2017). Furthermore, the leucine-rich repeat kinase 2 (LRRK2) gene was reported to be highly associated with both familial and sporadic PD and is accepted as the significantly susceptible locus for PD (Liu et al. 2015; Hui et al. 2018). The mRNA expression levels of pro-inflammatory cytokines were found to be significantly dysregulated in the ascending colon of PD patients, as evaluated utilizing real-time PCR to investigate colonic biopsies from 19 PD patients (Devos et al. 2013). Though, the expression levels of pro-inflammatory cytokine were reported to be considerably heterogeneous among PD patients, with certain subjects indicating comparative levels to control subjects and others displaying four- to sixfold upregulation compared with the healthy control group (Devos et al. 2013). Recently, a study has reported that the allele-specific for apolipoprotein-E (ApoE) can alleviate the risk of dementia in synucleinopathies disorders. This ApoE influences the bile acid secretion and thereby triggers the conditions that affect and alters the gut microbial composition, which increases the abundance of microorganisms causing aggregations of the misfolded proteins (Keshavarzian et al. 2015) (Fig. 3).
Schematic representation of the mechanism involved in gut inflammation-induced neuronal cell death in the brain. GI inflammation may increase intestinal epithelial permeability and thereby, allowing the leakage of microbes and their byproducts which may elicit α-syn accumulation and pro-inflammatory cytokine release in the ENS. Pathological α-syn may propagate to the midbrain through the vagal nerve and pro-inflammatory cytokines reach to the brain via the humoral pathway or stimulate the vagus nerve for the production of pro-inflammatory factors in the brain. Pro-inflammatory cytokines together with synucleiopathies in the brain might induce neuronal cell injury and eventually cell death, which further, increase acute inflammatory responses
Also, IL-1β, TNF-α, and glial fibrillary acidic (GFAP) Protein were over-expressed in the ascending colon when 6-hydroxydopamine was administered into the forebrain bundle of a Wistar rat PD model (Carolina et al. 2016; Clairembault et al. 2014). Ongoing studies likewise also found raised levels of IL-1β, IL-1α and C-reactive protein in the fecal samples of PD patients with a multiplex immunoassay, which additionally exhibited connections of this protein with age and span of PD (Houser et al. 1997). As per the current literature, inflammatory mediators in the ENS and CNS can be separated into four most probable mechanisms. (Heintz-Buschart et al. 2018a) CNS lymphatics; where dural sinuses transmit both immune cells and fluid from the cerebrospinal fluid, demonstrating a potential pathway between the GI tract and the CNS (Louveau et al. 2016). (Keshavarzian et al. 2015) Interaction between TOL and α-syn; the interaction may initiate microglial reactions, aggregation of α-syn, and results in an enhanced degeneration of dopaminergic neurons in the substantia nigra (Takeuchi and Akira 2010). (Lin et al. 2018a) Molecular mimicry: a mechanism where self-antigens share nucleotide sequences or structural similarities with foreign antigens (Friedland 2015). The mechanism in PD may start in the GM, where the secretion of extracellular amyloid antigens can lead to stimulation of the innate immune system (Schwartz and Boles 2013). (Mulak and Bonaz 2015) Inflammaging: Inflammaging: the concept that ageing is correlated to increased low-grade chronic inflammation, which usually appears due to latent infections along with enhanced levels of inflammatory markers, for example, IL-6, TNF-α and C-reactive protein. It is related to ageing and modifications of the GM (Biagi et al. 2013). Altogether, the entirety of the above results demonstrates that the GM is associated with gastrointestinal permeability and the inflammatory reaction in PD patients. Though there have been no consistent inferences concerning the specific effect of the GM imbalance in gut inflammation and mucosal permeability in PD, these studies are consistent with interpretations that the GM might be somehow involved in inflammatory responses in the GI tract and CNS in PD and hence, may influence PD pathology.
Clinical Evidence
Several case studies have reported the altered GM composition in PD patients when compared to healthy control. Keshavarzian et al. (2015), has reported the first study of abnormal and altered gut microbial structure, function and composition in PD patients (Keshavarzian et al. 2015). Later, approximately 15 more studies have come forward from USA, Eastern Europe, Asia and Western, Eastern Europe which confirm the role of gut microbiota in PD (Table 1).
Conclusion
Currently, GM is widely accepted as a vital modulator for human physiology. Even though it is a recent entrant of the scientific domain, GM’s influence on the human brain and behavior has attracted enormous consideration across the world. Research has shown that the gut microbiota is efficient in modulating the brain neurochemistry precisely through the biochemical mechanisms such as endocrine, neural, and immune system. Gut microbiota can influence various standard benchmarks of PD. It can modulate the striate gene expression, dopamine turnover, locomotion, dopaminergic receptor expression, oxidative stress, synaptic plasticity, and several other factors in various brain locations. Recent evidence has suggested that the GM is altered in PD patients and thus this gut dysbiosis is linked to motor fluctuations. These alterations can be manipulated in a way to use as biomarkers for PD pathophysiology. Although, certain links remain missing from the puzzle of “GMBA” in PD, because of which the need to foster a cause-and-effect relationship between the trifecta remains.
Recent approaches specified in the review article have set the basis for the identification of the biochemical mechanisms underlying the power of gut microbiota to modulate and thus influence the host’s brain and behavior. Further insights into this trifecta (microbiota-gut-brain) will enable identification of the missing links between GM and PD etiology. If this gut-brain axis hypothesis is validated and the gut microbiota is successfully proved to be involved in PD pathogenesis, a new therapeutic and alternative drug regimen could be considered with a specific focus on dietary interventions aiming to retain healthy GM.
References
Andrews ZB, Erion D, Beiler R (2009) Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci 29:14057–14065
Arumugam M, Raes J, Pelletier E, Paslier DL, Yamada T, Mende DR et al (2011) Addendum: enterotypes of the human gut microbiome. Nature 473:174–180
Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M (2011) Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25(3):397–407
Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, Pinelli G (2019) Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov Disord 34(3):396–405
Barrenschee M, Zorenkov D, Böttner M, Lange C, Cossais F, Scharf AB, Wedel T (2017) Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathologica Commun 5(1):1–14
Bedarf JR, Hildebrand F, Coelho LP et al (2017) “Functional implications of microbial and viral gut metagenome changes in early stage L-dopa-naïve Parkinson’s disease patients. Genome Med 9(1):39
Berer K, Krishnamoorthy G (2012) Commensal gut flora and brain autoimmunity: a love or hate affair? Acta Neuropathol 123(5):639–651
Bested AC, Logan AC, Selhub EM (2013) Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part I—autointoxication revisited. Gut Pathog 5:5
Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P (2013) Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res 69:11–20
Bialecka M, Kurzawski M, Klodowska-Duda G, Opala G, Juzwiak S, Kurzawski G et al (2007) CARD15 variants in patients with sporadic Parkinson’s disease. Neurosci Res 57:473–6
Bogunovic M, Davé SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Plevy SE (2007) Enteroendocrine cells express functional Toll-like receptors. Am J Physiol-Gastrointest Liver Physiol 292(6):G1770–G1783
Böttner M, Zorenkov D, Hellwig I, Barrenschee M, Harde J, Fricke T, Arlt A (2012) Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol Dis 48(3):474–480
Braak H, Del Tredici K, Rüb U, De Vos RA, Steur ENJ, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Burgueño JF, Abreu MT (2020) Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-019-0261-4
Çamci G, Oguz S (2016) Association between Parkinson’s disease and Helicobacter pylori. J Clin Neurol 12:147–150
Caputi V, Giron MC (2018) Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int J Mol Sci 19(6):1689
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203
Carolina P, Matteo F, Rocchina C, Erika T, Fabio B, Giovanna L et al (2016) Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J Neuroinflamm 13:1–13
Cassani E, Privitera G, Pezzoli G et al (2011) Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol 57(2):117–121
Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM, Son F (2016) Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci Rep 6(1):1–10
Chen QQ, Haikal C, Li W, Li JY (2019) Gut inflammation in association with pathogenesis of Parkinson’s disease. Front Mol Neurosci 12:218
Clairembault T, Kamphuis W, Leclair-Visonneau L, Rolli-Derkinderen M, Coron E, Neunlist M et al (2014) Enteric GFAP expression and phosphorylation in Parkinson’s disease. J Neurochem 130:805–815
Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F et al (2015) Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 3:12
Coimbra CG, Junqueira VB (2003) High doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson’s disease patients. Braz J Med Biol Res 36(10):1409–1417
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712
Darby TM, Owens JA, Saeedi BJ, Luo L, Matthews JD, Robinson BS, Naudin CR, Jones RM (2019) Lactococcuslactis subsp. cremoris Is an efficacious beneficial bacterium that limits tissue injury in the intestine. Iscience 12:356–367
De Vadder F, Kovatcheva-Datchary P, Goncalves D et al (2014) Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156(1–2):84–96
Derkinderen P, Shannon KM, Brundin P (2014) Gut feelings about smoking and coffee in Parkinson’s disease. Mov Disord 29:976–979
Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H et al (2013) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50:42–48
Dinan, T. G., & Cryan, J. F. (2017). Gut feelings on Parkinson’s and depression. In Cerebrum: the Dana forum on brain science (Vol. 2017). Dana Foundation.
Dodiya HB, Forsyth CB, Voigt RM et al (2018) Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol Dis 135:104352
Eisenhofer G, Åneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H et al (1997) Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 82:3864–3871
Elfil M, Kamel S, Kandil M, Koo BB, Schaefer SM (2020) Implications of the gut microbiome in Parkinson’s Disease. Mov Disord 35:921–933
Engen PA, Dodiya HB, Naqib A, Forsyth CB, Green SJ, Voigt RM, Keshavarzian A (2017) The potential role of gut-derived inflammation in multiple system atrophy. J Parkinson’s Dis 7(2):331–346
Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Cbc ER et al (2013) The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord 28:1241–1249
Feher J (2017) 8.3—Intestinal and colonic chemoreception and motility.
Fitzgerald E, Murphy S, Martinson HA (2019) Alpha-synuclein pathology and the role of the microbiota in Parkinson’s disease. Front Neurosci 13:369
Forsyth CB, Shannon KM, Kordower JH et al (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 6(12):e28032
Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S (2017) The evolution of the host microbiome as an ecosystem on a leash. Nature 548(7665):43–51
Freestone P (2013) Communication between bacteria and their hosts. Scientifica 2013:1–15
Friedland RP (2015) Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis 45:349–362
Friedland RP, Chapman MR (2017) The role of microbial amyloid in neurodegeneration. PLoS Pathog 13(12):e1006654
Galan JE, Collmer A (1999) Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322–1328
Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Bailey MT (2014) Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol 14(1):189
Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N (2013) Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol 13:869–874
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934
Gray MT, Munoz DG, Gray DA, Schlossmacher MG, Woulfe JM (2014) Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord 29(8):991–998
Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K et al (2015) Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS ONE 10:e0142164
Heintz-Buschart A, Pandey U, Wicke T et al (2018a) The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33(1):88–98
Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, Wilmes P (2018b) The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33(1):88–98
Hill-Burns EM, Debelius JW, Morton JT et al (2017a) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32(5):739–749
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD et al (2017b) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32:739–749
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R (2017c) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32(5):739–749
Hoffman BU, Lumpkin EA (2018) A gut feeling. Science 361:1203–1204
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Li JY (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128(6):805–820
Hopfner F, Künstner A, Müller SH et al (2017) Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 1667:41–45
Houser MC, Tansey MG (2017) The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis 3:3. https://doi.org/10.1038/s41531-016-0002-0
Houser MC, Chang J, Factor SA, Molho ES, Zabetian CP, Hillburns EM et al (1997) Stool immune profiles evince gastrointestinal inflammation in Parkinson’s disease. Neurobiol Dis 30:125–143
Houser MC, Chang J, Factor SA, Molho ES, Zabetian CP, Hill-Burns EM et al (2018) Stool immune profiles evince gastrointestinal inflammation in Parkinson’s disease. Mov Disord 33:793–804
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Patterson PH (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155(7):1451–1463
Hueck CJ (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62:379–433
Hui KY, Fernandez-Hernandez H, Hu J, Schaffner A, Pankratz N, Hsu NY et al (2018) Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci Transl Med 10:eaai7795
Ilie OD, Ciobica A, McKenna J, Doroftei B, Mavroudis I (2020) Minireview on the relations between gut microflora and Parkinson’s disease: further biochemical (oxidative stress), inflammatory, and neurological particularities. Oxid Med Cell Longev 2020:1–15
Johnson KVA, Foster KR (2018) Why does the microbiome affect behaviour? Nat Rev Microbiol 16(10):647–655
Kamada N, Seo SU, Chen GY, Núñez G (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13(5):321–335
Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA et al (2014) Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29:999–1009
Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG (2017) Cross talk: the microbiota and neurodevelopmental disorders. Front Neurosci 11:490
Keshavarzian A, Green SJ, Engen PA et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30(10):1351–1360
Klann E, Tagliamonte MS, Ukhanova M, Mai V, Vedam-Mai V (2020) Gut microbiota dynamics in Parkinsonian mice. ACS Chem Neurosci 11(20):3267–3276
König J, Mall JPM, Rangel I, Edebol H, Brummer RJ (2015) The role of the gut microbiota in brain function. In: Venama K, Carmo PA (eds) Probiotics and prebiotics: current research and future trends. Caister Academic Press, Norfolk, pp 381–389
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14(5):504–506
Latorre R, Sternini C, De Giorgio R, Greenwood-Van Meerveld B (2016) Enteroendocrine cells: a review of their role in brain–gut communication. Neurogastroenterol Motil 28(5):620–630
Levy M, Kolodziejczyk AA, Thaiss CA, Elinav EJNRI (2017) Dysbiosis and the immune system. Nat Rev Immunol 17:219–232
Li W, Wu X, Hu X, Wang T, Liang S, Duan Y, Jin F, Qin B (2017a) Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci 60(11):1223–1233
Li W, Wu X, Hu X et al (2017b) Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci 60(11):1223–1233
Li C, Cui L, Yang Y, Miao J, Zhao X, Zhang J, Zhang Y (2019) Gut microbiota differs between Parkinson’s disease patients and healthy controls in northeast China. Front Mol Neurosci 12:171
Liddle RA (2019) Neuropods. Cell Mol Gastroenterol Hepatol 7(4):739–747
Lin A, Zheng W, He Y et al (2018a) Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 53:82–88
Lin A, Zheng W, He Y, Tang W, Wei X, He R, Xie H (2018b) Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 53:82–88
Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M, Adler CH, Derkinderen P, Beach TG (2018) Does Parkinson’s disease start in the gut? Acta Neuropathol 135(1):1–12
Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A et al (2015) Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 47:979–986
Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, Wirdefeldt K (2017a) Vagotomy and Parkinson disease: a Swedish register–based matched-cohort study. Neurology 88(21):1996–2002
Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Xia H (2017b) Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 23(7):859
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD et al (2016) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341
Manrique P, Dills M, Young MJ (2017) The human gut phage community and its implications for health and disease. Viruses 9(6):141
Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, Tanner CM (2018) Prevalence of Parkinson’s disease across North America. NPJ Parkinson’s Dis 4(1):1–7
Martin CR, Osadchiy V, Kalani A, Mayer EA (2018) The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 6(2):133–148
Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12:5–9
Mayer EA, Savidge T, Shulman RJ (2014) Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 146:1500–1512
Mazzoli R, Pessione E (2016) The neuro-endocrinological role of microbial glutamate and GABA signaling. Front Microbiol 7:1934
McNaught KSP, Olanow CW, Halliwell B, Isacson O, Jenner P (2001) Failure of the ubiquitin–proteasome system in Parkinson’s disease. Nat Rev Neurosci 2(8):589–594
Mertsalmi TH, Aho VT, Pereira PA, Paulin L, Pekkonen E, Auvinen P, Scheperjans F (2017) More than constipation–bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur J Neurol 24(11):1375–1383
Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K et al (2017) Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. PLoS ONE 12:e0187307
Miranda-Morales E, Meier K, Sandoval-Carrillo A, Salas-Pacheco J, Vázquez-Cárdenas P, Arias-Carrión O (2017) Implications of DNA methylation in Parkinson’s disease. Front Mol Neurosci 10:225
Moeller AH, Caro-Quintero A, Mjungu D, Georgiev AV, Lonsdorf EV, Muller MN, PuseyPeetersHahnOchman AEMBHH (2016) Cospeciation of gut microbiota with hominids. Science 353(6297):380–382
Moro T, Rasmussen H, Hamaker B (2019) Potential of prebiotic butyrogenic fibers in Parkinson’s disease. Front Neurol 10:663
Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Gendelman HE (2006) Neuroinflammation, oxidative stress, and the pathogenesis of Parkinson’s disease. Clin Neurosci Res 6(5):261–281
Mukherjee A, Biswas A, Das SK (2016) Gut dysfunction in Parkinson’s disease. World J Gastroenterol 22:5742–5752
Mulak A, Bonaz B (2015) Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol 21(37):10609–10620
Nair AT, Ramachandran V, Joghee NM, Antony S, Ramalingam G (2018) Gut microbiota dysfunction as reliable non-invasive early diagnostic biomarkers in the pathophysiology of Parkinson’s disease: a critical review. J Neurogastroenterol Motil 24(1):30
Noble EE, Hsu TM, Kanoski SE (2017) Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci 11:9
Pan-Montojo F, Anichtchik O, Dening Y et al (2010) Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 5(1):e8762
Perez-Pardo P, Kliest T, Dodiya HB, Broersen LM, Garssen J, Keshavarzian A, Kraneveld AD (2017) The gut-brain axis in Parkinson’s disease: possibilities for food-based therapies. Eur J Pharmacol 817:86–95
Perez-Pardo P, Dodiya HB, Engen PA et al (2018) Gut bacterial composition in a mouse model of Parkinson’s disease. Benef Microb 9(5):799–814
Perez-Pardo P, Dodiya HB, Engen PA et al (2019) Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68(5):829–843
Qian Y, Yang X, Xu S et al (2018) Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70:194–202
Quigley EM, Quera R (2006) Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology 130:S78-90
Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, Fariñas I (2014) Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75(3):351–362
Rekdal VM, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP (2019) Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science 364(6445):6323
Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJ, Kraneveld AD (2017) Exploring Braak’s hypothesis of Parkinson’s disease. Front Neurol 8:37
Sachdev AH, Pimentel M (2013) Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis 4(5):223–231
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–80.e12
Santos SF, de Oliveira HL, Yamada ES, Neves BC, Pereira A (2019) The gut and Parkinson’s disease–a bidirectional pathway. Front Neurol 10:574
Scheperjans F (2018) The prodromal microbiome. Mov Disord 33(1):5–7
Scheperjans F, Aho V, Pereira PA et al (2015a) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30(3):350–358
Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E et al (2015b) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358
Schwartz K, Boles BR (2013) Microbial amyloids—functions and interactions within the host. Curr Opin Microbiol 16:93–99
Shen L (2020) Gut, oral and nasal microbiota and Parkinson’s disease. Microb Cell Fact 19(1):1–7
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H et al (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139
Smith MP, Fletcher-Turner A, Yurek DM, Cass WA (2006) Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res 31(4):533–539
Soto C, Pritzkow S (2018) Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci 21(10):1332–1340
Steiner JA, Quansah E, Brundin P (2018) The concept of alpha-synuclein as a prion-like protein: ten years after. Cell Tissue Res 373(1):161–173
Stolp HB, Dziegielewska KM, Ek CJ, Potter AM, Saunders NR (2005) Long-term changes in blood-brain barrier permeability and white matter following prolonged systemic inflammation in early development in the rat. Eur J Neurosci 22(11):2805–2816
Stopschinski BE, Diamond MI (2017) The prion model for progression and diversity of neurodegenerative diseases. Lancet Neurol 16(4):323–332
Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, Mroue N (2019) GABA-modulating bacteria of the human gut microbiota. Nat Microbiol 4(3):396–403
Sudo N (2012) Role of microbiome in regulating the HPA axis and its relevance to allergy. Chem Immunol Allergy 98:163–175
Suzuki M, Yoshioka M, Hashimoto M et al (2013) Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr 97(5):1004–1013
Takeuchi O, Akira SJC (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
Tan AH, Mahadeva S, Thalha AM, Gibson PR, Kiew CK, Yeat CM et al (2014) Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat Disord 20:535–540
Tan AH, Chong CW, Song SL, Teh CSJ, Yap IKS, Loke MF, Tan YQ, Yong HS, Mahadeva S, Lang AE, Lim SY (2018) Altered gut microbiome and metabolome in patients with multiple system atrophy. Mov Disord 33(1):174
Tetz G, Brown SM, Hao Y, Tetz V (2018) Parkinson’s disease and bacteriophages as its overlooked contributors. Sci Rep 8(1):10812
Tsigos C, Kyrou I, Kassi E, Chrousos GP (2016) Stress, endocrine physiology and pathophysiology. In: De Groot LJ, Chrousos G, Dungan K et al (eds) Endotext [Internet]. MDText. com Inc, South Dartmouth
Unger MM, Möller JC, Mankel K, Schmittinger K, Eggert KM, Stamelou M et al (2011) Patients with idiopathic rapid-eye-movement sleep behavior disorder show normal gastric motility assessed by the 13C-octanoate breath test. Mov Disord 26:2559–2563
Unger MM, Spiegel J, Dillmann KU et al (2016a) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Schäfer KH (2016b) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72
van Kessel SP, Frye AK, El-Gendy AO et al (2019) Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 10(1):310
Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C (2014) Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol 817:221–239
Yang D, Zhao D, Shah SZA, Wu W, Lai M, Zhang X, Gao H (2019) The role of the gut microbiota in the pathogenesis of Parkinson’s disease. Front Neurol 10:1155
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Hsiao EY (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161(2):264–276
Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G, Chen X (2020) The progress of gut microbiome research related to brain disorders. J Neuroinflamm 17(1):25
Acknowledgements
We acknowledge Dr. Ashok Chauhan, Founder, Amity University and honorable Chancellor Sir for providing us infrastructure and facilities and University Grant Commission (Government of India) for granting senior research fellowship to Ms Nitu Dogra to undertake this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dogra, N., Mani, R.J. & Katare, D.P. The Gut-Brain Axis: Two Ways Signaling in Parkinson’s Disease. Cell Mol Neurobiol 42, 315–332 (2022). https://doi.org/10.1007/s10571-021-01066-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01066-7