Abstract
Bone defects pose significant challenges in healthcare, with over 2 million bone repair surgeries performed globally each year. As a burgeoning force in the field of bone tissue engineering, 3D printing offers novel solutions to traditional bone transplantation procedures. However, current 3D-printed bone scaffolds still face three critical challenges in material selection, printing methods, cellular self-organization and co-culture, significantly impeding their clinical application. In this comprehensive review, we delve into the performance criteria that ideal bone scaffolds should possess, with a particular focus on the three core challenges faced by 3D printing technology during clinical translation. We summarize the latest advancements in non-traditional materials and advanced printing techniques, emphasizing the importance of integrating organ-like technologies with bioprinting. This combined approach enables more precise simulation of natural tissue structure and function. Our aim in writing this review is to propose effective strategies to address these challenges and promote the clinical translation of 3D-printed scaffolds for bone defect treatment.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Bone defects are a highly prevalent condition that often results from congenital or acquired factors, such as autoimmune diseases, genetic disorders, degenerative diseases, bone cancer, sports injuries, and traffic accidents [1]. Despite the intrinsic self-healing properties of bones, complete regeneration is not achievable in many cases [2]. Severe bone defects commonly occur in limb and facial bones, necessitating intervention and treatment. To date, certain bone scaffolds have clinical applications, but they often come in standardized shapes [3], failing to meet the demand for personalization. This limitation restricts the applicability of bone scaffolds in treating specific patients, especially those with complex or irregular bone defects. In bone tissue engineering (BTE), considering individual patient factors is crucial. These factors include unique defect shapes, sizes, locations, bone trabeculae, cortical thickness, and pore diameters.
Over the past four decades, there has been significant progress in the development of customized bone scaffolds using 3D printing technology, bringing benefits of cost-effectiveness and shortened manufacturing cycles [4]. Currently, FDA-approved cell-free 3D printing technology is available for large-scale production [5]. However, the products can only serve as supporting structures without bone inductivity or dynamic precision. The emerging 3D bioprinting methods can solve the above problems; however, these techniques are still at a relatively early stage in terms of clinical application. The recent level of technological maturity falls short of meeting clinical demands [6].
Current clinical scaffolds face significant challenges in restoring the complex structure and functionality of bone tissue. These challenges primarily manifest in three aspects. (1) Selecting scaffold materials that simultaneously meet mechanical performance and biological requirements can be difficult. Metal materials exhibit good load-bearing performance but poor biocompatibility, while ceramics and polymers offer good biocompatibility but weaker support capabilities [7]. (2) Printing methods encounter various challenges, including insufficient resolution, slow manufacturing speed, difficulty in creating complex shapes, limited material choices, and a lack of simulation for soft tissues [8]. (3) During the process of bioprinting bone scaffolds, incorporating regenerative cells into the construct and maturing them to tissue repair levels while ensuring recipient acceptance relies on local cell interactions and self-organization—a significant challenge [9].
In the field of regenerative medicine, organoid technology emerges as a promising approach for bone repair, showcasing robust self-renewal and self-organization capabilities that mimic the spatial organization and function of native organs [10, 11]. Bone organoids constructed through 3D bioprinting, with their spatial characteristics and mechanical structures, can withstand high levels of compression and shear loads in implants, demonstrating an inducible osteogenic function. Despite significant advancements in this field, the use of bioprinted organoids for orthopedic tissue engineering is still in its nascent stages. Challenges persist in aspects such as printing speed, resolution, material degradation properties and biocompatibility, as well as cell sourcing and co-culturing, necessitating further attention and resolution.
This review systematically explores the clinical applications and prospects of 3D printing in bone scaffold fabrication, addressing current materials, methods, and their limitations (Fig. 1). We comprehensively evaluated the clinical translation status of 3D-printed bone scaffolds, focusing on the three main challenges currently faced, along with their implications for research progress and potential directions for development. Additionally, we highlight the promising integration of bioprinting with bone organoids in BTE. The review aims to analyze the current applications, challenges, and future trends of 3D printed bone scaffolds, emphasizing the need for innovation in orthopedic treatment technologies through interdisciplinary research in regenerative medicine and tissue engineering.
Performance criteria for ideal bone scaffolds
Bone regeneration constitutes a multifaceted process, encompassing molecular, cellular, biochemical, and mechanical elements. Drawing influence from the natural structure of bones when designing scaffolds for bone tissue regeneration is sensible. Combining these fundamentals with common biomaterial design criteria allows the creation of a set of ideal AM bone scaffold structures and properties.
Composition of the bone microenvironment
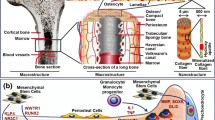
A deep understanding of the natural structure of the bone microenvironment is crucial for better constructing in vitro scaffolds. According to classical bone tissue research, the bone microenvironment is a unique and highly dynamic compartment composed of heterogeneous cells, extracellular matrix, soluble growth factors, and cytokines [12]. Bone tissue contains various types of bone cells with different functions, including osteoblasts that are responsible for bone formation and secrete bone matrix, osteoclasts that are involved in bone resorption and secrete various enzymes and acids to degrade the bone matrix, and osteocytes embedded deep within the bone matrix. Additionally, it includes other cells such as mesenchymal stem cells (MSCs), vascular endothelial cells, and immune cells [13].
One of the primary objectives in 3D printing bone scaffolds is to simulate the bone formation function. MSCs are the main source of bone-forming cells, which is why many studies on 3D-printed scaffolds choose MSCs as the seed cells [14]. The bone matrix, the main non-cellular component of bone tissue, is primarily composed of collagen (about 90%) and calcium phosphate salts (approximately 70% hydroxyapatite and 30% calcium carbonate), providing a survival environment and mechanical support for bone cells. The bone matrix endows bones with the necessary strength and elasticity to withstand mechanical loads.
Additionally, bone tissue contains numerous soluble growth factors and cytokines that regulate bone cell function and bone metabolism, such as bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs). These factors are important considerations when constructing bone scaffolds in vitro [15].
Biocompatibility: immune response avoidance and cellular response induction
First, the scaffold must possess biocompatibility, suggesting that it should not elicit acute or long-term toxic reactions in vitro and in vivo. Additionally, the scaffold surface wettability and chemistry are crucial for promoting early cell adhesion, spreading, and proliferation [16]. Therefore, optimizing scaffold surface hydrophilicity is highly desirable. Last, the scaffold design should be carefully formulated to prevent undesirable host immune responses, such as the development of fibrous tissue [17].
Mechanical properties: balancing strength and flexibility for bone repair
In optimal scenarios, bone tissue engineering scaffolds produced through AM should exhibit supportive attributes that align with the host bone characteristics, whether intended for weight-bearing or non-weight-bearing bone repair applications. This requirement calls for providing sufficient flexibility to enable regular mechanical transfer and sufficient mechanical strength to withstand stresses and maintain stability. Cortical and trabecular (spongy) bone both comprise the tissue of human bone. In contrast to trabecular bone, which has a permeability range of 50–90%, cortical bone demonstrates great density with 5–10% porosity. Approximately 80% of the weight of the human skeleton is composed of cortical bone, whereas the remaining 20% is composed of trabecular bone [18]. Notably, the compressive properties and Young’s modulus of cortical bone vastly outperform those of trabecular bone [19].
Porous structure: key influencing factors for nutrient transport and cell proliferation
In the field of bone tissue engineering, the pursuit of optimal scaffold design often revolves around replicating the hierarchical porous structure found in human bone. The size, number, shape and distribution characteristics of micropores are crucial in the design of an ideal bone scaffold. The 150–800-µm pores provide channels for nutrient transport and metabolite excretion [20]. The 40–100-µm pores facilitate the growth of nonmineralized tissues [21], whereas the 10–100-µm pores promote the growth of capillaries, which contribute to nutrient exchange and waste excretion. Nanosized pores have relatively large specific surface areas and active targets that facilitate cell nucleation and protein adsorption, thus creating a favorable cellular response that promotes tissue regeneration and development [22].
Degradability: matching degradation and new bone formation rates
An ideal bone scaffold should serve as a temporary mechanical support rather than a permanent implant, which suggests that they gradually degrade or are absorbed over time to create space for new bone formation. The scaffold rate of degradation should match the rate at which new tissue forms. If the scaffold degrades too quickly, mechanical failure may occur, while insufficient mechanical support during new bone formation can result in fracture [23]. Conversely, if the scaffold degrades slowly, the presence of foreign material may cause an inflammatory response, preventing tissue regeneration [24]. Of paramount significance is the requirement for degradation byproducts to be nontoxic and easily metabolized by the host. Concretely, these characteristics provide mechanical support for a period of 3–6 months [25] before degrading completely within 12–24 months [26].
Osteoinductivity: orchestrating cellular differentiation and bone regeneration
Concerning clinical applications, bone scaffolds manufactured through 3D printing must exhibit robust osteoinductivity. Osteoinductivity, which is a pivotal attribute, denotes the intrinsic ability of the scaffold to not only stimulate stem cells but also orchestrate their transformation into osteogenic cells, thereby catalyzing the genesis of new bone tissue. This facet of scaffold functionality holds paramount significance in tissue engineering, as it directly influences the potential for successful bone regeneration [27]. To date, numerous scholars have explored the use of 3D bioprinted bone scaffolds loaded with growth factors or stem cells [28]. Unfortunately, successful clinical translation of osteoinductive 3D bioprinted scaffolds has not yet been achieved.
Precision: transitioning from static to dynamic
The external contour of the best BTE scaffold should match the anatomical features of the human bone defect site to ensure the best fit [29]. Beyond its intricate three-dimensional architecture, tissue regeneration entails the orchestration of functional tissue generation through dynamic alterations in tissue configuration [30]. Specifically, the process of bone regeneration is delineated into four stages [31]: (1) Activation: initiation of bone regeneration at specific locations. (2)Resorption: bone resorption followed by the recruitment of osteoblasts and mesenchymal stem cells. (3) Formation: osteoblast differentiation and osteoid production. (4) Mineralization: mineralization of osteoid and the formation of lamellar bone. As per established patterns, this cycle typically spans 4–6 months. Consequently, an ideal bone scaffold should possess the capacity for dynamic transformation, adapting its morphology and properties to the surrounding microenvironment over time in response to shifts in the external conditions.
Biodegradable materials for 3D printing and clinical translation
Within the framework of the aforementioned six pivotal performance facets of ideal bone scaffolds, the biocompatibility, degradability, and mechanical properties are intricately interwoven with material behavior. These characteristics help to avoid secondary surgeries with nondegradable materials [32]. And the risks of toxic side effects and local tissue reactions caused by metal particles generated by long-term retention in the body [33]. A myriad of degradable materials, including metals, ceramics, and polymers, are utilizable in clinical bone scaffold applications. Generally, metallic materials have high mechanical properties and stability but may suffer from biocompatibility and degradation levels that are difficult to control. Ceramic materials have excellent biocompatibility and bioactivity, but they have poor mechanical properties and may not be suitable for use in load-bearing areas. Conversely, polymeric materials exhibit favorable degradation properties and biocompatibility, but they may encounter challenges in maintaining stability in load-bearing regions.
Evidently, no universally acknowledged optimal material for 3D-printed bone scaffolds exists to date, as the characteristics of these materials dictate their applicability and limitations in a clinical setting. To achieve a nuanced equilibrium between biocompatibility, mechanical attributes, and degradability, certain advanced biodegradable materials have demonstrated success in laboratory settings in recent years. Nevertheless, numerous unknown challenges remain for their clinical translation. A thorough understanding of the distinguishing qualities of various materials and their actual performance levels can help to progress the development of increasingly effective and durable options for bone tissue regeneration and repair applications.
Biodegradable metal for 3D printing
Biodegradable metals, which are exemplified by magnesium, zinc, and their alloy counterparts, have garnered considerable interest among researchers due to their remarkable biocompatibility and degradability levels [34]. First, these materials possess hardnesses that approach the cortical bone hardness, providing effective fixation strength to support early fracture healing during the initial implantation period. Second, the implants gradually degrade in the physiological environment, avoiding the damage caused by secondary surgeries required with traditional metal fixation devices [35]. Magnesium degradation also leads to the release of alkaline and enriched Mg2+ ions, which promote bone healing [36]. Last, their mass density and Young’s modulus are very similar, avoiding stress shielding [37]. Relevant studies have indicated that ions produced by magnesium degradation can stimulate the regeneration of surrounding bone tissue [38]. Dong et al. [39]. conducted an assessment that encompassed the mechanical characteristics, in vitro degradation dynamics, electrochemical responses, and biocompatibility levels of a 3D-printed Mg scaffold featuring a porosity of 55% and a strut density of 92%. These scholars clearly demonstrated the tremendous potential of 3D-printed porous Mg scaffolds for further development as bone implants.
However, magnesium faces significant challenges in rapid biodegradation due to its highly negative corrosion potential (-2.37 V) [25]. The first commercial magnesium screws (Magnezix, Syntellix, Hannover, Germany) were introduced in 2013, but they dissolve after one to two years of implantation [40]. This rapid degradation of the implant results in a deterioration of its mechanical properties before the newly formed bone can adequately withstand the requisite mechanical load [41]. Additionally, the intense generation of hydrogen gas during the rapid biodegradation process can lead to the formation of cavities around the fracture site, affecting the healing outcome. Scholars have reported hydrogen gas release rates of 0.01 mL/cm2/day for magnesium alloys containing zinc, aluminum, and manganese [42].
Scholars have found that surface coatings can slow the degradation of biodegradable metals, allowing the hydrogen gas produced during the degradation process to be absorbed by the body without forming cavities [43]. Ibrahim et al. [44] developed a novel Mg-Zn-Ca-Mn-based alloy, where a ceramic coating significantly reduced the corrosion rate from 10.37 mm/year for the uncoated alloy to 0.03 mm/year for the coated alloy. Additionally, the microhardness increased by 36.4%, and in vivo studies demonstrated improved biocompatibility. Kopp et al. [45] applied phosphate-based PEO surface modification (Kermasorb®) to magnesium alloy screws, confirming their biocompatibility and ability to reduce alloy degradation rates. This was tested for the first time in a long-term large animal model over 18 months, with the presence of residual material within the implanted area confirming these findings.
Even trace amounts of impurities, such as nickel, significantly increase the corrosion rate of magnesium, thereby accelerating its degradation. Moreover, nickel exhibits poor biocompatibility. Therefore, purifying magnesium alloys by removing impurities like nickel can enhance their biocompatibility while controlling degradation rates [46]. Additionally, nickel has demonstrated poor biocompatibility [47]. Hence, purifying magnesium alloys by eliminating nickel and other impurities can regulate degradation rates and improve biocompatibility.
Biodegradable ceramics for 3D printing
Compared to biodegradable metals, biodegradable ceramics have several advantages, including biocompatibility, corrosion resistance, and bioactivity. The most significant advantage is the gradual degradation of these ceramics through solution-driven and cell-mediated processes after implantation [18], avoiding the generation of hydrogen gas. Additionally, biodegradable ceramics have mineral compositions similar to natural bone tissue, allowing them to gain inherent bone bonding activity and to greatly promote bone growth and integration [48].
Hydroxyapatite (HA), denoted as Ca10(PO4)6(OH)2, constitutes approximately 65% of the total bone mass and represents a significant portion of the inorganic constituents within bone tissue [49]. Synthetic HA exhibits excellent osteoconductivity due to its high chemical similarity to natural HA. However, the biodegradation rate of HA is relatively slow, which may have adverse effects on bone defect repair. Brandt et al. [50]. implanted nanocrystalline HA into the distal femur of rabbits and observed minimal absorption at the edges of most implants.
Ca3(PO4)2 (TCP) has the advantage of a faster biodegradation rate than HA, which promotes the growth of surrounding new bone [51]. Zheng et al. [52]. used 3D printing to prepare TCP composite scaffolds, enhancing their mechanical properties and their bone induction and regeneration effects. However, TCP lacks sufficient mechanical strength to withstand physiological loads [53], limiting its applicability in large bone defects. To address this issue, recent research is mainly focused on doping TCP with other components to enhance its mechanical strength. Nandi et al. [54]. evaluated the effects of 3D-printed TCP scaffolds doped with silica and zinc oxide on the bone formation and healing characteristics in a rabbit tibia defect model. The results showed that the addition of dopants promotes bone formation, thus accelerating the healing process. Additionally, Ossiform™ has successfully produced bone scaffolds for maxillofacial bone defects by adjusting the formulation and printing methods of TCP, achieving successful clinical translation after thorough preclinical experiments and the attainment of regulatory approval [55]. Nevertheless, the mechanical strength of a TCP scaffold remains a limitation, making it more suitable for repairing craniofacial bone defects than other bone defects.
Biologically active glass (BAG) is another important type of bioceramic. The main difference between BAG and traditional ceramics arises because BAG is typically noncrystalline, while ceramics are crystalline. BAG displays short-range atomic ordering; this characteristic is in contrast to ceramics, which feature long-range atomic ordering [56]. Notably, 45S5 Bioglass® is an inaugural synthetic material capable of establishing a chemical bond with bone [57]. The mechanism of bone bonding entails a sequence of interfacial reactions, culminating in the creation of a hydroxyapatite layer akin to bone on the material surface. In parallel, released ions, such as calcium, phosphate, and silica, significantly contribute to its osteogenic potential. However, the disadvantages of BAG include poor fracture toughness, brittleness, and high rigidity, making it suitable for non-load-bearing bone defect repairs only. PerioGlas® is the first clinically used particulate BAG product that is mainly used for periodontal tissue reinforcement and jawbone defect repair [58]. Additionally, BAG exhibits a relatively gradual in vivo degradation rate that does not align with the pace of new bone formation. In a 14-year follow-up clinical trial for benign tumor excision-induced bone defects, some BonAlive® glass particles were still present [59]. Due to these limitations, present applications of BAG in bone repair are limited to coatings or particulate bone fillers. To broaden the scope of BAG utilization in bone tissue engineering, it is imperative to address these constraints by enhancing its mechanical properties and degradation rate, thus catering to a diverse array of clinical requirements.
Biodegradable polymers for 3D printing
Polymers are extensive molecules created through the covalent linkage of recurring monomers. In the field of bone implants, biodegradable polymers have become widely applicable due to their degradability [60]. Depending on their origin, polymers can be divided into natural and synthetic varieties. Peptides and proteins, polynucleotides, and polysaccharides are the three primary categories into which biodegradable polymers of natural origin can be generally divided [61]. Due to their biodegradability, bioactivity, and biocompatibility levels, these materials have drawn considerable interest as prospective treatments for bone defect healing.
Collagen, which is a pivotal constituent of the extracellular matrix (ECM), plays a vital role in governing cellular behavior [62]. Therefore, collagen is considered one of the most promising materials for 3D bioprinting [63]. Wang et al [64]. developed a reliable UV-curable 3D bioprinting bioink system using a novel water-soluble recombinant human collagen. The scholars demonstrated that the 3D-printed structures provide a microenvironment for cell diffusion, proliferation, and migration, and that collagen supports the expression of ECM or angiogenesis-related proteins by cells. Natural polymers have specific drawbacks, such as microbial contamination (e.g., endotoxins), reduced tunability, immune reactions, uncontrolled degradation rate, and low mechanical strength, which limit their applicability in hard tissue regeneration [41]. Presently, no exclusively natural polymer material exists that is exclusively utilized for bone scaffolds. Instead, natural polymers are primarily applicable as components within composite materials, thereby augmenting diverse types of scaffolds [65]. As a result, most of the collagen products presently marketed as bioinks, such as Fibercoll-Flex-A®, have been limited to scientific experiments [66], although they have demonstrated excellent performance and outstanding shape fidelity in the printing of complex scaffolds.
In contrast to their natural counterparts, synthetic polymers can be tailored for diverse applications through well-regulated design and synthesis parameters. Within the realm of 3D printing, synthetic polymers, such as aliphatic polyesters like polycaprolactone (PCL), PLA, Polyglycolic acid (PGA), and the copolymer poly(lactic-co-glycolic acid) (PLGA), hold notable relevance. Among these, PCL is noteworthy due to its cost-effectiveness, FDA approval, and inherent flexibility [67]. Despite its biodegradability and biocompatibility, PCL has been found to degrade slowly and exhibit poor mechanical properties, making it suboptimal for bone defect repair [68]. PGA is an uncomplicated aliphatic polyester distinguished by its consistent linear molecular arrangement. Due to its excellent tensile modulus and controllable solubility, PGA has been clinically used for many years as the first biodegradable suture thread [69]. However, because of its rapid degradation rate in the body, using PGA scaffolds alone is not suitable for bone defect repair.
PLGA is a copolymer created by the ring-opening polymerization of PLA and PGA. By adjusting the ratio of these two components, its degradation kinetics can be controlled, making PLGA a better option than PGA and PLA [70]. Unfortunately, the application of PLGA in bone repair is limited due to its poor bone inductivity and hydrophobicity [71]. Therefore, additional materials are required for modification. Liu T et al. [72]. utilized 3D printing to fabricate customized three-dimensional porous PLGA/CaSO4 scaffolds, resulting in improved mechanical properties, biocompatibility, and osteogenic performance. Fan et al. [73]. used poly(lactic-co-glycolic acid) and sodium alginate to create PLGA/SA scaffolds, demonstrating their ability to successfully repair joint cartilage and prevent adverse reactions such as osteoarthritis in rat knee cartilage. Liu Z et al. [74] modified the surface of 3D-printed PLGA/HA composite scaffolds with a novel fusion peptide, finding that it exhibited good biocompatibility, antibacterial activity, and promoted osteogenesis and angiogenesis. Despite many efforts to enhance PLGA, successful clinical translation of 3D-printed bone scaffolds has yet to be realized. To date, PLGA products are mainly used in the production of sutures, as well as cell and drug delivery systems [18, 75,76,77,78,79].
Although polymers have been extensively studied as bone scaffolds, the side effects and toxicity of their degradation products remain critical issues. The size, location, and shape of degradation products are crucial features. For instance, positively charged particles produced after PLA degradation can induce cell toxicity, oxidative stress, and even DNA damage. Excessive reactive oxygen species (ROS) can lead to local inflammation. Moreover, brittle nanoparticles may be enzymatically degraded once they enter the bloodstream, or they may undergo immune rejection through protein adsorption and aggregation [80]. The key to addressing inflammatory reactions from degradation products lies in controlling the degradation rate. Rapid degradation rates can increase crystalline substance concentrations in local tissues and enhance macrophage-mediated foreign body responses (FBRs) [81]. PLGA has become one of the most successful biodegradable polymers due to its controllable degradation rate. PLGA degrades into lactic acid and glycolic acid, both of which are endogenous monomers easily metabolized by the human body through the Krebs cycle. Therefore, the use of PLGA in biomedical applications minimizes systemic toxicity [82].
A common issue faced by synthetic polymers such as PCL, PGA, and PLGA is their poor biological activity, mainly due to their hydrophobicity, inertness, and lack of cell recognition/binding sites [17]. Therefore, for clinical bone scaffolds, it is often necessary to use bioactive materials for surface modification or reconstruction to provide cell recognition and binding sites, promote cell adhesion and proliferation, and facilitate new bone growth and regeneration. In Liu et al.‘s study [83], a hydrogel rich in angiogenic factors was introduced into a 3D-printed polylactic acid scaffold, greatly promoting bone regeneration.
To date, successful clinical translation of 3D-printed bone scaffolds is primarily dominated by synthetic polymer-based scaffolds. By manipulating the material composition and molecular weight, these polymer scaffolds can be fine-tuned to achieve targeted degradation rates and mechanical characteristics. However, challenges remain in terms of osteoinductivity and limited application in weight-bearing areas. Future research is shifting toward the composite modification of polymer scaffolds with other materials to enhance bone-forming and mechanical properties. Another noteworthy category with considerable clinical applicability is bioceramics, which comprise elements akin to those found in the human body. These ceramics exhibit relatively good bone-forming properties, but their mechanical performance is comparably inferior, and their degradation rates are relatively slow. Future research efforts should focus on improving ceramic formulations. Relative to the aforementioned materials, biodegradable metals, while possessing superior mechanical properties, have not yet achieved widespread clinical translation for 3D-printed bone scaffolds due to certain issues, such as hydrogen release and stress shielding effects. Future efforts are likely to concentrate on surface coating modifications to address these challenges(Fig. 2). Apart from these potential improvement approaches, the printing methods themselves are closely intertwined with the enhancement of mechanical and degradation properties.
Effectiveness of materials for printing stents in BTE. (A) Magnesium coating enhances osseointegration [84]. (a) Surface morphologies and elements of porous PEEK scaffolds in different groups examined by SEM and EDS. (a) Surface morphology and elements of porous PEEK scaffolds examined by SEM and EDS. (b, c) 3D reconstructed images of internal vessels and bone ingrowth in scaffolds determined by micro-CT. (Copyright 2023, Elsevier) (B) Functionalized silk-hydroxyapatite scaffolds for enhanced bone regeneration [85]. (a, b) Micro-CT of a 3D printed cube and anatomical structures. (c, d) SEM of a 3D-printed construct and immunofluorescent staining of osteopontin (OPN). (Copyright 2021, Elsevier) (C) 3D-printed PLGA/BP scaffold stimulates bone regeneration by modulating macrophage M2 polarization [86]. (a) Schematic of PLGA/BP scaffolds and proposed mechanism. (b, c) CLSM images of hBMSC cells and RAW264.7 cells on the scaffolds. (Copyright 2023, Advanced Science)
3D Printing techniques and clinical translation
To achieve the ideal bone scaffold for clinical application, the selection of the appropriate manufacturing technique is key to achieving a personalized design and a pore structure that matches the bone tissue. The chosen manufacturing method should align with the selected material to guarantee optimal scaffold performance and successful implementation. Specific aspects, such as resolution, fabrication speed, cost, and complexity associated with various technologies, must be taken into account during the selection of the appropriate fabrication approach.
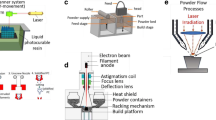
According to the standards of the American Society for Testing and Materials (ASTM), there are seven key 3D printing technologies: material extrusion, powder bed fusion, vat photopolymerization, material jetting, binder jetting, directed energy deposition, and sheet lamination [87] Based on relevant literature, we will briefly introduce material extrusion, powder bed fusion, vat photopolymerization, and binder jetting, which are highly relevant to BTE [88](Fig. 3). Vat photopolymerization bioprinting technologies offer significant advantages in rapid manufacturing, precise geometric control, and high resolution for BTE. However, limitations arise from material selection and printing processes, particularly in achieving multifunctional, multi-material printing. In contrast, ME (Material Extrusion) technology is user-friendly and widely used for commercial scaffold printing, albeit with relatively lower resolution. BJ (Binder Jetting) technology effectively reduces the risk of irregular shrinkage-induced fractures, especially suitable for printing special materials like bioceramics, although mechanical performance needs improvement. PBF (Powder Bed Fusion), as a preferred method for metal additive manufacturing, excels in quality and efficiency, but controlling pore structure and degradation rate remains challenging (Table 1).
Vat photopolymerization
The 3D printing technique Vat Photopolymerization is frequently utilized in bone tissue engineering. SLA uses photopolymer resins and constructs structures layer-by-layer after curing the resin with UV light [96]. As a result, these printing technologies have the potential to achieve personalized implants with anatomical shapes and optimized internal porous structures. This ability allows any 3D shape to be sliced into thin layers and to be constructed by layer-by-layer photopolymerization. Later, this process was improved by conducting continuous light curing in each layer region, known as digital light processing (DLP) or continuous DLP (cDLP), which offers a faster printing speed than standard SLA.
In recent years, advancements in chip manufacturing technology have significantly enhanced the speed and precision of SLA printing using DLP printers equipped with DMD (Digital Micromirror Device) chips [97]. A DMD consists of millions of tiny mirrors, each independently controllable to tilt and adjust the direction of light projection. Prior to printing, the DLP printer slices the 3D model data into numerous thin layers. During operation, the printer projects an image of each layer onto photosensitive resin. This image is composed of millions of tiny light spots, where each spot corresponds to a pixel. These spots selectively cure the resin, solidifying it into the desired shape. As the print platform incrementally rises, layer by layer, the entire model is built up [98]. The ability of DLP printers to project entire layers at once, rather than scanning point by point, significantly accelerates printing speed. Additionally, the high resolution of DMD enables finer detail in printed objects and facilitates the creation of complex geometric shapes.
SLA/DLP bioprinting technology has unique advantages in the field of BTE, such as fast manufacturing speed, precise control over scaffold geometry, and good resolution (< 100 μm) [99]. These advantages have propelled the extensive adoption of this technology within the field of bone tissue engineering, leading to the creation of numerous 3D-printed scaffolds. For example, Lam et al. [100] methacrylated gelatin (GelMA) and hyaluronic acid methacrylate (HAMA) through stereolithography-based bioprinting to create cartilage models with varying chondrocyte densities. Hong et al. [101]. mixed cellulose with methacrylated glycerol (silk-GMA) for DLP 3D printing to treat partial tracheal defects in a rabbit model. These in vivo experiments have demonstrated that SLA/DLP bioprinting technology can be used for treating cartilage defects and for adjusting the sizes and shapes of bioprinted structures based on individual patient needs.
However, it is important to note that although SLA/DLP bioprinting technology is advantageous in many ways, it may not be ideal for versatile multimaterial printing due to constraints in material choices and printing procedures. Additionally, there is a problem with ceramics settling in suspension during printing, which causes the density of the finished part to be uneven [102]. With the continuous development and improvement of the technology, the SLA/DLP bioprinting technique is anticipated to advance further, bringing new discoveries and developments to the fields of bone tissue engineering and medicine.
Materials extrusion
Materials extrusion (ME) stands out as a widely favored AM technology due to its straightforwardness and user-friendliness. In most material extrusion 3D printing systems, thermoplastic filaments are fed into a heated nozzle, where they are softened and extruded as the print head moves within the x-y plane. Other systems can utilize pellet or screw extrusion concepts to deposit the extruded material onto a build plate that can move along the z-direction [8]. This technique typically requires shear thinning or thermoplastic materials. Thermoplastic materials, such as PLLA, can be applied in the form of continuous filaments, which are melted in the extrusion head and deposited through a nozzle to build 3D structures. This process is called fused deposition modeling (FDM) [103]. Direct extrusion of ceramic or glass-based pastes, also known as robocasting or direct ink writing, is widely used to fabricate green bodies, which are then sintered to form 3D bioceramic scaffolds [104].
Based on the easy operation of ME, a large number of commercial scaffolds are currently printed using this method. Yang et al. [105]. investigated the effect of GENESIS-BCP™ scaffolds made of HAp and TCP on the creation of bones. They transplanted GENESIS-BCP™ scaffolds into rabbits and humans, and used Masson’s trichrome and hematoxylin-eosin staining to display the formation of new bone. The research findings substantiated the notable capacity of this bone graft material to expedite the generation of fresh bone tissue. In another study, Miramond et al. [106]. evaluated commercially available biphasic calcium phosphate (MBCP® and MBCP+®) scaffolds by implanting them in a nude mouse model with varying ratios of hydroxyapatite/β-tricalcium phosphate. The goal was to assess the osteoinductive properties of these scaffolds. These scholars showed that commercial ME-printed scaffolds have excellent capabilities for promoting bone formation and that their design and composition can be adjusted according to specific patient needs, providing a personalized and effective solution for bone defect repair.
ME 3D printing has a lower resolution (typically > 100 μm) than SLA, depending on certain factors, such as nozzle diameter and material viscosity [107]. Additionally, ME 3D printing faces another limitation: it cannot be used with soft or low-viscosity materials, as these materials tend to lose their shape after extrusion. However, a strategy known as freeform reversible embedding of suspended hydrogels (FRESH) has been proposed to overcome this limitation. FRESH involves depositing soft hydrogels in support channels and suspending the printing material in a movable base, temporarily maintaining the shape of the printed structure until it stabilizes through further crosslinking [108]. his approach overcomes the traditional ME 3D printing constraints on hard materials, increasing the range of materials to be used in ME 3D printing and enabling the manufacturing of increasingly complex and functional structures.
Binder jetting
Material jetting is a 3D printing process where tiny droplets of material are deposited and then cured or solidified on a build platform. It involves building objects layer by layer using photopolymer or wax droplets that solidify when exposed to light [109]. The nature of material jetting allows for printing different materials on the same object. Therefore, current applications of parts printed on commercial MJ printers are primarily limited to colored models and applications not subjected to heavy loads (such as molds) or used for prototyping purposes [110].
Binder jetting (BJ) is also an inkjet printing technique in which a liquid binder is strategically placed in layers of powder to fuse the particles and gradually form the desired structure in a layer-by-layer fashion [111]. A roller is used to evenly distribute the powder first onto the build plate and then onto a printhead, using a CAD file as guidance to precisely deposit the liquid binding agent. This binding agent assumes a glue-like role, temporarily fortifying the printed object.
In BJ technology, postprocessing steps after printing are crucial, including drying, curing, sintering, and infiltration, to enhance the strengths and stabilities of the printed objects. Because these postprocessing procedures are introduced selectively, BJ technology may effectively reduce the danger of fractures in printed products caused by irregular shrinkage, making it particularly suitable for printing with special materials, such as bioceramics. Ahn et al. [112]. used BJ-based 3D printing to manufacture partially hydrated calcium sulfate (CSH) scaffolds in their study. The best compressive strength, modulus, and toughness are in the scaffold, in which the fracture behavior shows a decrease in brittleness. This process of creating composite scaffolds with BJ technology presents a fresh perspective on the creation and use of bioceramic materials.
However, in the BJ technique, powders with diameters larger than 20 μm can be laid directly on the powder bed [102]. In addition, due to the usage of glue, BJ technology may not produce printed items with the same density and strength as other 3D printing techniques. This finding suggests that metal parts printed using BJ technology typically show poor mechanical properties, limiting their use in some high-strength and high-performance applications. Therefore, using BJ printing entails the consideration of material, application, and process constraints.
Powder bed fusion
Powder bed fusion (PBF) encompasses subtechnologies such as selective laser sintering (SLS), selective laser melting (SLM), and electron beam melting (EBM), akin to BJ [113]. SLS is primarily used for polymers, while SLM and EBM processes are used for metal parts. SLS and SLM can be used to print complex designs; a key advantage of this technology is that it does not require the addition of solvents or polymers, thereby avoiding potential structural shrinkage in further processing steps [114]. Additionally, unlike stereolithography and extrusion printing methods, SLS and SLM typically do not require temporary support structures when printing complex structures, as the powder bed maintains the integrity of the sintered region during the printing process [115]. While EBM has a lower resolution, larger minimum feature size, and rougher surface finish than SLS and SLM, it has lower oxygen absorption in the final printed part; this reduced oxygen absorption level improves the mechanical properties, particularly in titanium implant applications, due to the electron beam in a vacuum [8].
PBF has been recognized as one of the most suitable metal additive manufacturing methods in terms of quality and efficiency [116]. Liu et al. [117] utilized PBF to produce magnesium porous scaffolds and observed good biocompatibility and enhanced osteogenesis. Despite the success achieved with non-degradable metal scaffolds manufactured via PBF [118], the degradation rate of pure degradable metals remains relatively fast compared to fracture healing rates, resulting in void formation in the fracture area that hinders healing [119]. This challenge complicates the clinical application of PBF-manufactured bone scaffolds.
Future research could consider alloying degradable pure metals manufactured via PBF to provide customized mechanical, chemical, physical, and biological properties [47]. Additionally, depositing coatings on the surfaces of degradable metals could customize bone scaffolds to match fracture healing rates more effectively, thereby promoting better fracture healing [120].
The leap from 3D printing to bioprinting
3D printing of bone scaffolds represents a specific application of 3D printing technology in bone tissue engineering. These scaffolds are designed as three-dimensional structures intended to provide mechanical support and serve as templates for new bone growth. They are used for repairing or regenerating damaged or lost bone tissue. In this context, 3D printing is employed to manufacture porous scaffolds with precise geometric shapes tailored to meet the patient’s needs. These scaffolds can be made from biocompatible materials such as ceramics or polymers, and may include bioactive factors to promote bone regeneration. Derived from 3D printed bone scaffolds, 3D bioprinting is a novel technology that combines living cells, biomaterials, and growth factors to create complex three-dimensional structures mimicking natural tissues or organs.
Clinical translation of 3D bioprinting
3D bioprinting technology involves precise layer-by-layer deposition of bioinks containing cells to create functional biological constructs [121]. Unlike 3D printing which constructs supportive bone scaffolds, the goal of 3D bioprinting is to manufacture tissues or organs with biological activity. It typically requires specialized printers, bioinks, and cell culture techniques [122]. The focus of 3D bioprinting is on using bioinks to create complex biological tissues or organs, whereas traditional 3D printing of bone scaffolds concentrates on creating structures resembling bone tissue for tissue engineering applications. The former has broader applications in creating functional tissues, while the latter represents a specific application within the field of bone regeneration.
Bioprinting involves layer-by-layer deposition of specific bioinks that contain cells or growth factors with osteoinductive properties to build scaffolds, starting from a single cell type, thereby achieving control and high reproducibility in the scaffold manufacturing process [123]. Unlike acellular 3D printing, bioprinting eliminates the possibility of uneven cell distribution, offering new possibilities for precise scaffold construction and positioning [124]. Bioprinting enables precise control of scaffold porosity and pore size, allowing for the precise deposition of growth factors and cells in predetermined architectures, such as vascular networks, to improve scaffold integration with bone tissue [125].
Currently, single-material non-cellular 3D printed bone scaffolds, such as Ossiform™ and Cerabone®, have been preliminarily applied in clinical practice. Relative to acellular 3D printing, 3D bioprinting remains nascent, primarily concentrating on proof-of-concept studies conducted in vitro or with small animal models. Some prospective (although in their early stages to date) applications include manufacturing feasible tissue structures shaped according to anatomy for the restoration of non-load-bearing bones [126]; creating intermediate templates for bulk bone regeneration [127]; minimally invasive in vivo bioprinting for immediate defect repair [128]; and facile generation of personalized models for disease modeling and drug screening to complement in vivo experiments [129], among others. So far, there are no bioprinted tissue engineering products on the market, and even the number of products undergoing clinical trials that we could find is extremely limited, which is a concerning fact. In 2023, Hao and colleagues [130] introduced the first case of using a self-developed 3D bioprinted active bone for bone defect repair. They prepared a bone scaffold using bio-ink derived from the patient’s own platelet-rich plasma (PRP) combined with a polycaprolactone/β-tricalcium phosphate (PCL/β-TCP) composite scaffold material to repair the left tibia.
This situation is influenced by several complex underlying reasons. First, achieving mechanically strong, large-scale, and vascularized bone structures for addressing critical-sized defects is a significant challenge in bioprinting [131], especially when considering reasonable speed and cost. Additionally, biomanufactured implants often face the issue of immaturity, and after implantation, they may lose their specified functions, potentially leading to long-term failure [132]. Lastly, autologous bone remains the gold standard in bone transplantation [133], and currently, there is no research demonstrating that bioprinted bone scaffolds can achieve better therapeutic outcomes than autologous bone. Moreover, there are uncertainties in medical regulation and commercial models [134].(Table 2).
Challenges in the clinical translation of 3D bioprinting: limitations due to materials
Bioprinting grapples with substantial hurdles in achieving the desired balance between scaffold degradability and mechanical functionality. Addressing degradability challenges involves meticulous control over the scaffold degradation rate. Excessively rapid degradation can undermine the tissue support function, while overly slow degradation may trigger inflammatory responses, compromising tissue regeneration [135]. Moreover, ensuring satisfactory mechanical performance necessitates the consideration of multiple indicators, such as compressive strength, tensile strength, elasticity, and fatigue resistance, during scaffold fabrication [136].
Hydrogels, as water-swollen porous networks, are ideal materials for cell encapsulation, tissue construction, and 3D bioprinting. The hydrogels used in 3D bioprinting must also have adjustable substrate stiffness and be capable of network reconfiguration after printing, allowing for cell spreading, migration, proliferation, and interaction. However, hydrogels typically have poor mechanical properties [136]. Future researchers might explore the development of synthetic/natural hybrid bioinks by incorporating inorganic fillers and/or rigid thermoplastic frameworks to enhance mechanical properties. Yang et al. [137] proposed a post-bioprinting strategy involving mechanical assistance to load cells immediately after scaffold fabrication. They constructed hollow fiber hydrogel scaffolds (HHS) with mechanically responsive large-scale complex structures. Due to their hollow structure, HHS exhibited attractive elasticity, rapid shape recovery, and excellent fatigue resistance, making them highly responsive to load sensors in a rapid, uniform, and precise manner.
Challenges in the clinical translation of Bioprinting: limitations of Printing methods
Bioprinting technologies primarily utilize three main printing methods: VP, BJ, and ME. These methods operate on principles similar to traditional 3D printing but have specific limitations concerning cell viability. VP uses large vats of photoactivatable bioresin to create cell-laden 3D structures point by point, layer by layer, or volumetrically with computer assistance [138]. Different photoinitiators (PI) promote crosslinking to produce high-resolution complex tissues. However, excessive free radicals from PIs can damage cells, while insufficient PI concentration limits bioink photocuring, affecting the mechanical properties and resolution of the printed structures [139].
Drop-on-demand (DOD) material jetting is ideal for non-contact deposition and patterning of various cells and biomaterials, enhancing cell-cell and cell-matrix interactions. In DOD bioprinting, the impact of cell-laden bioink droplets affects print quality and cell viability. Higher cell concentrations slow droplet impact speed, improving cell survival and reducing splashing. Limiting print time per layer to 2 min prevents excessive droplet evaporation, maintaining high cell viability. Studies recommend a cell concentration of 4 million/mL and a print duration of 2 min [140]. Research by Ng et al. shows that higher bioink viscoelasticity stabilizes droplets before they break from the nozzle, improving deposition accuracy and reducing splashing [141].
Shear stress is the primary cause of cell damage/death in ME [142]. Cell damage increases exponentially with shear stress, starting from zero at no stress to 100% at high stress. This impact is controlled by material properties and printing parameters. Optimal bioinks have low viscosity under shear stress and higher viscosity post-printing to maintain shape and cell viability. Ataie et al. developed nanostructured bioinks using reversible nanoparticle assembly, enhancing printability and shape fidelity [143]. Key parameters affecting shear stress and cell viability include dispensing pressure, nozzle diameter, and geometry. Higher dispensing pressure reduces cell viability. Smaller nozzle diameters increase shear and tensile stress. Cells in cylindrical nozzles have ten times lower viability than those in conical nozzles [144].
Enhancing biomimicry in scaffolds is a critical bioprinting challenge. While imaging technologies help with 3D defect shape, replicating microstructures is difficult. The STL format represents models as a triangular mesh of surfaces, lacking internal structure and attribute information of objects. Because it only captures geometric shapes and cannot convey details like color and texture, printing complex models in STL may lead to issues such as truncation errors, inconsistent normals, incorrect intersections, and small planar degeneracies. Additionally, STL format lacks mechanisms to ensure geometric watertightness, making the repair of erroneous STL files time-consuming and error-prone, while lacking descriptions of topology, process-related, and auxiliary information [145]. In contrast, the AMF format builds upon STL by incorporating color, texture, material properties, and supports multiple 3D printing technologies. AMF also preserves internal structure and attribute information of objects, facilitating subsequent simulation and analysis [146]. However, the AMF format’s adoption in the AM industry has been limited primarily due to insufficient consultation with major AM system developers prior to its adoption [147].
In addition to rapid technological advancements, establishing a cohesive open-source bioink database can be highly beneficial for addressing this issue. Such a database would allow researchers to access existing bioink formulations and printing parameters quickly and efficiently, thereby improving the manufacturing efficiency and accuracy of scaffolds. As community-driven databases continue to grow, designing innovative yet relatively complex bioinks with predictable performance should be facilitated, thus promoting the development of bioprinting technology [148].
Challenges in the clinical translation of 3D bioprinting: limitations due to cells
BTE therapy based on bioprinting revolves around key principles: isolating and cultivating a large quantity of regenerative-capable cells, integrating them into bioprinted structures, ensuring these structures reach a mature level suitable for tissue repair, and ensuring recipient acceptance post-transplantation [112]. Cells are pivotal participants in bone tissue engineering, crucial for bone formation and regeneration [149,150,151]. On one hand, cells serve as fundamental units for new bone tissue formation. By introducing bone cells (such as osteoblasts, chondrocytes, etc.) or stem cells capable of differentiation into bone cells, they can promote the formation and growth of new bone tissue supported by biomaterials, thereby accelerating the healing process of fractures or bone defects [152]. On the other hand, the presence of cells enables the engineered constructs in bone tissue engineering to interact with surrounding host tissues. Through the release of growth factors and extracellular matrix, they regulate the local environment, aiding in the maintenance and promotion of new bone tissue growth [153, 154]. Additionally, cells can assist in material degradation, supporting the steady degradation of biomaterials [155]. In the process of preparing cells for bone tissue engineering, at least three aspects need consideration: cell selection, cell source, and cell expansion.
As for cell selection, despite the explosion of various methods for generating stem or functional cells for medical purposes, the most effective cell type has not been definitively determined. Primary-derived cells exhibit lower immunogenicity, enhanced self-assembly capabilities, and a genetic background similar to recipients, which potentially allows them to form complex microstructures. However, they have limited lifespans and raise ethical concerns. Isolated primary cells are typically fragile and prone to high mortality rates due to the stresses of temperature, pressure, and shear forces during 3D bioprinting processes [156]. Considering that cell lines can be cloned and expanded massively, generating cell populations with identical genetic characteristics [157], this uniformity permits the manufacture of homogeneous tissues and organs, ensuring consistent and reliable outcomes of printed structures. Furthermore, cell lines are easier to handle and cost-effective, capable of withstanding environmental changes and mechanical stress [158]. Cell lines thus emerge as an alternative, yet their significant drawback lies in often being unable to fully replicate the mature and specialized functions of corresponding cells in vivo. Moreover, carrying gene mutations increases the risk of tumor formation [159].
Stem cells, due to their ability for self-renewal and multilineage differentiation, are favored seed cells for 3D printing applications. Their advantage of sharing the same genetic background helps significantly reduce the risk of immune rejection after transplantation into the body. However, the proliferative capacity of stem cells decreases as they differentiate, limiting their ability to fill 3D constructs. Induced pluripotent stem cells (iPSCs), derived from adult cells through genetic reprogramming techniques, also present a promising cell source. Yet, iPSC generation is complex, time-consuming, and carries risks of epigenetic abnormalities and potential tumorigenicity of undifferentiated pluripotent stem cells. Another strategy involves using specific differentiated cells to aid in constructing tissue or organ models, but this approach may be more expensive and complex. Moreover, the relatively low efficiency of differentiation hinders its application [160].
It is worth noting that recent research has discovered cell-derived exosomes carrying genetic characteristics of their parent cells. In bone tissue engineering, combining cell-derived exosomes with 3D printing technology holds promise as a new “cell-free” regenerative medicine approach. Han et al.‘s [161] study successfully released exosomes derived from periodontal ligament cells loaded into 3D bioprinted hyaluronic acid scaffolds. This approach significantly enhanced cell attachment of mesenchymal stromal cells from human buccal fat pad and promoted ligament, osteogenic, and dentinogenic differentiation. (Fig. 4)
Regarding cell source, cells can be obtained from the patient’s own body (autologous) or from donors (allogeneic), and each source has its pros and cons, with factors like the risk of rejection and ethical considerations needing to be taken into account. In terms of cell expansion, a persistent challenge is how to effectively expand these cells while preserving their phenotype and function. Some solutions, including exploring alternative sources of cells such as induced pluripotent stem cells (iPSCs) and improving culture systems and protocols [162], may help address this issue.
The long-term outcomes of a two-week osteogenic and ligamentous differentiation in hBFP-MSCs cultured on bioprinted sEV scaffolds were assessed. (a) Osteogenic assay showed increased ALP staining, indicating more ALP-positive cells after 2 weeks on bioprinted GelMA/hPDLCs-sEV scaffolds. (b) Alizarin Red staining and (c) its quantification demonstrated enhanced osteogenic differentiation on bioprinted sEV constructs. (d) Toluidine blue staining showed ligamentous differentiation of hBFP-MSCs on bioprinted sEV constructs after 2 weeks. (Copyright 2024, Biomaterials Advances)
Challenges in the clinical translation of 3D bioprinting: limitations due to regulatory
When preclinical research is successful and ready for clinical translation, obtaining regulatory approval from relevant management authorities worldwide is necessary. These regulatory agencies may include the U.S. Food and Drug Administration (FDA), the National Institutes for Food and Drug Control (NIFDC) in China, and the European Medicines Agency (EMEA) among others. The application process encompasses the entire lifecycle of the product, covering conceptualization, prototype development, preclinical research, clinical trials, pre-market assessment, industrialization and commercialization, as well as post-market surveillance [163].
In particular, the “concept to product” translation of biomaterial devices worldwide largely depends on the successful execution of the following steps [164]: (1)Conceptualization: New device concepts are developed based on the expertise of academic researchers and specific patient needs in clinical settings. (2)Feasibility Testing: The device’s feasibility is validated in the laboratory environment and on a larger scale to ensure its functionality and practicality. (3)Regulatory Testing: The device is subjected to rigorous testing according to established regulatory standards to collect convincing data on biocompatibility, efficacy, and performance through benchtop and in vitro/in vivo experiments. (4)Regulatory Approval: The collected data is submitted to designated regulatory agencies for a comprehensive multi-criteria review, aiming to clear regulatory hurdles and obtain approval. (5)Manufacturing and Quality Control: Once the device receives approval (often through patents and trademarks), it undergoes appropriate quality control measures and is manufactured according to Good Manufacturing Practices (GMP) before entering the market and clinical settings.
Completion of the above steps for bioprinted scaffolds requires a prolonged clinical follow-up and application process. As an example, FDA review standards require 11, 9 and 6 years for drug, biologic and medical device approvals, respectively [165]. To expedite the approval process and facilitate successful clinical translation of bioprinted medical devices, it is essential to conduct thorough safety and efficacy evaluations and define clear instructions for use in advance. This includes accurately describing the intended purpose, patient population, and usage conditions/places. By providing such well-defined information, the approval process can be accelerated, leading to smoother clinical translation and faster delivery of bioprinted medical devices to patients, ultimately improving healthcare and treatment outcomes [166].
Frontier of 3D printing: 3D Bioprinted Organoids
While bioprinting technology has made rapid advancements, our ability to print biologically meaningful, clinically relevant, and appropriately sized bio-replacements remains limited to a few types of tissues. The central challenge persists: how to incorporate regenerative-capable cells into bioprinted constructs that mature to a level suitable for tissue repair and ensure acceptance by the recipient during subsequent transplantation. These tissues still rely on the body’s ability to remodel tissue structures and populate them with cells [167].
For those aiming to bioprint solid organs with multiple cell types, such as bones, co-culturing poses challenges [168]. In vivo tissue formation heavily relies on precisely regulated morphogenetic processes, allowing local interactions among cell populations and self-organization. Iterative interactions between these local developmental units guide continuous cycles of cell differentiation and pattern formation, ultimately establishing biological complexity on a large scale [169,170,171,172]. Despite extensive research, the optimal timing and proportions of these cell interactions remain largely unknown. Current bioprinting practices involve dispersing cells of different lineages together, yet they fall short of achieving effective and predictable functionality.
Organoids, on the other hand, offer a promising avenue. These multicellular three-dimensional structures are cultivated in vitro and exhibit microscopic anatomical features reminiscent of source organs [173]. While not true organs, organoids closely mimic organ-specific cellular composition and spatial organization [174]. They contain various organ-specific cell types arranged in patterns similar to their source organs. Additionally, they exhibit some of the specific functions associated with their source organs. In recent years, the development of organoids has provided new opportunities to address the challenges faced in bioprinting.
Organoid manufacturing and innovative bioprinting
Currently, organoids represent the most accurate in vitro model systems closely mimicking the in vivo tissue context, offering a promising avenue for personalized medicine [175]. Defined as “3D cell clusters entirely derived from embryonic stem cells, induced pluripotent stem cells, or native tissues capable of self-renewal and self-organization, exhibiting organ-like functions similar to native tissues,” organoids may “rely on artificial extracellular matrix (ECM) to promote their self-organization into structures resembling native tissue“ [176]. Leveraging their unique self-organizing potential, stem cell-derived organoids hold the potential to construct tissue structures and cellular compositions with local features unmatched by engineering approaches [177].
Bioprinting enables precise manipulation of structural characteristics, including structure, mechanical properties, cell arrangement, and biological cues, allowing the imitation of natural tissues [178]. In comparison to traditional 2D and 3D cultured organ systems, 3D bioprinting offers advantages such as high throughput, high precision, and automation [179]. By mimicking developmental biological pathways, 3D bioprinting can maximally restore in vivo microenvironments, allowing the design of multi-layered tissue structures closely mimicking joint anatomical geometry. Furthermore, due to its spatial characteristics and mechanical structure, bone organoids constructed through 3D bioprinting can withstand high levels of compression and shear loads present in joint implants.
Research by Little et al. [180] demonstrates that the use of 3D bioprinting technology for the automated fabrication of self-organizing kidney organoids matches the morphology, cellular composition, and gene expression levels achieved through manual construction, significantly increasing production efficiency by nine times (approximately 200 organoids in 10 min). Professor Humphreys [181] notes that one challenge in organoid processes is batch-to-batch variations, which bioprinted organoids greatly improve, exhibiting a very low size variation coefficient of 1–4%.
Taking innovation further, Lutolf et al. [182]. combined organoid manufacturing and 3D bioprinting in the Bioprinting-Assisted Tissue Emergence (BATE) technique. Leveraging stem cells and organoids as self-organizing building units, they spatially arranged these units to form interconnected and continuously evolving cellular structures. Their real-time observation bioprinting system, integrating a microextrusion system with a microscope equipped with a 3D motion platform, opens up exciting possibilities for spatiotemporally combined bioprinting based on automatic microscopy.
Cells for 3D bioprinted organoids
Establishing the cell source is a pivotal step in constructing bone organoids. Both pluripotent stem cells (PSCs) and adult stem cells (ASCs) demonstrate the potential to form organoids by mimicking the required biochemical and physical signals for tissue development and homeostasis [183].
PSCs, known for their ability to differentiate into various cell types, possess remarkable developmental potential, theoretically capable of generating any cell constituting the entire organism [184]. Zhang et al. innovatively utilized hPSCs to generate spheroids, successfully inducing neural lineage differentiation and reproducing the structure of the neural tube in vitro [185]. While various protocols for cultivating organoids from PSCs exist, these methods often take months, requiring precise addition of specific growth factor combinations at each step [186, 187].
In terms of cell sourcing, induced pluripotent stem cells (iPSCs) offer significant advantages. Easily isolated from autologous fibroblasts, iPSCs can be reprogrammed into various cell types, facilitating the construction of genetically consistent complex systems. Crucially, iPSCs retain the patient’s genetic information, providing a powerful tool for establishing patient-specific models of bone diseases, drug testing, and discovery [177].
The Clevers team achieved the first derivation of intestinal organoids from AdSCs after identifying intestinal stem cells [188]. In comparison to PSC-derived organoids, AdSC-derived organoids can be obtained more rapidly and directly from regenerative human adult tissues [174]. However, due to the limited differentiation potential of AdSCs, the organoids they derive typically only contain a single epithelial cell type. Despite this limitation, these organoids are closer to adult tissue in terms of maturity, presenting potential applications in adult tissue repair [186].
Clinical Ttanslation of 3D bioprinted organoids
Organoids and 3D bioprinting stand out as two major research frontiers in the field of tissue engineering. While the utilization of 3D bioprinting to manufacture organoids is still in its early stages, the combination of these technologies has already demonstrated significant potential, hinting at broad developments and applications in the future. In the realm of bone tissue engineering, the successful transformation enabled by organoids is particularly noteworthy, especially in constructing models of bone organs. Traditional 2D and 3D cell culture models have substantial limitations in simulating the complexity and physiological functions of joint tissues in the body, whereas organoid technology can more authentically replicate the pathological states of joints at the organ level.
Moreover, establishing animal models for bone diseases relies on time-consuming, labor-intensive, and costly methods such as surgery, drug administration, and gene knockout [189]. In contrast, building bone organoids is expected to significantly reduce the development cycle, decrease research costs, and simulate realistic bone defect scenarios without sacrificing animals. Notably, 3D bioprinting has become a crucial means of constructing bone organoids. It can accurately replicate the intricate structure of bones, providing robust technical support for in vitro regenerative bone organ development [180].
Currently, preclinical evaluations of bone implant biomaterials (such as medical metals, ceramics, and polymers) heavily rely on animal experiments, which not only sacrifice numerous animal lives but also often yield inconsistent results in human clinical trials due to inter-species differences [190]. In comparison, organoids constructed from a patient’s own tissues can effectively avoid the data mismatch problem caused by species differences, thus achieving more accurate preclinical assessments of drugs and bone implant biomaterials [191]. This undoubtedly opens up new research directions and possibilities for the treatment of bone diseases and the regeneration and repair of bone tissues.
The ultimate goal of bioprinting is to achieve bone regeneration, replacing damaged or normal organs with healthy tissues in vitro to achieve immunosuppression-free, disease-free, and reduced-toxicity effects. This approach aims to avoid the substantial economic burden associated with lifelong immunosuppressive therapy. Chang et al. [192] achieved rapid bone regeneration in a bone defect model by combining DLP printing technology with hydrogel microspheres loaded with BMSCs. The engineered bone callus-like organ demonstrated effective bone defect regeneration in rabbits within 4 weeks, a significant improvement over previous tissue engineering studies which typically required 2–3 months. Furthermore, Gabriella et al. [193]reported an innovative engineered wound healing tissue organ. The bone callus organ they synthesized can spontaneously undergo bioassembly, forming large engineered tissues and consequently healing critical-size long bone defects in mice (Fig. 5).
In addition to the mentioned applications, the bone-related cells and bone growth factors produced by bone organoids can serve as bioactive components, inducing the bone regeneration process. For instance, Diomede et al. [194] found that cultivating stem cells on cortical spongy scaffolds enhances osteogenic activity, thereby accelerating bone regeneration. However, to achieve clinical translation of bioprinted bone organoids, more research is needed. This includes in-depth investigation of the biological characteristics of bone organoids, optimizing bioprinting techniques to improve precision and efficiency, and exploring suitable clinical application scenarios.
Bioprinted organoid for repairing bone defects in BTE. (A) Assembly of osteo-callus organoids into bone-like tissue [192]. New bone formation 4 weeks after implantation with (a) H&E and (b) Masson staining. (Copyright 2022, Elsevier). (B) The organoid promotes long bone healing [193]. In vivo implantation of (a) Hematoxylin and eosin (H&E), (b) Safranin O, (c) Masson’s Trichrome (M’s T), and (d) hOCN. (Copyright 2019, Advanced Science)
Strategies for Bioprinting to enable clinical translation
To date, repairing irregular bone defects remains a major challenge because of the high treatment costs and suboptimal outcomes. In this regard, 3D-printed scaffolds are playing an increasingly important role. Certain acellular 3D-printed bone scaffolds have already achieved successful clinical translation through FDA approval, such as 3D-printed titanium spinal implants, sacroiliac joints, and total knee joints. Similarly, specific biodegradable 3D-printed bone scaffolds, such as Osteoplug™, OsteoplugTM−C, and Osteopore®, have been successfully clinically translated [195].
However, acellular 3D-printed scaffolds are limited to offering mechanical support, and they lack the ability to foster bone regeneration. In recent years, new research has focused on 3D bioprinting techniques that involve incorporating cells and growth factors to improve the results of bone regeneration. However, there are no cases of 3D-bioprinted bone scaffolds that have successfully translated to clinical applications to date. Recently, there have been studies combining bioprinting with organoids. Unfortunately, these new technologies require considerable development until clinical translation can be achieved. To overcome the obstacles and achieve clinical translation of 3D-printed bone scaffolds, future research is anticipated to prioritize the following areas.
Composite material advancements
Thus far, there is no single material that can perfectly meet all the ideal standards for bone scaffolds. Many criteria for achieving the ideal performance of bone scaffolds are conflicting in materials. For instance, while many applications require scaffolds to have good mechanical stability, rapid degradation capability is necessary to promote tissue growth. The complexities and challenges in material selection arise from conflicting requirements.
The amalgamation of optimal mechanical attributes and cell-friendly traits within a singular tissue engineering construct has demonstrated potential [23]. Researchers should focus on exploring deeply to develop composite materials with higher bioactivity. The development of composite materials aims to overcome inherent limitations of biomaterials by integrating different types of biomaterials to harness synergistic effects from their unique advantages [150]. These scaffolds incorporate a variety of matrices, among which polymer-ceramic composites stand out. They effectively combine the toughness of polymers with the compressive strength of ceramics, mimicking the natural properties of bone tissue. This integration results in outstanding mechanical performance and ideal degradation rates [196].
Wang et al. [197] used fused deposition modeling (FDM) technology to create poly(lactic acid) (PLA) and nano-hydroxyapatite (n-HA) composite scaffolds. The printed scaffolds exhibited adjustable mechanical strength, depending on the proportion of the n-HA component. Their biocompatibility and osteoinductive properties were superior to pure PLA scaffolds. Janmohammadi [198] developed 3D-printed polycaprolactone scaffolds (M-PCL) integrated with tragacanth gum-45S5 bioactive glass (TG-BG) to treat critical-sized cranial defects in adult female Wistar rats. This integration enhanced the biomechanical properties during bone remodeling, promoting bone integration and repair, and increased the expression of Runx2 and type I collagen.
Improved printing methods
To accurately replicate the bone microenvironment, it is vital to enhance the resolution of printing technologies to the submicrometer scale. However, traditional 3D printing devices typically use fixed-size print heads or nozzles, which inevitably sacrifice printing resolution due to limitations in nozzle size and precision [199]. Additionally, many materials suitable for high-resolution printing often do not meet the requirements for biocompatibility. 3D printing usually involves building objects by layer-by-layer deposition, and the thickness of these layers also limits the printing resolution. Research by Rachel Schwartz et al. [200] reported that encapsulating cells reduced the stable shear viscosity of gelatin-based bioinks by approximately 50%, ultimately compromising the resolution of bioprinting. In summary, the resolution achieved by existing printing technologies remains relatively limited due to constraints in printer hardware, ink performance, and other factors [201]. Additive manufacturing solutions can enhance resolution by integrating existing technologies or developing new methods. With continuous advancements in printing technology and deeper research, these limitations are expected to gradually be overcome, leading to higher-resolution bioprinting.
Li et al. [202] proposed a method for 3D printing concentrated nanocrystal solutions without the addition of polymers or monomer additives. In this approach, nanocrystals are inherently encapsulated with ligands, and then local selective covalent bonds are formed between adjacent nanocrystals using two-photon radiation. This method enables direct writing of 3D structures with micron-level resolution without altering the intrinsic properties of the nanocrystals.
Bio-printed organoids
In the process of bioprinting bone scaffolds, achieving self-organization and co-culture of cells remains the greatest challenge. The complex interactions between different types of cells make it difficult to precisely control their differentiation directions within bone tissue. This significantly impedes the successful integration of bioprinted scaffolds carrying cells by recipients [203].
Compared to traditional cell-free 3D printed scaffolds, organoids can more accurately simulate physiological cell composition and behavior, mimicking real organ structure and function to a greater extent, and allowing for long-term stable passage culture. The integration of bioprinting with organoids is crucial for the development of 3D printed bone scaffolds.
Wang et al. [204]. utilized a novel bioink composed of GelMA/AlgMA/HAP to manufacture highly complex bone-like organs via bioprinting. The bioprinted scaffolds facilitate long-term cultivation and gradual maturation of a wide range of bioprinted bone-like organs, promoting multicellular differentiation and offering valuable insights into the initial stages of bone formation. The inherent self-mineralization properties of the bioink closely mimic natural bone characteristics, enhancing the bone repair capability of these organoids for both in vitro and in vivo applications.
Artificial Intelligence and Machine Learning
Artificial Intelligence (AI) and Machine Learning (ML) applications continue to advance, bringing numerous innovations to the field of 3D printing of bone scaffolds. In the realm of 3D printing bone scaffolds, the introduction of AI/ML technologies has made medical procedures more precise and personalized. Using patients’ medical imaging data such as CT scans, MRI scans, etc., doctors can obtain detailed information about the patient’s bone structure and employ AI techniques to process and analyze this data [205]. These technologies can automatically identify and extract key features, such as the size, shape, and location of bone defects, providing precise guidance for scaffold design. Furthermore, machine learning algorithms can predict optimal scaffold design parameters based on individual patient differences and recovery needs, such as porosity and material type, enabling truly personalized treatments [206].
AI/ML technologies play a crucial role in the design process of bone scaffolds. Through algorithms like deep learning and neural networks, machines can autonomously learn and optimize scaffold design parameters such as structural strength, biocompatibility, and biomechanical performance [207]. These algorithms can generate a large number of design options in a short time and assess their feasibility and effectiveness through simulation analysis. This efficient design and optimization process significantly shortens product development cycles, enhancing the quality and performance of bone scaffolds [208].
Moreover, machine learning algorithms can monitor and analyze various stages of the manufacturing process in real-time, promptly identifying potential issues and making adjustments to ensure the quality and consistency of bone scaffolds [209, 210].
Conclusions and prospects
To date, repairing irregular bone defects remains a major challenge because of the high treatment costs and suboptimal outcomes. In this regard, 3D-printed scaffolds are playing an increasingly important role. Certain acellular 3D-printed bone scaffolds have already achieved successful clinical translation through FDA approval, such as 3D-printed titanium spinal implants, sacroiliac joints, and total knee joints. Similarly, specific biodegradable 3D-printed bone scaffolds, such as Osteoplug™, OsteoplugTM−C, and Osteopore®, have been successfully clinically translated [195]. However, acellular 3D-printed scaffolds are limited to offering mechanical support, and they lack the ability to foster bone regeneration. In recent years, new research has focused on 3D bioprinting techniques that involve incorporating cells and growth factors to improve the results of bone regeneration. However, there are no cases of 3D-bioprinted bone scaffolds that have successfully translated to clinical applications to date.
In this article, we provide a comprehensive review of the published research up to the time of writing, focusing on 3D printed scaffolds and bio-3D printed scaffolds. We detail the current state of research and development challenges in the field of bone tissue engineering from multiple perspectives, including materials, printing methods, and cell culture. Traditional 3D printed scaffolds rely solely on materials to provide structural support and mechanical stability, without promoting cell growth and tissue regeneration. In contrast, bio-3D printing integrates biomaterials with living cells, successfully creating more complex and functional bone scaffolds, but it still faces numerous challenges: on one hand, how to achieve cell self-organization and co-culture during the 3D printing process is a pressing issue; on the other hand, clinical translation remains another significant hurdle. The rapid development of organoid technology has brought new opportunities and challenges to the advancement of bio-3D printing technology. Combining bioprinting with organoid technology is the future of bio-3D printing, a topic we highlight in this article. Beyond laboratory research, we also explore in depth the three core challenges facing the clinical translation of 3D printing technology and offer detailed suggestions for addressing these challenges.
In summary, although the prospects for 3D bioprinting are promising, many technical issues remain to be resolved to achieve this vision, such as optimizing the biocompatibility of printing materials, improving printing resolution and speed, and precisely controlling cell distribution. With ongoing scientific research and technological advancements, we believe that bio-3D printing technology will gradually become a crucial pillar in the future of tissue engineering and regenerative medicine, providing more effective and personalized treatment options for patients, which is truly exciting.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AM:
-
Additive manufacturing
- BTE:
-
bone tissue engineering
- FDA:
-
U.S. Food and Drug Administration
- HA:
-
Hydroxyapatite
- TCP:
-
Ca3(PO4)2
- BAG:
-
Biologically active glass
- ECM:
-
extracellular matrix
- PCL:
-
polycaprolactone
- PGA:
-
Polyglycolic acid
- PLGA:
-
poly(lactic-co-glycolic acid)
- SLA:
-
stereolithography
- DLP:
-
digital light processing
- HAMA:
-
hyaluronic acid methacrylate
- ME:
-
Materials extrusion
- FDM:
-
fused deposition modeling
- FRESH:
-
freeform reversible embedding of suspended hydrogels
- BJ:
-
Binder jetting
- PBF:
-
Powder bed fusion
- SLS:
-
selective laser sintering
- SLM:
-
selective laser melting
- EBM:
-
electron beam melting
- MSCs:
-
mesenchymal stem cells
- iPSCs:
-
induced pluripotent stem cells
References
Lu J, Wang Q-Y, Sheng J-G, Guo S-C, Tao S-CJBMD. A 3D-printed, personalized, biomechanics-specific beta-tricalcium phosphate bioceramic rod system: personalized treatment strategy for patients with femoral shaft non-union based on finite element analysis[J]. BMC Musculoskelet Disord. 2020;21(1):1–9.
Park J, Kaliannagounder VK, Jang SR, Yoon D, Rezk AI, Bhattarai DP, Kim CS. Electroconductive Polythiophene Nanocomposite Fibrous scaffolds for enhanced osteogenic differentiation via Electrical Stimulation[J]. ACS Biomater Sci Eng. 2022;8(5):1975–86.
Sculco PK, Wright T, Malahias M-A, Gu A, Bostrom M, Haddad F et al. The diagnosis and treatment of acetabular bone loss in revision hip arthroplasty: an international consensus symposium[J]. HSS Journal®, 2022, 18(1):8–41
A TDN, A AK, A GI AKTQN. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges[J]. Compos Part B: Eng. 2018;143:172–96.
Toh EMS, Thenpandiyan AA, Foo ASC, Zhang JJY, Lim MJR, Goh CP et al. Clinical outcomes of 3D-Printed bioresorbable scaffolds for bone tissue Engineering-A Pilot Study on 126 patients for Burrhole covers in subdural Hematoma[J]. Biomedicines, 2022, 10(11).
Ashammakhi N, GhavamiNejad A, Tutar R, Fricker A, Roy I, Chatzistavrou X, et al. Highlights on advancing frontiers in tissue engineering[J]. Tissue Eng Part B: Reviews. 2022;28(3):633–64.
Li Z, Wang Q, Liu G. A review of 3D printed bone Implants[J]. Micromachines (Basel), 2022, 13(4).
Bandyopadhyay A, Mitra I, Bose S. 3D Printing for Bone Regeneration[J]. Curr Osteoporos Rep. 2020;18(5):505–14.
Gu Z, Fu J, Lin H, He YJAJPS. Development of 3D bioprinting: from printing methods to biomedical applications[J]. Asian J Pharm Sci. 2020;15(5):529–57.
Rossi G, Manfrin A, Lutolf MPJNRG. Progress and potential in organoid research[J]. Nat Rev Genet. 2018;19(11):671–87.
Obeid DA, Mir TA, Alzhrani A, Altuhami A, Shamma T, Ahmed S, et al. Using Liver organoids as models to study the pathobiology of Rare Liver Diseases[J]. Biomedicines. 2024;12(2):446.
Chen S, Chen X, Geng Z, Su J. The horizon of bone organoid: a perspective on construction and application[J]. Bioactive Mater. 2022;18:15–25.
Teitelbaum SL. Bone resorption by osteoclasts[J]. Science. 2000;289(5484):1504–8.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement[J]. Cytotherapy. 2006;8(4):315–7.
Chan CK, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, et al. Identification of the human skeletal stem cell[J]. Cell. 2018;175(1):43–56. e21.
Wang C, Huang W, Zhou Y, He L, He Z, Chen Z, et al. 3D printing of bone tissue engineering scaffolds[J]. Bioact Mater. 2020;5(1):82–91.
Jia Z, Xu X, Zhu D, Zheng Y. Design, printing, and engineering of regenerative biomaterials for personalized bone healthcare[J]. Prog Mater Sci, 2023, 134.
Wei S, Ma JX, Xu L, Gu XS, Ma XL. Biodegradable materials for bone defect repair[J]. Mil Med Res. 2020;7(1):54.
Zhang J, Liu W, Schnitzler V, Tancret F, Bouler J-MJA. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties[J]. Acta Biomater. 2014;10(3):1035–49.
Tabia Z, Meriame B, Mabrouk KE, Vaudreuil SJJMS. Manufacturing of a metallic 3D framework coated with a bioglass matrix for implant applications[J]. J Mater Sci, 2021, 56(2).
Khoramgah MS, Ranjbari J, Abbaszadeh HA, Sadat F, Ghanbarian HJB. Freeze-dried multiscale porous nanofibrous three dimensional scaffolds for bone regenerations[J]. BioImpacts: BI. 2020;10(2):73.
Borowiec J, Hampl J, Singh S, Haefner S, Friedel K, Mai P, et al. 3D microcontact printing for combined chemical and topographical patterning on porous cell culture membrane[J]. ACS Appl Mater Interfaces. 2018;10(26):22857–65.
Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, Li B, Shu W. 3D bioactive composite scaffolds for bone tissue engineering[J]. Bioact Mater. 2018;3(3):278–314.
Mountziaris PM, Mikos AGJTEPBR. Modulation of the inflammatory response for enhanced bone tissue regeneration[J]. Tissue Eng Part B: Reviews. 2008;14(2):179–86.
Karunakaran R, Ortgies S, Tamayol A, Bobaru F, Sealy MPJB. Additive manufacturing of magnesium alloys[J]. Bioactive Mater. 2020;5(1):44–54.
B WL AYL. A JZ, a AAZJAB. Corrosion fatigue behavior of additively manufactured biodegradable porous zinc[J]. Acta Biomater. 2020;106:439–49.
Feng P, Zhao R, Tang W, Yang F, Tian H, Peng S, Pan H, Shuai C. Structural and functional adaptive Artificial bone: materials, fabrications, and Properties[J]. Adv Funct Mater, 2023, 33(23).
Huan Y, Zhou D, Wu X, He X, Chen H, Li S, et al. 3D bioprinted autologous bone particle scaffolds for cranioplasty promote bone regeneration with both implanted and native BMSCs[J]. Biofabrication. 2023;15(2):025016.
Oladapo BI, Zahedi SA, Ismail SO, Omigbodun FT, Bowoto OK, Olawumi MA, Muhammad MAJB-D. Manufacturing. 3D printing of PEEK–cHAp scaffold for medical bone implant[J]. Bio-Design Manuf. 2021;4:44–59.
Wan Z, Zhang P, Liu Y, Lv L, Zhou Y. Four-dimensional bioprinting: current developments and applications in bone tissue engineering[J]. Acta Biomater. 2020;101:26–42.
Owen R. Reilly GCJFib, biotechnology. In vitro models of bone remodelling and associated disorders[J]. Front Bioeng Biotechnol. 2018;6:134.
Herber V, Labmayr V, Sommer NG, Marek R, Wittig U, Leithner A, Seibert F, Holweg PJI. Can hardware removal be avoided using bioresorbable Mg-Zn-Ca screws after medial malleolar fracture fixation? Mid-term results of a first-in-human study[J]. Injury. 2022;53(3):1283–8.
Eger M, Sterer N, Liron T, Kohavi D, Gabet Y. Scaling of titanium implants entrains inflammation-induced osteolysis[J]. Sci Rep. 2017;7:39612.
Han HS, Sergio L, Indong J, James E, Yu-Chan K, Hyun-Kwang S, Frank W, Diego M, Sion GJJMT. Current status and outlook on the clinical translation of biodegradable metals[J]. Mater Today, 2018:S136970211830347X-.
Zhang J, Shang Z, Jiang Y, Zhang K, Li X, Ma M, Li Y, Ma B. Biodegradable metals for bone fracture repair in animal models: a systematic review[J]. Regen Biomater. 2021;8(1):rbaa047.
Gu X, Zheng Y, Cheng Y, Zhong S, Xi TJB. In vitro corrosion and biocompatibility of binary magnesium alloys[J]. Biomaterials. 2009;30(4):484–98.
Yy A, Ch A, De A, Wy A, Fq A, Dx B, et al. Mg bone implant: features, developments and perspectives[J]. Mater Design. 2020;185:108259.
Cheng M-q, Wahafu T, Jiang G-f, Liu W, Qiao Y-q et al. Peng X-c,. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration[J]. Scientific reports, 2016, 6(1):1–14
Dong J, Tumer N, Putra NE, Zhu J, Li Y, Leeflang MA, et al. Extrusion-based 3D printed magnesium scaffolds with multifunctional MgF(2) and MgF(2)-CaP coatings[J]. Biomater Sci. 2021;9(21):7159–82.
Windhagen H, Radtke K, Weizbauer A, Diekmann J, Noll Y, Kreimeyer U, Schavan R, Stukenborg-Colsman C. Waizy HJBeo. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study[J]. Biomed Eng Online. 2013;12:1–10.
Tsakiris V, Tardei C. Clicinschi FMJJoM, Alloys. Biodegradable mg alloys for orthopedic implants–A review[J]. J Magnesium Alloys. 2021;9(6):1884–905.
Avedesian MM, Baker H. ASM specialty handbook: magnesium and magnesium alloys. Volume 274. ASM international Materials Park, OH; 1999.
Agarwal S, Curtin J, Duffy B, Jaiswal SJMS. Biodegradable magnesium alloys for orthopaedic applications: a review on corrosion, biocompatibility and surface modifications[J]. Mater Sci Engineering: C. 2016;68:948–63.
Ibrahim H, Billings C, Abdalla M, Korra A, Anderson DE. Vivo Assessment of High-Strength and Corrosion-Controlled Magnesium-based bone Implants[J]. Bioengineering. 2023;10(7):877.
Kopp A, Fischer H, Soares AP, Schmidt-Bleek K, Leber C, Kreiker H, et al. Long-term in vivo observations show biocompatibility and performance of ZX00 magnesium screws surface-modified by plasma-electrolytic oxidation in Göttingen miniature pigs[J]. Acta Biomater. 2023;157:720–33.
Niranjan C, Raghavendra T, Rao MP, Siddaraju C, Gupta M, Jain VKS, Aishwarya R. Magnesium alloys as extremely promising alternatives for temporary orthopedic implants–A review[J]. J Magnesium Alloys, 2023.
Liu Y, Zheng Y, Chen XH, Yang JA, Pan H, Chen D, et al. Fundamental theory of biodegradable metals—definition, criteria, and design[J]. Adv Funct Mater. 2019;29(18):1805402.
Zafar MJ, Zhu D, Zhang Z. 3D Printing of Bioceramics for Bone tissue Engineering[J]. Mater (Basel), 2019, 12(20).
Chemistry VG-CJPSS. Calcium phosphates as substitution of bone tissues[J]. Prog Solid State Chem. 2004;32(1–2):1–31.
Brandt J, Henning S, Michler G, Hein W, Bernstein A, Schulz MJJMSMM. Nanocrystalline hydroxyapatite for bone repair: an animal study[J]. J Mater Science: Mater Med. 2010;21:283–94.
Ribas RG, Schatkoski VM, do Amaral Montanheiro TL, de Menezes BRC, Stegemann C, Leite DMG, Thim GPJCI. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: a review[J]. Ceram Int. 2019;45(17):21051–61.
Zheng SY, Liu ZW, Kang HL, Liu F, Yan GP, Li F. 3D-Printed scaffolds based on poly(trimethylene carbonate), poly(epsilon-Caprolactone), and beta-tricalcium phosphate[J]. Int J Bioprint. 2023;9(1):641.
Liu X, Rahaman MN, Hilmas GE, Bal BSJA. Mechanical properties of bioactive glass (13–93) scaffolds fabricated by robotic deposition for structural bone repair[J]. Acta Biomater. 2013;9(6):7025–34.
Nandi SK, Fielding G, Banerjee D, Bandyopadhyay A. Bose SJJomr. 3D-printed β-TCP bone tissue engineering scaffolds: effects of chemistry on in vivo biological properties in a rabbit tibia model[J]. J Mater Res. 2018;33(14):1939–47.
Thygesen T, Slots C, Jensen MB, Ditzel N, Kassem M, Langhorn L, Andersen MJB. Comparison of off-the-shelf β-tricalcium phosphate implants with novel resorbable 3D printed implants in mandible ramus of pigs[J]. Bone. 2022;159:116370.
Fernandes HR, Gaddam A, Rebelo A, Brazete D, Stan GE, Ferreira JMJM. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering[J]. Materials. 2018;11(12):2530.
Hench LL, Wilson JJS. Surface-active biomaterials[J]. Science. 1984;226(4675):630–6.
Jones JRJA. Reprint of: review of bioactive glass: from Hench to hybrids[J]. Acta Biomater. 2015;23:S53–82.
Jones JR, Brauer DS, Hupa L, Greenspan DCJIJAGS. Bioglass and bioactive glasses and their impact on healthcare[J]. Int J Appl Glass Sci. 2016;7(4):423–34.
van der Heide D, Cidonio G, Stoddart MJ, D’Este M. 3D printing of inorganic-biopolymer composites for bone regeneration[J]. Biofabrication, 2022, 14(4).
Reddy MSB, Ponnamma D, Choudhary R, Sadasivuni KKJP. A comparative review of natural and synthetic biopolymer composite scaffolds[J]. Polymers. 2021;13(7):1105.
Lin WJP. An overview on collagen and gelatin-based cryogels: fabrication, classification, properties and Biomedical Applications[J]. Polymers, 2021, 13.
Osidak EO, Karalkin PA, Osidak MS, Parfenov VA, Sivogrivov DE, Pereira F, et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting[J]. J Mater Sci Mater Med. 2019;30(3):31.
Wang Z, Yang Y, Gao Y, Xu Z, Yang S, Jin M. Establishing a novel 3D printing bioinks system with recombinant human collagen[J]. Int J Biol Macromol. 2022;211:400–9.
Zhai P, Peng X, Li B, Liu Y, Sun H, Li XJI. The application of hyaluronic acid in bone regeneration[J]. Int J Biol Macromol. 2020;151:1224–39.
Garcia-Villen F, Guembe A, José MR, Zúñiga T, Ruiz-Alonso S, Saenz-del-Burgo L, Jesús MI, José IR, Pedraz JLJIJoB. Characterization and assessment of new fibrillar collagen inks and bioinks for 3D printing and bioprinting[J]. Int J Bioprinting, 2023, 9(3).
Okamoto M, John BJPPS. Synthetic biopolymer nanocomposites for tissue engineering scaffolds[J]. Prog Polym Sci. 2013;38(10–11):1487–503.
Mondrinos MJ, Dembzynski R, Lu L, Byrapogu VK, Wootton DM, Lelkes PI, Zhou J. Porogen-based solid freeform fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineering[J]. Biomaterials. 2006;27(25):4399–408.
Tan L, Yu X, Wan P, Yang KJJoMS. Technology. Biodegradable materials for bone repairs: a review[J]. J Mater Sci Technol. 2013;29(6):503–13.
Albertsson A-C, Varma IKJB. Recent developments in ring opening polymerization of lactones for biomedical applications[J]. Biomacromolecules. 2003;4(6):1466–86.
Lee SJ, Khang G, Lee YM, Lee HBJJBSPE. Interaction of human chondrocytes and NIH/3T3 fibroblasts on chloric acid-treated biodegradable polymer surfaces[J]. J Biomater Sci Polym Ed. 2002;13(2):197–212.
Liu T, Li Z, Zhao L, Chen Z, Lin Z, Li B, et al. Customized design 3D printed PLGA/Calcium sulfate Scaffold enhances mechanical and Biological properties for Bone Regeneration[J]. Front Bioeng Biotechnol. 2022;10:874931.
Fan L, Teng W, He J, Wang D, Liu C, Zhao Y, Zhang L. [Retracted] value of 3D printed PLGA scaffolds for cartilage defects in terms of Repair[J]. Evidence-Based Complement Altern Med. 2022;2022(1):3561430.
Liu Z, Tian G, Liu L, Li Y, Xu S, Du Y, et al. A 3D-printed PLGA/HA composite scaffold modified with fusion peptides to enhance its antibacterial, osteogenic and angiogenic properties in bone defect repair[J]. J Mater Res Technol. 2024;30:5804–19.
lin Liang Z, Chen L, quan Wu Y. 3D printed PLGA scaffold with nano-hydroxyapatite carrying linezolid for treatment of infected bone defects[J]. Biomed Pharmacother. 2024;172:116228.
Chereddy KK, Vandermeulen G, Préat V. PLGA based drug delivery systems: promising carriers for wound healing activity[J]. Wound Repair Regeneration. 2016;24(2):223–36.
Alghareeb S, Asare-Addo K, Conway BR, Adebisi AO. PLGA nanoparticles for nasal drug delivery[J]. J Drug Deliv Sci Technol, 2024:105564.
Xu S, Tian G, Zhi M, Liu Z, Du Y, Lu X, et al. Functionalized PLGA Microsphere loaded with Fusion peptide for therapy of bone Defects[J]. ACS Biomaterials Sci Eng. 2024;10(4):2463–76.
Li Z, Huang W, Zhang M, Huo Y, Li F, Song L, et al. Minocycline-loaded nHAP/PLGA microspheres for prevention of injury-related corneal angiogenesis[J]. J Nanobiotechnol. 2024;22(1):134.
Li G, Zhao M, Xu F, Yang B, Li X, Meng X, Teng L, Sun F, Li Y. Synthesis and biological application of polylactic acid[J]. Molecules. 2020;25(21):5023.
Ramot Y, Haim-Zada M, Domb AJ, Nyska A. Biocompatibility and safety of PLA and its copolymers[J]. Adv Drug Deliv Rev. 2016;107:153–62.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications[J]. J Controlled Release. 2012;161(2):505–22.
Liu K, Li L, Chen J, Li Y, Wen W, Lu L, et al. Bone ECM-like 3D printing scaffold with liquid crystalline and viscoelastic microenvironment for bone regeneration[J]. ACS Nano. 2022;16(12):21020–35.
Wei X, Zhou W, Tang Z, Wu H, Liu Y, Dong H, et al. Magnesium surface-activated 3D printed porous PEEK scaffolds for in vivo osseointegration by promoting angiogenesis and osteogenesis[J]. Bioactive Mater. 2023;20:16–28.
Fitzpatrick V, Martín-Moldes Z, Deck A, Torres-Sanchez R, Valat A, Cairns D, Li C, Kaplan DLJB. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization[J]. 2021, 276:120995.
Long J, Yao Z, Zhang W, Liu B, Chen K, Li L, et al. Regulation of Osteoimmune Microenvironment and Osteogenesis by 3D-Printed PLAG/black phosphorus scaffolds for bone Regeneration[J]. Adv Sci. 2023;10(28):2302539.
Askari M, Hutchins DA, Thomas PJ, Astolfi L, Watson RL, Abdi M, et al. Additive manufacturing of metamaterials: a review[J]. Additive Manuf. 2020;36:101562.
Ng WL, An J, Chua CK. Process, material, and regulatory considerations for 3D printed medical devices and tissue constructs[J]. Engineering, 2024.
Chen Z, Li Z, Li J, Liu C, Lao C, Fu Y, et al. 3D printing of ceramics: a review[J]. J Eur Ceram Soc. 2019;39(4):661–87.
Trombetta R, Inzana JA, Schwarz EM, Kates SL. Awad HAJAobe. 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery[J]. Ann Biomed Eng. 2017;45:23–44.
Quan H, Zhang T, Xu H, Luo S, Nie J, Zhu XJB. Photo-curing 3D printing technique and its challenges[J]. Bioactive Mater. 2020;5(1):110–5.
Awad A, Fina F, Goyanes A, Gaisford S, Basit AWJADDR. Advances in powder bed fusion 3D printing in drug delivery and healthcare[J]. Adv Drug Deliv Rev. 2021;174:406–24.
Li Z, Wang Q, Liu GJM. A review of 3D printed bone implants[J]. Micromachines. 2022;13(4):528.
Attarilar S, Ebrahimi M, Djavanroodi F, Fu Y, Wang L, Yang J. 3D Printing technologies in metallic implants: a thematic review on the techniques and Procedures[J]. Int J Bioprint. 2021;7(1):306.
Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering[J]. Nat Reviews Mater. 2020;5(8):584–603.
Mirkhalaf M, Men Y, Wang R, No Y, Zreiqat H. Personalized 3D printed bone scaffolds: a review[J]. Acta Biomater. 2023;156:110–24.
Wu Y, Su H, Li M, Xing H. Digital light processing-based multi‐material bioprinting: processes, applications, and perspectives[J]. J Biomedical Mater Res Part A. 2023;111(4):527–42.
Amini A, Guijt RM, Themelis T, De Vos J, Eeltink S. Recent developments in digital light processing 3D-printing techniques for microfluidic analytical devices[J]. J Chromatogr A. 2023;1692:463842.
Li W, Wang M, Ma H, Chapa-Villarreal FA, Lobo AO, Zhang YSJI. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication[J]. Iscience, 2023, 26(2).
Lam T, Dehne T, Krüger JP, Hondke S, Endres M, Thomas A, Lauster R, Sittinger M, Kloke LJJBMRPBAB. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage[J]. 2019, 107(8):2649–57.
Hong H, Seo YB, Lee JS, Lee YJ, Lee H, Ajiteru O, et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering[J]. J Biomedical Mater Res Part B: Appl Biomaterials. 2020;232:119679.
Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay AJP. Additive manufacturing of biomaterials[J]. Prog Mater Sci. 2018;93:45–111.
Grémare A, Guduric V, Bareille R, Heroguez V, Latour S, L’heureux N, Fricain JC, Le Catros S, Nihouannen DJJoBMRPA. Characterization of printed PLA scaffolds for bone tissue engineering[J]. J Biomedical Mater Res Part A. 2018;106(4):887–94.
Peng E, Zhang D, Ding JJAM. Ceramic robocasting: recent achievements, potential, and future developments[J]. Adv Mater. 2018;30(47):1802404.
Yang DH, Park HN, Bae MS, Lee JB, Heo DN, Lee WJ, et al. Evaluation of GENESIS-BCP™ scaffold composed of hydroxyapatite and β-tricalcium phosphate on bone formation[J]. Macromol Res. 2012;20:627–33.
Miramond T, Corre P, Borget P, Moreau F, Guicheux J, Daculsi G, Weiss PJJBA. Osteoinduction of biphasic calcium phosphate scaffolds in a nude mouse model[J]. J Biomater Appl. 2014;29(4):595–604.
Placone JK, Engler AJJA. Recent advances in extrusion-based 3D printing for biomedical applications[J]. Adv Healthc Mater. 2018;7(8):1701161.
Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR. Feinberg AWJSa. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels[J]. Sci Adv. 2015;1(9):e1500758.
Attarilar S, Ebrahimi M, Djavanroodi F, Fu Y, Wang L, Yang J. 3D printing technologies in metallic implants: a thematic review on the techniques and procedures[J]. Int J Bioprinting, 2021, 7(1).
Elkaseer A, Chen KJ, Janhsen JC, Refle O, Hagenmeyer V, Scholz SG. Material jetting for advanced applications: a state-of-the-art review, gaps and future directions[J]. Additive Manuf. 2022;60:103270.
Zhou L, Miller J, Vezza J, Mayster M, Raffay M, Justice Q et al. Additive Manufacturing: A Comprehensive Review[J]. Sensors, 2024, 24(9):2668
Ahn J-H, Kim J, Han G, Kim D, Cheon K-H, Lee H, et al. 3D-printed biodegradable composite scaffolds with significantly enhanced mechanical properties via the combination of binder jetting and capillary rise infiltration process[J]. Additive Manuf. 2021;41:101988.
Lowther M, Louth S, Davey A, Hussain A, Ginestra P, Carter L, Eisenstein N, Grover L, Cox SJAM. Clinical, industrial, and research perspectives on powder bed fusion additively manufactured metal implants[J]. Additive Manuf. 2019;28:565–84.
Brunello G, Sivolella S, Meneghello R, Ferroni L, Gardin C, Piattelli A, Zavan B. Bressan EJBa. Powder-based 3D printing for bone tissue engineering[J]. Biotechnol Adv. 2016;34(5):740–53.
Duan B, Wang MJM. Selective laser sintering and its application in biomedical engineering[J]. MRS Bull. 2011;36(12):998–1005.
Sing SL, An J, Yeong WY, Wiria FEJJOR. Laser and electron-beam powder‐bed additive manufacturing of metallic implants: a review on processes, materials and designs[J]. J Orthop Res. 2016;34(3):369–85.
Liu J, Liu B, Min S, Yin B, Peng B, Yu Z, et al. Biodegradable magnesium alloy WE43 porous scaffolds fabricated by laser powder bed fusion for orthopedic applications: process optimization, in vitro and in vivo investigation[J]. Bioactive Mater. 2022;16:301–19.
Van der Stok J, Van der Jagt OP, Amin Yavari S, De Haas MF, Waarsing JH, Jahr H, et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects[J]. J Orthop Res. 2013;31(5):792–9.
Zhang J, Shang Z, Jiang Y, Zhang K, Li X, Ma M, Li Y, Ma B. Biodegradable metals for bone fracture repair in animal models: a systematic review[J]. Regenerative Biomaterials. 2021;8(1):rbaa047.
Li L-Y, Cui L-Y, Zeng R-C, Li S-Q, Chen X-B, Zheng Y, Kannan MB. Advances in functionalized polymer coatings on biodegradable magnesium alloys–A review[J]. Acta Biomater. 2018;79:23–36.
Raees S, Ullah F, Javed F, Akil HM, Khan MJ, Safdar M, et al. Classification, processing, and applications of bioink and 3D bioprinting: a detailed review[J]. Int J Biol Macromol. 2023;232:123476.
Sabetkish S, Currie P, Meagher L. Recent trends in 3D Bioprinting Technology for skeletal muscle Regeneration[J]. Acta Biomater, 2024.
Ashammakhi N, Hasan A, Kaarela O, Byambaa B, Sheikhi A, Gaharwar AK, Khademhosseini A. Advancing frontiers in bone Bioprinting[J]. Adv Healthc Mater. 2019;8(7):e1801048.
Gao G, Cui XJBL. Three-dimensional bioprinting in tissue engineering and regenerative medicine[J]. Biotechnol Lett. 2016;38(2):203–11.
Freeman FE, Burdis R, Kelly DJ. Printing New bones: from print-and-Implant devices to Bioprinted Bone Organ Precursors[J]. Trends Mol Med. 2021;27(7):700–11.
Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala, AJNb. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity[J]. Nat Biotechnol. 2016;34(3):312–9.
Daly AC, Cunniffe GM, Sathy BN, Jeon O, Alsberg E, Kelly. DJJAhm. 3D bioprinting of developmentally inspired templates for whole bone organ engineering[J]. Advanced healthcare materials, 2016, 5(18):2353–2362.
Di Bella C, Duchi S, O’Connell CD, Blanchard R, Augustine C, Yue Z, et al. In situ handheld three-dimensional bioprinting for cartilage regeneration[J]. J Tissue Eng Regen Med. 2018;12(3):611–21.
Daly AC, Prendergast ME, Hughes AJ, Burdick JAJC. Bioprinting Biologist[J] Cell. 2021;184(1):18–32.
Hao Y, Cao B, Deng L, Li J, Ran Z, Wu J et al. The first 3D-bioprinted personalized active bone to repair bone defects: a case report[J]. Int J Bioprinting, 2023, 9(2).
Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, Li B. Shu WJBm. 3D bioactive composite scaffolds for bone tissue engineering[J]. Bioactive Mater. 2018;3(3):278–314.
Kang C-W, Fang F-ZJAM. State of the art of bioimplants manufacturing: part I[J]. Adv Manuf. 2018;6:20–40.
Zhang J, Li S, He H, Han L, Zhang S, Yang L, et al. Clinical guidelines for indications, techniques, and complications of autogenous bone grafting[J]. Chin Med J. 2024;137(01):5–7.
Mladenovska T, Choong PF, Wallace GG, O’connell CD. The regulatory challenge of 3D bioprinting[J]. Regen Med. 2023;18(8):659–74.
Zan J, Qian G, Deng F, Zhang J, Zeng Z, Peng S. Shuai CJJoMR, Technology. Dilemma and breakthrough of biodegradable poly-l-lactic acid in bone tissue repair[J]. J Mater Res Technol. 2022;17:2369–87.
Li G, Yang H, Zheng Y, Chen X-H, Yang J-A, Zhu D, Ruan L. Takashima KJAb. Challenges in the use of zinc and its alloys as biodegradable metals: perspective from biomechanical compatibility[J]. Acta Biomater. 2019;97:23–45.
Yang J, Chen Z, Gao C, Liu J, Liu K, Wang X, et al. A mechanical-assisted post-bioprinting strategy for challenging bone defects repair[J]. Nat Commun. 2024;15(1):3565.
Levato R, Dudaryeva O, Garciamendez-Mijares CE, Kirkpatrick BE, Rizzo R, Schimelman J, et al. Light-based vat-polymerization bioprinting[J]. Nat Reviews Methods Primers. 2023;3(1):47.
Ng WL, Lee JM, Zhou M, Chen Y-W, Lee K-XA, Yeong WY, Shen Y-F. Vat polymerization-based bioprinting—process, materials, applications and regulatory challenges[J]. Biofabrication. 2020;12(2):022001.
Ng WL, Huang X, Shkolnikov V, Goh GL, Suntornnond R, Yeong WY. Controlling droplet impact velocity and droplet volume: key factors to achieving high cell viability in sub-nanoliter droplet-based bioprinting[J]. Int J Bioprinting, 2022, 8(1).
Ng WL, Huang X, Shkolnikov V, Suntornnond R, Yeong WY. Polyvinylpyrrolidone-based bioink: influence of bioink properties on printing performance and cell proliferation during inkjet-based bioprinting[J]. Bio-Design Manuf. 2023;6(6):676–90.
Xu H-Q, Liu J-C, Zhang Z-Y, Xu C-X. A review on cell damage, viability, and functionality during 3D bioprinting[J]. Military Med Res. 2022;9(1):70.
Ataie Z, Kheirabadi S, Zhang JW, Kedzierski A, Petrosky C, Jiang R, Vollberg C, Sheikhi A. Nanoengineered granular hydrogel bioinks with preserved interconnected microporosity for extrusion bioprinting[J]. Small. 2022;18(37):2202390.
Boularaoui S, Al Hussein G, Khan KA, Christoforou N, Stefanini C. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability[J]. Bioprinting. 2020;20:e00093.
Qin Y, Qi Q, Scott PJ, Jiang X. Status, comparison, and future of the representations of additive manufacturing data[J]. Comput Aided Des. 2019;111:44–64.
Godec D, Pilipović A, Breški T. General process Workflow in Additive Manufacturing. A guide to Additive Manufacturing. Springer International Publishing Cham; 2022. pp. 45–57.
Kumar A, Kumar P, Mittal RK, Singh H. Printing file formats for additive manufacturing technologies[J]. Adv Additive Manuf, 2023:87–102.
Mahadik B, Margolis R, McLoughlin S, Melchiorri A, Lee SJ, Yoo J, Atala A, Mikos AG, Fisher JPJB. An open-source bioink database for microextrusion 3D printing[J]. Biofabrication. 2022;15(1):015008.
Ma S, Wang Z, Guo Y, Wang P, Yang Z, Han L, Sun J, Xia Y. Enhanced osteoinduction of electrospun scaffolds with assemblies of hematite nanoparticles as a bioactive interface[J]. Int J Nanomed, 2019:1051–68.
Ferraz MP. An overview on the big players in bone tissue Engineering: Biomaterials, scaffolds and Cells[J]. Int J Mol Sci. 2024;25(7):3836.
Takabatake K, Matsubara M, Yamachika E, Fujita Y, Arimura Y, Nakatsuji K, Nakano K, Nagatsuka H, Iida S. Comparing the osteogenic potential and bone regeneration capacities of dedifferentiated fat cells and adipose-derived stem cells in vitro and in vivo: application of DFAT cells isolated by a mesh method[J]. Int J Mol Sci. 2021;22(22):12392.
Gao X, Ruzbarsky JJ, Layne JE, Xiao X, Huard J. Stem Cells Bone Tissue Engineering[J] Life. 2024;14(3):287.
Aghali A. Craniofacial bone tissue engineering: current approaches and potential therapy[J]. Cells. 2021;10(11):2993.
Oliveira ÉR, Nie L, Podstawczyk D, Allahbakhsh A, Ratnayake J, Brasil DL, Shavandi A. Advances in growth factor delivery for bone tissue engineering[J]. Int J Mol Sci. 2021;22(2):903.
Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves[J]. J Cell Biol. 2015;210(1):153–68.
Cuvellier M, Rose S, Ezan F, Jarry U, de Oliveira H, Bruyère A, et al. In vitro long term differentiation and functionality of three-dimensional bioprinted primary human hepatocytes: application for in vivo engraftment[J]. Biofabrication. 2022;14(3):035021.
Jeon MJ, Haugen BR. Preclinical models of follicular cell-derived thyroid cancer: an overview from cancer cell lines to mouse models[J]. Endocrinol Metabolism. 2022;37(6):830.
Clark CC, Yoo KM, Sivakumar H, Strumpf K, Laxton AW, Tatter SB, Strowd RE, Skardal A. Immersion bioprinting of hyaluronan and collagen bioink-supported 3D patient-derived brain tumor organoids[J]. Biomed Mater. 2022;18(1):015014.
Wu Y, Chen X, Bao W, Hong X, Li C, Lu J, Zhang D, Zhu A. Effect of humantenine on mRNA m6A modification and expression in human colon cancer cell line HCT116[J]. Genes. 2022;13(5):781.
Ma Y, Deng B, He R, Huang P. Advancements of 3D bioprinting in regenerative medicine: exploring cell sources for organ fabrication[J]. Heliyon, 2024.
Han P, Raveendran N, Liu C, Basu S, Jiao K, Johnson N, Moran CS, Ivanovski S. 3D bioprinted small extracellular vesicles from periodontal cells enhance mesenchymal stromal cell function[J]. Biomaterials Adv. 2024;158:213770.
Bastami F, Nazeman P, Moslemi H, Rezai Rad M, Sharifi K, Khojasteh AJCP. Induced pluripotent stem cells as a new getaway for bone tissue engineering: a systematic review[J]. Cell Prolif. 2017;50(2):e12321.
Song X, Hu M, Li B, Zhang K, Zhang X, Wang L. Advancing medical device regulatory reforms for innovation, translation and industry development in China[J]. J Orthop Translation. 2022;37:89–93.
Morrison RJ, Kashlan KN, Flanangan CL, Wright JK, Green GE, Hollister SJ, Weatherwax KJ. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices[J]. Clin Transl Sci. 2015;8(5):594–600.
Naghshineh N, Brown S, Cederna PS, Levi B, Lisiecki J, D’Amico RA, Hume KM, Seward W, Rubin JP. Demystifying the US Food and Drug Administration: understanding regulatory pathways[J]. Plast Reconstr Surg. 2014;134(3):559–69.
Ghelich P, Kazemzadeh-Narbat M, Hassani Najafabadi A, Samandari M, Memić A, Tamayol A. (Bio) manufactured solutions for treatment of bone defects with an emphasis on US-FDA regulatory science perspective[J]. Adv Nanobiomed Res. 2022;2(4):2100073.
Murphy SV, De Coppi P, Atala AJN. Opportunities and challenges of translational 3D bioprinting[J]. Nat Biomedical Eng. 2020;4(4):370–80.
Miki Y, Ono K, Hata S, Suzuki T, Kumamoto H. Sasano HJTJosb, biology m. The advantages of co-culture over mono cell culture in simulating in vivo environment[J]. J Steroid Biochem Mol Biol. 2012;131(3–5):68–75.
Brassard JA, Nikolaev M, Hübscher T, Hofer M, Lutolf MP. Recapitulating macro-scale tissue self-organization through organoid bioprinting[J]. Nat Mater. 2020;20(1):22–9.
Lai J, Liu Y, Lu G, Yung P, Wang X, Tuan RS, Li ZA. 4D bioprinting of programmed dynamic tissues[J]. Bioactive Mater. 2024;37:348–77.
Wang J, Yang X, Xu M, Liu H, Liu L, Tan Z. Distinct cellular microenvironment with cytotypic effects regulates orderly regeneration of vascular tissues[J]. Mater Today Bio. 2024;26:101033.
Maharjan S, Ma C, Singh B, Kang H, Orive G, Yao J, Zhang YS. Advanced 3D imaging and organoid bioprinting for biomedical research and therapeutic applications[J]. Adv Drug Deliv Rev, 2024:115237.
[118] Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, et al. Organoid Model Tumor Immune microenvironment[J] Cell. 2018;175(7):1972–88. e1916.
Brassard JA, Lutolf MPJC. Engineering stem cell self-organization to build better organoids[J]. Cell Stem Cell. 2019;24(6):860–76.
Akiva A, Melke J, Ansari S, Liv N, van der Meijden R, van Erp M, et al. An organoid for woven bone[J]. Adv Funct Mater. 2021;31(17):2010524.
Fatehullah A, Tan SH, Barker NJN. Organoids as an in vitro model of human development and disease[J]. Nat Cell Biol. 2016;18(3):246–54.
Chen S, Chen X, Geng Z, Su JJBM. The horizon of bone organoid: a perspective on construction and application[J]. Bioactive Mater. 2022;18:15–25.
Bertassoni LE. Bioprinting of Complex Multicellular organs with Advanced functionality-recent Progress and challenges Ahead[J]. Adv Mater. 2022;34(3):e2101321.
Chakraborty J, Chawla S, Ghosh SJCOB. Developmental biology-inspired tissue engineering by combining organoids and 3D bioprinting[J]. Curr Opin Biotechnol. 2022;78:102832.
Lawlor KT, Vanslambrouck JM, Higgins JW, Chambon A, Bishard K, Arndt D, et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation[J]. Nat Mater. 2021;20(2):260–71.
Humphreys BDJNM. Bioprinting better kidney organoids[J]. Nat Mater. 2021;20(2):128–30.
Brassard JA, Nikolaev M, Hübscher T, Hofer M, Lutolf MPJNM. Recapitulating macro-scale tissue self-organization through organoid bioprinting[J]. Nat Mater. 2021;20(1):22–9.
Lancaster MA, Knoblich JAJS. Organogenesis in a dish: modeling development and disease using organoid technologies[J]. Science. 2014;345(6194):1247125.
Watanabe N, Santostefano KE, Yachnis AT, Terada NJLI. A pathologist’s perspective on induced pluripotent stem cells[J]. Lab Invest. 2017;97(10):1126–32.
Zhang S-C, Wernig M, Duncan ID, Brüstle O. Thomson JAJNb. In vitro differentiation of transplantable neural precursors from human embryonic stem cells[J]. Nature biotechnology, 2001, 19(12):1129–1133.
Tang X-Y, Wu S, Wang D, Chu C, Hong Y, Tao M, et al. Human organoids in basic research and clinical applications[J]. Signal Transduct Target Therapy. 2022;7(1):168.
Lee H, Son M-Y. Current challenges associated with the use of human induced pluripotent stem cell-derived organoids in regenerative medicine[J]. Int J Stem Cells. 2021;14(1):9.
[131] Sato T, Vries RG, Snippert HJ, Van De Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche[J]. Nature. 2009;459(7244):262–5.
Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK, Mikos AGJNp. Evaluation of bone regeneration using the rat critical size calvarial defect[J]. Nat Protoc. 2012;7(10):1918–29.
Gao C, Peng S, Feng P, Shuai CJB. Bone biomaterials and interactions with stem cells[J]. Bone Res. 2017;5(1):1–33.
Zhang Y, Li G, Wang J, Zhou F, Ren X, Su JJS. Small Joint Organoids 3D Bioprinting: Construction Strategy and Application[J]. Small, 2023:2302506.
Xie C, Liang R, Ye J, Peng Z, Sun H, Zhu Q, et al. High-efficient engineering of osteo-callus organoids for rapid bone regeneration within one month[J]. Biomaterials. 2022;288:121741.
Nilsson Hall G, Mendes LF, Gklava C, Geris L, Luyten FP, Papantoniou IJAS. Developmentally engineered callus organoid bioassemblies exhibit predictive in vivo long bone healing[J]. Adv Sci. 2020;7(2):1902295.
Diomede F, Zini N, Gatta V, Fulle S, Merciaro I, D’Aurora M, et al. Human periodontal ligament stem cells cultured onto cortico-cancellous scaffold drive bone regenerative process[J]. Eur Cells Mater. 2016;32:181–201.
Toh EM, Thenpandiyan AA, Foo AS, Zhang JJ, Lim MJ, Goh CP et al. Clinical outcomes of 3D-printed bioresorbable scaffolds for bone tissue engineering—A pilot study on 126 patients for burrhole covers in subdural hematoma[J]. Biomedicines, 2022, 10(11).
Martelli A, Bellucci D, Cannillo V. Additive manufacturing of polymer/bioactive glass scaffolds for regenerative medicine: a review[J]. Polymers. 2023;15(11):2473.
Wang W, Zhang B, Li M, Li J, Zhang C, Han Y, et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering[J]. Compos Part B: Eng. 2021;224:109192.
Janmohammadi M, Doostmohammadi N, Bahraminasab M, Nourbakhsh MS, Arab S, Asgharzade S, Ghanbari A, Satari A. Evaluation of new bone formation in critical-sized rat calvarial defect using 3D printed polycaprolactone/tragacanth gum-bioactive glass composite scaffolds[J]. Int J Biol Macromol, 2024:132361.
Guastaldi FP, Mahadik B. Bone tissue engineering: bench to bedside using 3d printing[J]. 2022.
Schwartz R, Malpica M, Thompson GL, Miri AK. Cell encapsulation in gelatin bioink impairs 3D bioprinting resolution[J]. J Mech Behav Biomed Mater. 2020;103:103524.
Zandrini T, Florczak S, Levato R, Ovsianikov A. Breaking the resolution limits of 3D bioprinting: future opportunities and present challenges[J]. Trends Biotechnol. 2023;41(5):604–14.
Li F, Liu S-F, Liu W, Hou Z-W, Jiang J, Fu Z, et al. 3D printing of inorganic nanomaterials by photochemically bonding colloidal nanocrystals[J]. Science. 2023;381(6665):1468–74.
Daly AC, Lim KS. High resolution lithography 3D bioprinting[J]. Trends Biotechnol. 2023;41(3):262–3.
Wang J, Wu Y, Li G, Zhou F, Wu X, Wang M et al. Engineering large-scale self‐mineralizing bone organoids with bone matrix‐inspired Hydroxyapatite Hybrid Bioinks[J]. Adv Mater, 2024:2309875.
Lee J, Chung SW. Deep learning for orthopedic disease based on medical image analysis: Present and future[J]. Appl Sci. 2022;12(2):681.
Kolomenskaya E, Butova V, Poltavskiy A, Soldatov A, Butakova M. Application of Artificial Intelligence at all stages of bone tissue Engineering[J]. Biomedicines. 2023;12(1):76.
Choi AH. Artificial Intelligence, Machine Learning, and Neural Network. In: Bone Remodeling and Osseointegration of Implants Springer; 2023: 83–96.
Babu SS, Mourad A-HI, Harib KH, Vijayavenkataraman S. Recent developments in the application of machine-learning towards accelerated predictive multiscale design and additive manufacturing[J]. Virtual Phys Prototyp. 2023;18(1):e2141653.
Bonatti AF, Vozzi G, De Maria C. Enhancing quality control in bioprinting through machine learning[J]. Biofabrication. 2024;16(2):022001.
Monfared V. Application of Artificial Intelligence (Machine Learning) in Additive Manufacturing, Bio-Systems, Bio-medicine, and composites. Additive Manufacturing for biocomposites and Synthetic composites. CRC; 2023. pp. 152–203.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China (82071155, 82271023, 82301052), the Project of Shanxi Province Key Laboratory of Oral Diseases Prevention and New Materials [grant number RC2021-02 and RC202301], the Shanxi Applied Basic Research Program Outstanding Youth Cultivation Project Fund [grant numbers 202203021212368, 202203021222266], the Health Commission of Shanxi Province (2022XM14), the Shanxi Provincial Education Department (2022L165), Graduate Education Innovation Project of Shanxi Province(2023SJ170).
Author information
Authors and Affiliations
Contributions
XZ, NL and ZZ conducted the Investigation and wrote the original draft. YH, JW and QX prepared the tables and figures. JH, XZ and PZ checked the different sections of the manuscript. HL, ML and YZ conducted the literature search and organized the references. XZ, NL and XW edited and revised the manuscript. XR and XW gave the conceptualization and contributed to funding acquisition.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, X., Li, N., Zhang, Z. et al. Beyond hype: unveiling the Real challenges in clinical translation of 3D printed bone scaffolds and the fresh prospects of bioprinted organoids. J Nanobiotechnol 22, 500 (2024). https://doi.org/10.1186/s12951-024-02759-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02759-z