Abstract
Background
The association between triglyceride–glucose (TyG) index and poor prognosis remains controversial. Whether renal function status affects the ability of the TyG index to predict poor prognosis has not yet been elucidated and merits further studies.
Methods
This retrospective cohort study included 22,031 participants from communities in the U.S. By juxtaposing the TyG categories with the estimated glomerular filtration rate (eGFR, either < 60 mL/min/1.73m2 or ≥ 60 mL/min/1.73m2), participants were categorized into four distinct groups: (1) TyG_L/eGFR_H; (2) TyG_H/eGFR_H; (3) TyG_L/eGFR_L; and (4) TyG_H/eGFR_L. The endpoint was the all-cause mortality rate. Standard Kaplan–Meier plots were constructed and multifactor Cox regression analyses were carried out and restricted cubic spline regression analysis was utilized to assess the association between death and the TyG index for different renal function statuses.
Results
No statistical differences were found in the TyG groups in participants with normal renal function after adjustment for all covariates (P = 0.070). However, in the high TyG index group with renal insufficiency, the risk of all-cause mortality rates was reduced by 18%. (HR, 0.82; CI, 0.69–0.98). The TyG index (high vs. low) and renal function (eGFR < 60 vs. eGFR ≥ 60) had statistically significant interactions with death (P < 0.001). When all covariates were adjusted, the risk of mortality for the TyG_L combined with eGFR_L group was 56% higher than that for the TyG_L combined with eGFR_H group (HR, 1.56; CI, 1.33–1.82). In the renal insufficiency population, a nonlinear relationship was observed between mortality and the TyG index, albeit with a differing pattern (P for nonlinearity < 0.001).
Conclusions
While it has been known that TyG index was a prognosis marker of CVD, this research highlights that higher TyG index was associated with higher all-cause mortality rates for all participants. Furthermore, renal function status significantly moderates the effect of the TyG index on all-cause mortality in community-dwelling adults.
Similar content being viewed by others
Background
Insulin resistance (IR), typically characterized by the diminished responsiveness of target organs to circulating insulin, is a well-acknowledged marker for both metabolic disorders and systemic inflammation [1, 2]. Recently, the triglyceride-glucose (TyG) index, calculated as the logarithmic product of fasting triglyceride (TG) and glucose concentrations, has emerged as a potential alternative to IR and was superior to HOMA-IR [3, 4].
Over recent years, TyG index has gained attention due to its potential in predicting clinical outcomes like atrial fibrillation, cardiovascular disease (CVD), and stroke [5,6,7]. Although the association between poor prognosis and a high TyG index has been reported in most contemporary studies [8], there remains a notable discrepancy in the literature. Some studies failed to cement a clear connection between it and prognosis [9,10,11], and others even showed an absence of significant correlation [12]. This divergence in findings raised questions about potential external factors that might influence TyG index’s predictive capacity. Perhaps there are underlying conditions or states that modulate its accuracy [13].
With these queries in mind, our attention was drawn to a recent study that documented the dysregulation of renal insulin receptors. This dysregulation not only impacts local renal metabolism but also disrupts systemic glucose homeostasis, leading to systemic metabolic abnormalities [14]. This revelation suggested a potential intertwined relationship among IR, renal insufficiency, and poor prognosis. Given that both insulin resistance and renal insufficiencies are pervasive conditions with well-established links to adverse clinical outcomes, it became imperative to investigate their potential influence on TyG index’s prognostic capability.
Thus, arising from these concerns and the highlighted knowledge gap in the interplay between renal function and the TyG index, this research aimed to determine whether the accuracy of using the TyG as a predictor of poor prognosis is altered by the renal function status.
Methods
Study design and participants
The National Health and Nutrition Examination Survey (NHANES) is a national survey, which initiative of the U.S. National Center for Health Statistics (NCHS). It employs a stratified multistage random sampling strategy, with data being released in biennial cycles starting from 1999. After obtaining informed consent, selected participants complete a personal questionnaire at home, which is followed by physical and laboratory examinations as well as a 24-h dietary recall conducted at a mobile screening center. More comprehensive details about this survey are available on the NHANES website. And a retrospective analysis of publicly accessible NHANES data was performed in the present study.
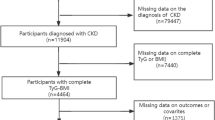
In this study, participants ≥ 18 years of age from U.S. communities were included. Of these, participants for whom fasting triglycerides, glucose, or estimated glomerular filtration rate (eGFR) data were not available were excluded. Also excluded are those who were Pregnant women, patients undergoing dialysis, and 25 participants who were not followed up. Consequently, 22031 individuals were enrolled in this study (Fig. 1).
Exposure
In this research, the TyG index was the exposure of interest, which was calculated at the time of study inclusion based on parameters measured while fasting. This index was specific calculations based on the formula of Luis E et al.: TyG index = Ln [1/2(TG (mg/dL) × FPG (mg/dL)] [4]. Blood samples for measuring plasma glucose and triglycerides were exclusively taken in the morning. We ensured that these measurements were obtained only from participants aged 12 and above who had fasted for a minimum of 8 h and not exceeding 24 h. Renal function, represented by eGFR, was calculated from serum creatinine levels using the Chronic Kidney Disease (CKD) Epidemiology Collaborative’s creatinine equation as a means to establish the baseline CKD status, participants were considered to have CKD if their eGFR was less than 60 mL/min/1.73m2 or if their Urinary Albumin to Creatinine Ratio was greater than or equal to 30 mg/g [15]. A restricted cubic spline function analysis determined the optimal TyG index threshold was to be 8.6, which led to the categorization of the participants into two groups: the TyG_L group with a TyG index of < 8.6, and the TyG_H group with a TyG index of ≥ 8.6. By juxtaposing the TyG categories with eGFR (either < 60 mL/min/1.73m2 or ≥ 60 mL/min/1.73m2), four distinct groups were obtained: (1) TyG_L/eGFR_H; (2) TyG_H/eGFR_H; (3) TyG_L/eGFR_L; and (4) TyG_H/eGFR_L.
Definition of variables of interest
For this study, the following factors were selected as covariates: the confounding factors of gender (male or female), age, education level (less than high school diploma, high school diploma, college or university degree or above), and race (Mexican American, non-Hispanic black, non-Hispanic white, other Hispanic, and other races) were used as confounders related to the demographic characteristics. The factors related to lifestyle included smoking (former or current smoker) and drinking habits. Body mass index (BMI) was measured by using the formula of measured weight in kilograms (kg) divided by the height squared (m2). The clinical comorbidities included hypertension (defined as having a physician diagnosed history of hypertension or a history of use of antihypertensive medication, the measured mean systolic blood pressure ≥ 140 mmHg, the measured mean diastolic blood pressure ≥ 90 mmHg), diabetes mellitus (defined as a history of physician-diagnosed diabetes mellitus or the use of antihyperglycemic medication, measured fasting blood glucose ≥ 7.0 mmol/L, measured glycosylated hemoglobin [HbA1c] ≥ 6.5%), hyperlipidemia (yes/no), and a history of cardiovascular disease defined as self-reported diagnosis by a physician of congestive heart failure, coronary heart disease, angina pectoris, heart attack, or stroke. Information on the levels of fasting triglycerides (mg/dL), LDL-C (mg/dL), HDL-C (mg/dL), blood pressure (mmHg), and fasting blood glucose (mg/dL) were determined at the time of the medical center visit. The categories of chronic kidney disease were defined in accordance with the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines. Detailed information about the data on potential confounders were available on the NCHS website.
Mortality outcomes
All-cause mortality during follow-up was the outcome of this study. The International Statistical Classification of Diseases, 10th edition, was used as the basis for categorizing all causes of death in this study. Mortality follow-up data for NHANES participants from 1999 to 2018 were available until December 31, 2019.
Statistical analyses
The weights suggested by NHANES were used in the analysis of this study. Continuous variables were subjected to ANOVA testing and were reported as mean standard error (SE). Assessment of categorical variables was assessed through the use of the chi-squared test.
To assess the relationship between death and the TyG index and to further determine whether TyG was differentially predictive of mortality in individuals with different renal function status, standard Kaplan–Meier plots and a single multifactor Cox regression analysis were used. Sensitivity analysis of cardiovascular and non-cardiovascular mortality risk according to the TyG index and different renal functions using multifactorial Cox regression analysis. Model 1 was a basic design that was not adjusted for potential confounders. On the contrary, Model 2 included adjustments for age, sex, and race, and potential confounders were fully taken into account in Model 3, such as alcohol consumption, smoking states, BMI, education level, HDL cholesterol level, LDL cholesterol level, eGFR, hypertension, DM, and CVD. To investigate the nonlinear relationship between mortality and TyG in the general population and in those with different renal function statuses, restricted cubic spline regression analysis was performed.
A two-sided p-value of less than 0.05 was defined as statistically significant in this study. The R programming environment (version 4.2.2; Vienna, Austria) was used for data analysis.
Results
The TyG index was used to separate the research participants based on their basic characteristics, as shown in Table 1. Participants in the TyG_H group were typically elder, overweight, and apt to the male sex compared with the TyG_L group (all P < 0.001). Based on the CKD G category results, the TyG_H group displayed a higher prevalence of CKD, with 36.0% in stage 2, 2.9% in stage 3a, 2.0% in stage 3b, 0.6% in stage 4, and 0.4% in stage 5. Furthermore, participants in the TyG_H group exhibited higher systolic and diastolic blood pressure, FPG, LDL-C, and TG levels; smoking prevalence; and incidence of DM, hypertension, and CVD (all P < 0.001).
The study population was further categorized according to the TyG index and eGFR (eGFR_L vs. eGFR_H). Table 2 demonstrates that the TyG_H combined with the eGFR_L group had the highest proportion of older patients and those with comorbidities, such as DM, hypertension, hyperlipidemia, and CVD (all P < 0.001).
Kaplan–Meier curves indicated a powerful connection between death and the TyG index in our research participants (all P < 0. 001; Fig. 2). When all covariates were adjusted, a high TyG index corresponded to a 53% increased risk of mortality (P = 0.047). Using Cox proportional risk analysis, it revealed that the TyG index increased by 1 unit was associated with a 13% increase in mortality risk (P = 0.010; Table 3).
Using univariate Cox proportional hazard analysis, it showed that the TyG index increased by 1 unit was associated with an 18% increase in mortality risk (P = 0.004) for participants with normal renal function. However, no statistically significant increase in the mortality risk was observed for individuals with renal insufficiency (P = 0.980). When all covariates were considered, no significant difference was noted among the TyG groups in participants with normal renal function (P = 0.070); however, the high TyG index group with renal insufficiency had an 18% reduced risk of death (HR, 0.82; CI, 0.69–0.98; Table 3). The TyG index (high vs. low) and renal function (eGFR < 60 vs. eGFR ≥ 60) had statistically significant interactions with all-cause mortality (P < 0.001).
Further categorization of the participants based on renal function status revealed distinct all-cause mortality trends within the TyG groups (all P < 0.001; Fig. 3). In the analysis of all-cause mortality, a significant difference was not perceived between the TyG_H combined with the eGFR_H group and the TyG_L combined with the eGFR_H group (P = 0.090) after adjusting for all covariates. However, the risk of mortality was 56% higher in the TyG_L combined with the eGFR_L group compared with the TyG_L combined with the eGFR_H group (HR, 1.56; CI, 1.33–1.82; Table 4). When we further disaggregated the causes of death, we found that the relationship between the TyG and the risk of cardiovascular or non-cardiovascular mortality in adults with renal insufficiency was generally consistent with the results for all-cause mortality (Supplementary Table 1).
The results of restricted cubic spline regression analysis alluded to a nonlinear relationship between mortality and TyG in the general population and in those with normal renal function (P for nonlinearity < 0.001; Fig. 4A and B). A similar nonlinear trend was also noted in individuals with renal insufficiency, albeit with a differing pattern (P for nonlinearity < 0.001; Fig. 4C).
In subgroups stratified by eGFR, a nonlinear correlation was observed between TyG and death, with a sinusoidal association between the TyG and mortality in participants with eGFR levels of 50–60 mL/min/1.73m2 (P for nonlinearity < 0.001; Fig. 5A). In those with eGFR levels of 40–50 mL/min/1.73m2, the nonlinear demonstrated an approximately U-shaped relationship (P for nonlinearity < 0.001; Fig. 5B). In participants with eGFR levels of 30–40 mL/min/1.73m2 too, a nonlinear relationship between them was observed, but with an inverse spoon-shaped pattern (P for nonlinearity < 0.001; Fig. 5C). Nonetheless, in participants with eGFR < 30 mL/min/1.73m2, a monotonically increasing relationship was observed between them (P for nonlinearity < 0.001; Fig. 5D).
Discussion
This retrospective analysis demonstrated that the correlation between all-cause mortality and the a high TyG index was not straightforward and that this association was affected by fluctuations in kidney function.
A range of clinical conditions, such as prediabetes, lipodystrophy, polycystic ovary syndrome, and nonalcoholic fatty liver disease, especially pertaining to metabolic syndrome and obesity, are associated with IR [16,17,18,19]. The TyG index is an economically viable and readily available indicator and can hence be used as a surrogate for IR. Liu et al. stated that an elevated TyG index, which is indicative of pronounced IR, is nonlinearly related to all-cause mortality [20], which agrees with the findings of the present study. A recent meta-analysis was unable to establish an independent link between the TyG index and death [10]. Nonetheless, such associations were influenced by factors such as diet, aging, and low BMI. As most studies have calculated the TyG index which based on the parameters that were measured during fasting in healthy individuals [4], it may be affected by hyperlipidemia or DM, which makes it challenging to exclude these confounders [21]. These observations support the idea that the application of this index is subject to variations among different populations. In the current study, in healthy individuals, the TyG index exhibited an optimal value in accordance with its original definition.
IR and renal insufficiency are tightly interwoven and potentially interact with each other via diverse mechanisms [14]. Moreover, both high TyG index and renal insufficiency have been reported to be risk factors for CVD and mortality [22, 23]. Several studies from China have observed that an elevated risk of CKD was associated with a high TyG index [24, 25], a claim supported by a recent meta-analysis [26]. Thus, the confluence of these risk factors should exacerbate the mortality risk. However, this hypothesis has not been tested previously, and the present investigation fills this research gap. This study examined the effect of renal function as a combined variable on the TyG index in two main ways. The findings revealed that the combination of a high TyG index and normal renal function incrementally increased all-cause mortality risk.
Furthermore, this survey found another intriguing result. The combination of a low TyG index and renal insufficiency exerted the greatest incremental effect on all-cause mortality risk, and a comprehensive evaluation indicated that renal insufficiency modifies the nonlinear relationship between the TyG index and death. The discovery significantly furthers the development of the TyG index in studies on adverse prognosis and may explain why its utility varies across studies. Moreover, the integration of the TyG index with renal function could augment its clinical utility considerably. Clinicians usually do not stratify patients when attempting to determine the prognosis and merely rely on findings from simple population-wide studies. This approach might lead to the misclassification of high-risk groups owing to the differential effects of the TyG index in individuals with renal insufficiency. Previous research had examined the combined effect of the TyG index with systolic blood pressure and BMI on all-cause mortality [13, 27]. Nevertheless, studies on the role and its optimal targets of TyG in different subpopulations are currently limited. Therefore, future research should delve into the application of IR across various subpopulations.
IR is often subclinical and is characterized by reduced insulin receptor levels in key metabolic tissues such as the liver, muscle, and adipose. This reduction leads to compromised insulin signaling, impairing glucose uptake at the cellular level. Emerging evidence indicates that insulin receptors are not only prevalent in the liver, muscle, and adipose tissues but are also notably present in the kidney [28, 29]. These receptors in the kidney play a crucial role in overseeing glucose homeostasis through various mechanisms [30,31,32]. Such discoveries highlight the kidney’s significant contribution to systemic glucose regulation. Moreover, the kidney is vital in modulating lipid metabolism in vivo [2], and a marked reduction in insulin receptor expression was noted in the cortex of the kidney of high-fat diet-fed rats and in patients with diabetes mellitus type 2 [33,34,35,36]. Consequently, individuals with renal insufficiency are susceptible to both glucose and lipid metabolic disorders, pushing the body towards insulin resistance. This shift not only influences the TyG index, derived from blood glucose and lipid levels but also heightens the risk of all-causes mortality. The exact impact of varying renal function on the TyG index’s ability to predict mortality risk remains unclear. It’s hypothesized that renal insufficiency might cause toxin buildup, inciting inflammation and oxidative stress [37], which in turn increases IR and affects the TyG index. Additionally, renal issues might alter lipid metabolism [2], further impacting the TyG index. Given the TyG index’s strong association with chronic disease risks, these alterations might jeopardize its predictive accuracy for mortality risk.
Study strengths and limitations
The TyG index was considered to be a surrogate for IR in the current study to determine the impact of renal function on the ability of the index to predict all-cause mortality. The NHANES has laid down stringent regulations for assessing fasting lipid and glucose levels, which precludes the exclusion of confounding factors, such as hyperlipidemia and DM. Finally, to minimize the possibility of errors, the data were adjusted for actual and possible confounders.
There are certain limitations which are worth recognizing. Firstly, the current study collected the single relative index at the beginning, and this snapshot may not entirely reflect the long-term trajectory of the participants’ renal function and IR status. In addition, retrospective cohort design was used, and although multivariate risk regression modeling was performed to account for potential confounding factors, their influence on the results could not be completely ruled out. Moreover, samples with missing data were excluded, which might have introduced selection bias. Finally, despite using a nationally representative U.S. sample, the results may not be generalizable to other regions or individuals with specific diseases.
Conclusion
It is well-known that higher TyG index is associated with higher all-cause mortality rates in community-dwelling adults. Taking into consideration that renal function could modulate the effect of the TyG index on all-cause mortality in this research, further work should involve investigation of the associations of renal function trajectories with the long-term cardiovascular outcomes.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CKD:
-
Chronic kidney disease
- CVD:
-
Cardiovascular diseases
- DM:
-
Diabetes mellitus
- FPG:
-
Fasting plasma glucose
- HDL-C:
-
High-density lipoprotein cholesterol
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- TG:
-
Triglycerides
- TyG:
-
Triglyceride-glucose
References
DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 Diabetes. Diabetes Care. 2009;32(Suppl 2):157–63.
Vaziri ND. Dyslipidemia of chronic Renal Failure: the nature, mechanisms, and potential consequences. Am J Physiol Ren Physiol. 2006;290(2):F262–272.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Wang A, Tian X, Zuo Y, Chen S, Meng X, Wu S, Wang Y. Change in triglyceride-glucose index predicts the risk of Cardiovascular Disease in the general population: a prospective cohort study. Cardiovasc Diabetol. 2021;20(1):113.
Wang A, Wang G, Liu Q, Zuo Y, Chen S, Tao B, Tian X, Wang P, Meng X, Wu S, et al. Triglyceride-glucose index and the risk of Stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. 2021;20(1):46.
Liu X, Abudukeremu A, Jiang Y, Cao Z, Wu M, Ma J, Sun R, He W, Chen Z, Chen Y, et al. U-shaped association between the triglyceride-glucose index and atrial fibrillation incidence in a general population without known Cardiovascular Disease. Cardiovasc Diabetol. 2023;22(1):118.
da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, Bressan J. Triglyceride-glucose index is associated with symptomatic coronary artery Disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89.
Hou Z, Pan Y, Yang Y, Yang X, Xiang X, Wang Y, Li Z, Zhao X, Li H, Meng X, et al. An analysis of the potential relationship of triglyceride glucose and body Mass Index with Stroke Prognosis. Front Neurol. 2021;12:630140.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, Ma J, Zhao Y, Zhu W, Wang J. Relationship between the triglyceride-glucose index and risk of Cardiovascular Diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124.
Yan F, Yan S, Wang J, Cui Y, Chen F, Fang F, Cui W. Association between triglyceride glucose index and risk of Cerebrovascular Disease: systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):226.
Yang J, Tang YD, Zheng Y, Li C, Zhou Q, Gao J, Meng X, Zhang K, Wang W, Shao C. The impact of the triglyceride-glucose index on poor prognosis in NonDiabetic patients undergoing percutaneous coronary intervention. Front Endocrinol. 2021;12:710240.
Chen W, Ding S, Tu J, Xiao G, Chen K, Zhang Y, Huang R, Liao Y. Association between the insulin resistance marker TyG index and subsequent adverse long-term cardiovascular events in young and middle-aged US adults based on obesity status. Lipids Health Dis. 2023;22(1):65.
Singh S, Sharma R, Kumari M, Tiwari S. Insulin receptors in the kidneys in health and Disease. World J Nephrol. 2019;8(1):11–22.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Berge RK, Tronstad KJ, Berge K, Rost TH, Wergedahl H, Gudbrandsen OA, Skorve J. The metabolic syndrome and the hepatic fatty acid drainage hypothesis. Biochimie. 2005;87(1):15–20.
Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta. 2006;368(1–2):1–19.
Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A. 2004;101(28):10422–7.
Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14(3):137–45.
Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear Associated with all-cause and Cardiovascular Mortality in the General Population. Front Cardiovasc Med. 2020;7:628109.
Fujita F, Diener E, Sandvik E. Gender differences in negative affect and well-being: the case for emotional intensity. J Pers Soc Psychol. 1991;61(3):427–34.
Provenzano M, Coppolino G, Faga T, Garofalo C, Serra R, Andreucci M. Epidemiology of cardiovascular risk in chronic Kidney Disease patients: the real silent killer. Rev Cardiovasc Med. 2019;20(4):209–20.
Freeman AM, Pennings N. Insulin Resistance. In: StatPearls edn. Treasure Island (FL) ineligible companies. Disclosure: Nicholas Pennings declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023.
Shi Y, Hu L, Li M, Zhou W, Wang T, Zhu L, Bao H, Cheng X, Li P. Association between the Surrogate Markers of Insulin Resistance and chronic Kidney Disease in Chinese hypertensive patients. Front Med (Lausanne). 2022;9:831648.
Xu X, Tang X, Che H, Guan C, Zhao N, Fu S, Liu L, Ye Y, Guo M, Min H, et al. [Triglyceride-glucose product is an Independent risk factor for predicting chronic Kidney Disease in middle-aged and elderly population: a prospective cohort study]. Nan Fang Yi Ke Da Xue Xue Bao. 2021;41(11):1600–8.
Ren X, Jiang M, Han L, Zheng X. Association between triglyceride-glucose index and chronic Kidney Disease: a cohort study and meta-analysis. Nutr Metab Cardiovasc Dis. 2023;33(6):1121–8.
Yu Y, Gu M, Huang H, Cheng S, Deng Y, Cai C, Chen X, Niu H, Ning X, Hua W. Combined association of triglyceride-glucose index and systolic blood pressure with all-cause and cardiovascular mortality among the general population. J Transl Med. 2022;20(1):478.
Sechi LA, De Carli S, Bartoli E. In situ characterization of renal insulin receptors in the rat. J Recept Res. 1994;14(6–8):347–56.
Nakamura R, Emmanouel DS, Katz AI. Insulin binding sites in various segments of the rabbit nephron. J Clin Invest. 1983;72(1):388–92.
Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, Ecelbarger CM. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci USA. 2008;105(17):6469–74.
Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J. Abnormal renal and hepatic glucose metabolism in type 2 Diabetes Mellitus. J Clin Investig. 1998;102(3):619–24.
Cersosimo E, Judd RL, Miles JM. Insulin regulation of renal glucose metabolism in conscious dogs. J Clin Investig. 1994;93(6):2584–9.
Gatica R, Bertinat R, Silva P, Carpio D, Ramírez MJ, Slebe JC, San Martín R, Nualart F, Campistol JM, Caelles C, et al. Altered expression and localization of insulin receptor in proximal tubule cells from human and rat diabetic kidney. J Cell Biochem. 2013;114(3):639–49.
Tiwari S, Halagappa VK, Riazi S, Hu X, Ecelbarger CA. Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J Am Soc Nephrology: JASN. 2007;18(10):2661–71.
Eid A, Bodin S, Ferrier B, Delage H, Boghossian M, Martin M, Baverel G, Conjard A. Intrinsic gluconeogenesis is enhanced in renal proximal tubules of Zucker diabetic fatty rats. J Am Soc Nephrology: JASN. 2006;17(2):398–405.
Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic Nephropathy promotes cell death. Kidney Int. 2008;73(12):1385–93.
Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, Rahman M, Lash JP, Townsend RR, Ojo A, et al. Inflammation and progression of CKD: the CRIC Study. Clin J Am Soc Nephrology: CJASN. 2016;11(9):1546–56.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HQL designed the study. HLL, WHC, and SHH analyzed the data. XQL, WQC, and TZX interpreted the results. HLL, WHC, and XQL prepared the first draft of the manuscript. KHC, HQL, and SHH revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The NCHS Research Ethics Review Board (ERB) approved the NHANES study protocol and participants provided written informed consent at the time of their enrollment. The NCHS IRB/ERB protocols for the 1999–2018 National Health and Nutrition Surveys are numbered “#2021-05”, “#2018-01”, “#2011-17”, “#2005-06”, and “#98 − 12”.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Sup Table 1
. The HR (95% CI) of cardiovascular and non- cardiovascular mortality according to TyG and different renal function from the three models
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, H., Chen, W., Lin, X. et al. Influence of renal function on the ability of TyG Index to predict all-cause mortality. Lipids Health Dis 22, 193 (2023). https://doi.org/10.1186/s12944-023-01958-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01958-1