Abstract
Background
The degree of contamination of healthy tissue with tumor cells during a biopsy in bone or soft tissue sarcomas is clearly dependant on the type of biopsy. Some studies have confirmed a clinically relevant contamination of the biopsy tract after incisional biopsies, as opposed to core-needle biopsies. The aim of our prospective study was to evaluate the risk of local recurrence depending on the biopsy type in extremity and pelvis sarcomas.
Methods
We included 162 patients with a minimum follow-up of 6 months after wide resection of extremity sarcomas. All diagnostic and therapeutic procedures were performed at a single, dedicated sarcoma center. The excision of the biopsy tract after an incisional biopsy was performed as a standard with all tumor resections. All patients received their follow-up after the conclusion of therapy at our center by means of regional MRI studies and, at a minimum, CT of the thorax to rule out pulmonary metastatic disease. The aim of the study was the evaluation of the influence of the biopsy type and of several other clinical factors on the rate of local recurrence and on the time of local recurrence-free survival.
Results
One hundred sixty-two patients with bone or soft tissue tumors of the extremities and the pelvis underwent either an incisional or a core-needle biopsy of their tumor, with 70 sarcomas (43.2%) being located in the bone. 84.6% of all biopsies were performed as core-needle biopsies. The median follow-up time was 55.6 months, and 22 patients (13.6%) developed a local recurrence after a median time of 22.4 months. There were no significant differences between incisional and core-needle biopsy regarding the risk of local recurrence in our subgroup analysis with differentiation by kind of tissue, grading of the sarcoma, and perioperative multimodal therapy.
Conclusions
In a large and homogenous cohort of extremity and pelvic sarcomas, we did not find significant differences between the groups of incisional and core-needle biopsy regarding the risk of local recurrence. The excision of the biopsy tract after incisional biopsy in the context of the definitive tumor resection seems to be the decisive factor for this result.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
The scientific discussion regarding the standards for diagnostic biopsies in sarcomas of the extremities is ongoing. The choice between core-needle biopsy (CNB) and incisional biopsy (IB) depends not only on medical indications (e.g., location of the tumor, proximity of the nerve and vessel routs), but also on the experience of the surgeon and the pathologist and on the infrastructure. The diagnostic accuracy appears to be equal with both types of biopsy and for both, soft tissue and bone sarcomas [1,2,3]. Other studies have shown that no relevant tumor cell seeding occurs in the biopsy tract during CNB and that not excising the biopsy tract does not increase the risk of local recurrence (LR) [4, 5]. As a consequence, if excising the biopsy tract is not regarded as mandatory in CNB, less patients have to undergo plastic surgery for closure of resulting larger defects. Tissue sampling can also be done much faster and without general anesthesia, if CNB is used.
Previous studies have investigated the contamination of the biopsy tracts in soft tissue and bone sarcomas with both kinds of biopsies. The significantly higher tumor contamination of the biopsy tracts in IB cases suggests a significantly higher risk of LR [6]. While an excision of the tract in CNB cases does not seem to be necessary with respect to local recurrence-free survival (LRFS), the wide excision of IB tracts is important. There are not many studies investigating the influence of biopsy type on local recurrence with a relevant number of included cases [7,8,9]. Other factors influencing LRFS or overall survival (OS), like margins, grading, and perioperative multidisciplinary approach, are well known [10].
We prospectively recorded the follow-up of surgically treated patients at our institution, who had a biopsy between 2013 and 2018. The subject of this study was two questions: Does the type of biopsy influence the risk of LR and LRFS? Are there differences between subgroups (bone/soft tissue sarcoma, perioperative therapy) at our cohort?
Methods
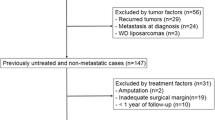
Patients, treated between 2013 and 2018 at our department, were evaluated. We included 174 patients in this study. Inclusion criteria were:
-
Final diagnosis of a primary sarcoma of bone or soft tissue of the extremities and pelvis
-
Biopsy performed at our institution
-
Final surgery performed at our institution
-
Wide (R0) resection of the tumor
-
Follow-up > 6 months
A team of two experienced surgeons performed all biopsies and resections of the tumors. Two different biopsy approaches were used: CNB and IB. A CNB without guidance was performed in cases of well-palpable tumors without risk of injury of vessels or nerves. Computed tomography (CT) or sonographic guidance in CNB was used for deep tumors. IB was used in cases with thick cortical bone that required drilling or with anatomically complex location of soft tissue sarcomas. After confirmation of the diagnosis, staging investigations were performed by means of magnetic resonance imaging (MRI) of the complete affected compartment and CT of the thorax and abdomen. In cases of Ewing sarcomas or osteosarcomas, positron-emitting tomography-CT (PET/CT) was used for staging. Depending on the entity, resection margins, and additional factors, neo-/adjuvant local radiotherapy or chemo-/targeted therapy was individually decided in the setting of an interdisciplinary tumor board.
Bone sarcomas were treated according to the established standards: chondrosarcomas underwent primary surgical resection (only dedifferentiated chondrosarcomas underwent an interdisciplinary approach). High-grade osteosarcomas were treated according to the EURO-B.O.S.S [11]. protocol or EURAMOS-1 protocol [12]; Ewing sarcomas underwent an interdisciplinary therapy according to the Euro-EWING 99 trial [13].
High-grade soft tissue sarcomas were treated interdisciplinary according to the guidelines of the European Society for Medical Oncology (ESMO) [14] with the additional option of regional hyperthermia [15]. Neo- or adjuvant radiotherapy was added in dependence of the entity, location, size, grading, and resection margins.
Two sarcoma-experienced pathologists performed all histopathological studies. Every sarcoma biopsy sample and specimen was characterized according to the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system: G1 as low-grade sarcoma and G2 and G3 as high-grade sarcoma. Tumor-free resection margins were defined as R0 resection, contaminated margins as R1, and intralesional margins as R2 resections. Only patients with R0 resections were included in this study (see the inclusion criteria).
In cases of CNB, we did not excise the biopsy tract during the tumor resection. In IB, we included the biopsy tract into a wide resection with a safety distance of ≥ 2 cm in all directions.
For follow-up, every sarcoma patient underwent regional MRI investigations (in some cases, e.g., myxoid liposarcoma additional extended MRI of the spine and pelvis) and CT of the thorax (in some cases also CT of the abdomen) in regular intervals: for the first year, every 3 months postoperatively, then either every 3 or 6 months depending on the type of sarcoma.
To examine our results for statistical significance, analysis was performed using the chi-square test set at a 95% confidence interval. For groups smaller than 20 individuals, we used Fisher’s exact test. Multivariate analysis (Cox proportional hazards regression) was used for the evaluation of a potential influence of patient characteristics on the risk of LR and on LRFS. The level of significance was set at less than 0.05. Local recurrence-free survival was calculated according to the Kaplan-Meier method, using the log-rank test for significance testing. The data analysis software used was IBM® SPSS® Statistics 25.
This study was approved by the institutional ethics committee. Written consent was obtained from all surviving patients included in this study. For non-surviving patients, data were irreversibly anonymized as recommended by the ethics committee.
Results
Total cohort
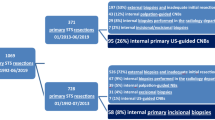
We included 174 patients, who met our inclusion not our exclusion criteria. Twelve patients were excluded because of a follow-up period of less than 6 months (lost to follow-up or died due to their tumor within 6 months after resection). Hence, complete data of 162 patients were available for analysis. The median follow-up time was 55.6 months (mean 52.9, range 7–103 months). Eighty-seven patients (53.7%) were male and the median age of all patients was 52.9 years (mean 50.0, range 10–86 years). Further characteristics of the patient cohort are summarized in Table 1; anatomic distribution of tumor sites and entities are demonstrated in Figs. 1 and 2. One hundred thirty-seven biopsies (84.6%) were performed as CNB and 25 (15.4%) as IB. Seventy tumors (43.2%) were located in the bone and 92 (56.8%) in soft tissue.
Radio-/chemotherapy (in some cases combined with local hyperthermia) was performed in 118 cases (72.8%). Twenty-two patients (13.6%) developed local recurrence in a median of 22.4 months (mean 28.7, range 4–48) after tumor resection.
The cohort was divided into two groups with respect to the type of biopsy: CNB and IB (Fig. 3). Both groups were comparable regarding the major clinical factors as kind of tissue (p = 0.254), grading (p = 0.069), and perioperative therapy (p = 0.681). There were 20 LR in 137 cases (14.6%) in patients with CNB and 2 LR in 25 cases (8%) of IB (p = 0.376).
Bone sarcomas
Seventy patients with bone sarcomas were included in this study. Fifty-eight (82.9%) biopsies were performed as CNB and 12 (17.1%) as IB. There were 11 (15.7%) cases with a LR. Ten (17.2%) of these occurred after CNB and one (8.3%) after IB (p = 0.675). The median LRFS of all patients with bone sarcomas was 54.9 months (range 4–104 months). The median LRFS in CNB was 49.5 months and in IB 69.1 months (p = 0.476; Fig. 4). Seven of 11 LR were chondrosarcomas (23.3% of all chondrosarcomas), 3 osteosarcomas (11.5%), and 1 Ewing sarcoma (7.7%) (p = 0.440).
Grading and perioperative multimodal therapy are factors that potentially could have influenced the occurrence of LR independently of the type of biopsy. They were evaluated in a univariate analysis and showed no significant influence on LR rate in this highly selected group of patients (grading p = 0.224; multimodal therapy p = 0.448).
If grading and multimodal therapy were used as separating factors, the type of biopsy also showed no influence on LR risk (3 groups of grading p = 0.440; two groups of multimodal therapy p = 0.395). Multivariate analysis of the data returned the same result (grading p = 0.442, 95% confidence interval −0.219–0.097; multimodal therapy p = 0.859, 95% confidence interval −0.220–0.263). The type of biopsy also had no influence within the subgroups of chondrosarcoma (p = 0.468), osteosarcoma (p = 0.592), and Ewing sarcoma (p = 0.923).
Soft tissue sarcomas
Ninety-two patients with STS underwent biopsy and sarcoma resection. The biopsy was performed as CNB in 79 patients (85.9%) and 13 (14.1%) underwent IB. LR occurred in 11 (12%) cases. In 79 CNBs, 10 (12.7%) cases with LR were seen; in 13 IBs, one LR (7.7%) was evident (p = 0.517). Six of 11 LR were non otherwise specified (NOS) sarcomas, 2 malignant peripheral nerve sheath tumor (MPNST), and each one fibrosarcoma, rhabdomyosarcoma, and leiomyosarcoma.
The comparison of LR rate between low-grade and high-grade tumors showed a trend towards a higher rate in high-grade sarcomas (13.8% vs 0%); however, this trend did not reach significance (p = 0.195). Multimodal therapy also did not influence the risk of LR (p = 0.472). The median LRFS in CNB was 48.5 months and in IB 60.9 months (p = 0.611; Fig. 5).
Also in STS, there was no significant influence of tumor grading (p = 0.517) and multimodal treatment (p = 0.505) on the rate of LR in both types of biopsies using univariate analysis. Multivariate analysis for grading (p = 0.165, 95% confidence interval −0.173–0.030) and perioperative therapy (p = 0.869, 95% confidence interval −0.213–0.180) showed the same.
Discussion
A multidisciplinary approach in the therapy of STS and BS is well-established [16, 17]. The influencing risk factors for survival of the patients as well as clinical outcomes have been the subject of some studies. However, the number of publications investigating the role of biopsy for the risk of local recurrence in sarcoma treatment is limited. The basis of this discussion is findings in the histopathological evaluation of the biopsy tracts with contaminating tumor cells. Some published data clearly show a significant difference between a high tumor cell load in an IB tract and a hardly existent tumor cells in CNB tracts [6, 18]. Therefore, there is a change in opinion regarding the excision of biopsy tracts in CNB and an increasing number of authors are not advocating such a strategy, including our institution [4, 7]. However, those studies included also cases with positive resection margins.
We were able to investigate a homogenous cohort of sarcoma patients, which underwent diagnostic and treatment procedures in a dedicated sarcoma center under the care of only two surgeons. Due to exclusion of patients with “whoops surgery,” positive margins, and non-standard treatment, we resulted in a good comparability of subgroups.
We were unable to identify any significant differences between CNB and IB regarding LR risk within the whole cohort and within individual subgroups. Our results do not show a significant difference in the LR rate between CNB and IB; however, we observed a trend towards a disadvantage of CNB with LR in 14.6% of cases, compared to 8% in IB cases. This finding represents a contradiction to the published literature: Barrientos-Ruiz et al. [6] showed a significantly higher degree of biopsy tract seeding with tumor cells in the case of IB. In their study, the biopsy tract was resected in every case, also in CNB. However, at the end, an IB was associated with an increased risk of LR and the study by Oliveira et al. found the same results [8]. One might conclude that non-resection of the biopsy tract in cases with CNBs in our study cohort causes the non-significant, but in trend higher rates of LR. The literature stands opposed to this perception. Berger-Richardson et al. [9] pooled 3 studies with a total of 63 patients in their review. In one of those studies, a contamination of excised biopsy tracts after CNB was seen in 13%. In another described cohort with 30 patients without excision of the biopsy tract after CNB, there were no cases of LR. Not excising the biopsy tract after CNB carried a risk of LR of 8% in the study by Binitie et al. [4]. The authors concluded, the same number in other studies including patients with resected biopsy tracts prove no influence on LR rate.
The risk of LR depends on many factors, such as entity, grading, and multimodal therapy. The value of multimodal therapy and its influence on the local recurrence rate is well known [16, 19]. However, there are tumor entities without further therapy options, except surgery (e.g., chondrosarcoma, superficial low-grade soft tissue sarcomas). In both groups and with or without multimodal treatment, we did not see any significant influence of the biopsy type on the LR rate or on LRFS.
In addition, the influence of grading on the LR rate is also well established. In cases with low-grade sarcoma, the risk of LR is significantly lower [20, 21]. Due to the low numbers of patients in this particular subgroup, we were unable to duplicate these findings in our highly selected study group. However, within both subgroups of low- and high-grade tumors, almost identical results for CNB and IB were seen.
The biological characteristics and behavior of bone and soft tissue sarcomas are different [22, 23]. However, we did not observe any differences between STS and BS regarding the type of biopsy. The evaluation of differences in entity subgroups of STS was not feasible because of the high number of entities and the consecutively very small numbers of patients in each subgroup. The subgroup analysis in BS showed a high rate of LR in chondrosarcomas (23.3%); however, this difference did not reach statistical significance in relation to other entities. A possible explanation for the observed difference could be the location of these tumors. Chondrosarcomas often arise in the pelvis with known worse results as compared to LR in extremity tumors. Atypical cartilaginous tumors (chondrosarcomas G1 of the extremities) did not undergo biopsy and were diagnosed by correlation of radiological and histological investigation results. Those cases are hence not included in this study.
Until today, published studies have shown that CNB and IB provide similar sensitivity regarding the diagnosis of sarcomas [3] and that IB has a higher risk for complications [24] and a potentially higher risk of spreading tumor cells [6]. The excision of IB tracts with tumor resection causes larger soft tissue defects and a higher risk of complications [2, 25].
The most important factor for lowering the risk of LR in incisional biopsy seems to be the excision of the biopsy tract. Our analysis of IB and CNB demonstrate an equivalence of both biopsy methods regarding the risk of LR and of LRFS, even if the biopsy tract was not excised in CNB.
Conclusions
We were unable to demonstrate different rates of LR or LRFS in patients with excised IB tracts and patients with non-excised CNB tracts in bone and soft tissue sarcomas of the extremities. Additional factors such as multimodal treatment (including radiotherapy) and grading did not influence this result. Based on our results, the excision of the biopsy tract in cases of CNB does not appear to be necessary.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CNB:
-
Core-needle biopsy
- IB:
-
Incisional biopsy
- LR:
-
Local recurrence
- LRFS:
-
Local recurrence-free survival
- OS:
-
Overall survival
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- PET/CT:
-
Positron-emitting tomography-CT
- STS:
-
Soft tissue sarcoma
- BS:
-
Bone sarcoma
References
Yang J, Frassica FJ, Fayad L, Clark DP, Weber KL. Analysis of nondiagnostic results after image-guided needle biopsies of musculoskeletal lesions. Clin Orthop Relat Res. 2010;468(11):3103–11.
Traina F, Errani C, Toscano A, Pungetti C, Fabbri D, Mazzotti A, et al. Current concepts in the biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 2015;97(1):e7.
Klein A, Fell T, Birkenmaier C, Fromm J, Jansson V, Knösel T, et al. Relative sensitivity of core-needle biopsy and incisional biopsy in the diagnosis of musculoskeletal sarcomas. Cancers. 2021;13(6):1393.
Binitie O, Tejiram S, Conway S, Cheong D, Temple HT, Letson GD. Adult soft tissue sarcoma local recurrence after adjuvant treatment without resection of core needle biopsy tract. Clin Orthop Relat Res. 2013;471(3):891–8.
Siddiqi MA, Kim HS, Jede F, Han I. Association of core needle biopsy tract resection with local recurrence in extremity soft tissue sarcoma. Skelet Radiol. 2017;46(4):507–12.
Barrientos-Ruiz I, Ortiz-Cruz EJ, Serrano-Montilla J, Bernabeu-Taboada D, Pozo-Kreilinger JJ. Are biopsy tracts a concern for seeding and local recurrence in sarcomas? Clin Orthop Relat Res. 2017;475(2):511–8.
Saghieh S, Masrouha KZ, Musallam KM, Mahfouz R, Abboud M, Khoury NJ, et al. The risk of local recurrence along the core-needle biopsy tract in patients with bone sarcomas. Iowa Orthop J. 2010;30:80–3.
Oliveira MP, Lima PM, de Mello RJ. Tumor contamination in the biopsy path of primary malignant bone tumors. Rev Bras Ortop. 2012;47(5):631–7.
Berger-Richardson D, Swallow CJ. Needle tract seeding after percutaneous biopsy of sarcoma: risk/benefit considerations. Cancer. 2017;123(4):560–7.
Bilgeri A, Klein A, Lindner LH, Nachbichler S, Knösel T, Birkenmaier C, et al. The effect of resection margin on local recurrence and survival in high grade soft tissue sarcoma of the extremities: how far is far enough? Cancers. 2020;12(9):E2560.
Ferrari S, Bielack SS, Smeland S, Longhi A, Egerer G, Sundby Hall K, et al. EURO-B.O.S.S.: a European study on chemotherapy in bone-sarcoma patients aged over 40: outcome in primary high-grade osteosarcoma. Tumori. 2018;104(1):30–6.
Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. (Oxford, England: 1990). 2019;109:36–50.
Paulussen M, Craft AW, Lewis I, Hackshaw A, Douglas C, Dunst J, et al. Results of the EICESS-92 Study: two randomized trials of Ewing’s sarcoma treatment--cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol. 2008;26(27):4385–93.
Casali PG, Blay JY. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198–203.
Issels RD, Lindner LH, Verweij J, Wessalowski R, Reichardt P, Wust P, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483–92.
Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70(3):200–29.
Casali PG, Bielack S, Abecassis N, Aro HT, Bauer S, Biagini R, et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv79–95.
Oliveira MP, Lima PM, da Silva HJ, de Mello RJ. Neoplasm seeding in biopsy tract of the musculoskeletal system. A Syst Rev. 2014;22(2):106–10.
Crompton JG, Ogura K, Bernthal NM, Kawai A, Eilber FC. Local control of soft tissue and bone sarcomas. J Clin Oncol. 2018;36(2):111–7.
Fromm J, Klein A, Baur-Melnyk A, Knösel T, Lindner L, Birkenmaier C, et al. Survival and prognostic factors in conventional central chondrosarcoma. BMC Cancer. 2018;18(1):849.
Smolle MA, Andreou D, Tunn PU, Szkandera J, Liegl-Atzwanger B, Leithner A. Diagnosis and treatment of soft-tissue sarcomas of the extremities and trunk. EFORT Open Rev. 2017;2(10):421–31.
Sleijfer S, Gelderblom H. Current clinical trials for advanced osteosarcoma and soft tissue sarcoma. Cur Opin Oncol. 2014;26(4):434–9.
Miwa S, Yamamoto N, Hayashi K, Takeuchi A, Igarashi K, Tsuchiya H. Therapeutic targets for bone and soft-tissue sarcomas. Int J Mole Sci. 2019;20(1):170.
Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78(5):656–63.
Winkler D, Fritzsche H, Schaser KD, Hofbauer C. Biopsy of musculoskeletal tumors: complications and avoidable errors. Der Orthopade. 2020;49(2):88–97.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study did not have any grants or funding despite the academic setting of the institutions and authors.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: AK and HRD; writing—original draft preparation: AK; writing, review, and editing: CB, DDG, JF, TK, and HRD; supervision: HRD, DDG, and TK. Each author has contributed significantly to and is willing to take public responsibility for this study: its design, data acquisition, and analysis and interpretation of data. All authors have been actively involved in the drafting and critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written consent was obtained from all surviving patients included in this study. In accordance with the ethics committee, the data from non-surviving patients were irreversibly anonymized for statistical evaluation.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical Faculty, University of Munich (ID 17-89).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Klein, A., Birkenmaier, C., Fromm, J. et al. Sarcomas of the extremities and the pelvis: comparing local recurrence after incisional and after core-needle biopsy. World J Surg Onc 20, 14 (2022). https://doi.org/10.1186/s12957-021-02481-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-021-02481-2