Abstract
Cinnamomum zeylanicum and Ocimum basilicum are two plants used by many cultures for food and medicinal purposes. The phenolic composition of ethanol extracts of both plants were determined by HPLC–DAD. A total of seventeen compounds were identified in C. zeylanicum with trans cinnamic acid (179.90 ± 0.45 µg/g) and coumarin (84.50 ± 0.41 µg/g) as major constituents while ten compounds were detected in O. basilicum with rosmarinic acid (360.40 ± 1.28 µg/g) and vanillic acid (36.30 ± 0.25 µg/g) as major constituents. C. zeylanicum exhibited higher inhibition on AChE (54.30 ± 0.97%) and BChE (66.43 ± 0.84%) than O. basilicum with lower AChE (23.43 ± 0.51%) and BChE (33.83 ± 0.75%) inhibitions. Both extracts showed moderate inhibition of tyrosinase and urease enzymes. The quorum sensing (QS) inhibition of O. basilicum and C. zeylanicum was evaluated by two assays: violacein inhibition on Chromobacterium violaceum CV12472 and QS inhibition on Chromobacterium violaceum CV026. Excellent inhibition of violacein synthesis in CV12472 was exhibited by C. zeylanicum with 100% inhibtion at MIC to MIC/4 and further inhibitions of 48.0 ± 2.0% (MIC.8) and 27.9 ± 1.2% (MIC/16). QS inhibition diameter zones on C. violaceum CV026 at MIC were 13.0 ± 1.0 mm and 10.5 ± 1.0 mm for C. zeylanicum and O. basilicum respectively. Since both extracts could inhibit violacein synthesis in CV12472 and QS in CV026, they could block signal production and signal reception in QS mediated processes in bacteria. These results indicate that both plants can be used to remedy microbial resistance and Alzheimer’s diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microbial resistance to antibiotics is a growing problem faced by medicine and food industry, since bacteria can mutate under adverse conditions and increase drug-resistance. A novel strategy to eliminate virulence factors and development of microbial resistance is to disrupt quorum-sensing network, a process of cell-to-cell communication used by many bacterial species to monitor their milieu [1]. The traditional antimicrobial agents that usually kill or inhibit bacteria are gradually getting out of use and researchers are turning towards medicinal plants for suitable alternatives [1,2,3]. Alzheimer’s disease (AD) is a neurodegenerative disorder resulting from reduction in acetylcholine, and the inhibition of the two types of cholinesterase enzymes (AChE and BChE) is remedy but recently, there is a growing interest in cholinesterase inhibitors from natural sources due to the draw backs of synthetic cholinesterase inhibitors such as gastro intestinal disorders, moderate to low effectiveness, high cost and short half-life [4,5,6]. In both cases of bacterial resistance and Alzheimer’s disease, medicinal plants proposes an alternative solution. Indigenous plants from different parts of the world are used as foods as well as medicines to remedy various ailments and investigating their chemical composition and bioactivities has become an interesting field of research, revealing important information with possible applications in food and medicine [7]. The nutritional habits of individuals show important linkages with their health and many global health problems arise from food related causes such as poor nutrition and food contamination by microorganisms and for this reason, an appropriate and health-promoting diet with scientific evidence is very important in maintaining the body in good health and free from illnesses [8, 9]. Cinnamomum zeylanicum Blume., and Ocimum basilicum Linn., are two plants which have been used as spices and food flavoring around the world and the usage is not only for the flavors, but also for their health benefits.
Cinnamon, the inner barks of C. zeylanicum a plant from Lauraceae family is widely used as food spice and therapeutic agent in various cultures and with a long history. Different parts of the cinnamon tree C. zeylanicum and including flowers, fruits, bark, leaves and roots have culinary uses as well as medicinal properties and are used as remedy for gynaecological, urinary tract problems, respiratory troubles, diabetes, acne and digestive illnesses [10, 11]. Different parts of cinnamomum have been shown to possess a wide range of phytochemical compounds amongst which are flavonoids, terpinenes, butanolides, saponins, steroids, lignans, coumarins, phenols, anthraquinones, alkaloids, tannins, procyanidins, catechins, hydrocarbons, fatty acids, carboxylic acid, phenylpropenoids and kaempferol glycosides as well as many volatile constituents [12,13,14]. C. zeylanicum has showed many biological applications.
O. basilicum Linn., also known as sweet Basil, is a food and medicinal plant belonging to the family of Lamiaceae. O. basilicum has been reported numerously in areas related to agriculture, food, cosmetic and pharmacology. O. basilicum is a medicinal plant traditionally used for the treatment of respiratory, headaches, diarrhea, warts, coughs, worms, constipation, kidney malfunction and some reports show that it can treat digestive disorders, cardiovascular disorders, diabetes, neuro-degenerated disorders menstrual cramps and cancer [15, 16]. The chemical composition of essential oils of Ocimum spp. has been well studied. Basil essential oils contained flavonoids, tannins, sterols, phenols, terpenoids, glycosides, alkaloids, carbohydrates, phlobatannins, phenyl propanoid derivatives and monoterpenes derivatives including camphor, limonene, 1, 8-cineole, linalool, geraniol, eugenol, methyleugenol, chavicol, estragole, methyl-cinnamate [16,17,18,19] while the ethanol extracts of O. basilicum is rich in phenolic compounds and has good antioxidant capacities [20].
There is increasing interest in phenolic compounds in foods such as C. zeylanicum and O. basilicum due to their nutritive values and bioactivities such as antimicrobial and anticholinesterase activities. Polar and non-toxic solvents such as ethanol are suitable for extraction of phenolic compounds contained in food plants. In this work ethanol extracts were obtained from C. zeylanicum and O. basilicum and the phenolic composition was determined and quantified using HPLC–DAD. The inhibition of quorum-sensing in bacteria and enzyme (Acetylcholinesterase, Butyrylcholinesterase, Tyrosinase, Urease) inhibitory potentials of the extracts were evaluated.
2 Materials and Methods
2.1 Plant material and Extraction
The plant materials were procured in ready-for-consumption form from grocery in the Mugla market, Mugla Turkey. Cinnamomum zeylanicum (50 g) and Ocimum basilicum (50 g) were extracted with ethanol at room temperature (24 h × 3), filtered, and evaporated in a vacuum to dryness. Both extracts were stored at + 4 °C until analysis of phenolic composition and bioactivity studies were performed.
2.2 Determination of Phenolic Composition
The HPLC–DAD method was used to analyze the phenolic compounds. For HPLC–DAD, the obtained extracts were dissolved in water:methanol (80:20) and filtered through a 0.20 μm disposable LC filter disk and separation was on an Intertsil ODS-3 reverse phase C18 column [21, 22]. The solvent flow rate was 1.0 mL/min and injection volume of sample was 20 μL. Mobile phase A was consisting of 0.5% acetic acid in water and mobile phase B was 0.5% acetic acid in methanol. The elution gradient was as follows: 0–10% B (0–0.01 min); 10–20% B (0.01–5 min); 20–30% B (5–15 min); 30–50% B (15–25 min); 50–65% B (25–30 min); 65–75% B (30–40 min); 75–90% B (40–50 min) 90–10% B (50–55 min). The detection was carried out using a photodiode array detector (PDA) with a chosen wavelength of 280 nm. The phenolic compounds were characterized by comparing UV data and retention times with commercial standards. The analysis was repeated three times. To identify and quantify of the phenolic compounds, the calibration curve was established via the injection of known concentrations (0.0, 0.00782, 0.01563, 0.03125, 0.0625, 0.125, 0.25, 0.5 and 1.0 ppm) of standard compounds. Totally 26 phenolic compounds were used namely, gallic protocatechuic, chlorogenic, p-hydroxy benzoic, vanillic, 3-hydroxy benzoic, syringic, p-coumaric, ferulic, ellagic, rosmarinic, trans-cinnamic acids, catechin, pyrocatechol, 6,7-dihydroxy coumarin, vanillin, taxifolin, coumarin, rutin, myricetin, quercetin, luteolin, hesperetin, kaempferol, apigenin and chrysin. The results were given in μg per g of dry weight.
2.3 Anticholinesterase Activity
Anticholinesterase activity was measured spectrophotometrically by determining acetylcholinesterase and butyrylcholinesterase enzyme inhibition method defined by Ellman with minor modifications [9, 23]. Shortly, 130 μL of 100 mM sodium phosphate buffer (pH 8.0), 10 μL of sample solution dissolved in ethanol at various concentrations, and 20 μL of enzyme (AChE or BChE) solution in buffer were mixed and incubated for 15 min at 25 °C, followed by 20 μL of 0.5 mM DTNB (5,5′-Dithio-bis(2-nitrobenzoic) acid) was added. The reaction was then initiated by addition of 0.71 mM, 20 μL of acetylthiocholine iodide, or 0.2 mM, 20 μL of butyrylthiocholine chloride. The formation of yellow 5-thio-2-nitrobenzoate anion as a result of the reaction of DTNB with thiocholine, released by the enzymatic hydrolysis of acetylthiocholine iodide or butyrylthiocholine chloride, respectively, was monitored spectrophotometrically using a 96-well microplate reader at a wavelength of 412 nm. The results were expressed as a percentage inhibition (%) of the enzyme 200 μg/mL concentration of the extracts.
2.4 Anti-urease Activity
Urease enzyme inhibition activity was analyzed by measuring ammonia production using the indophenol method [24]. Briefly, 100 mM sodium phosphate buffer (pH 8.2), 25 μL of urease enzyme (Jack bean source) solution and 50 μL of urea (100 mM) were combined and incubated at 30 °C for 15 min after adding of extracts (10 μL). Then, 45 μL of phenol reagent and 70 μL of alkali reagent was added to each well. After 50 min of incubation, absorbance was measured at 630 nm using a microplate reader. The reference compound was thiourea. The findings were expressed as a 50% inhibition concentration (IC50).
2.5 Anti-tyrosinase Activity
Tyrosinase enzyme inhibitory activity was determined by the spectrophotometric method described previously [25]. The enzyme used was mushroom tyrosinase, and the reaction's substrate was L-DOPA. 150 μL of 100 mM sodium phosphate buffer (pH 6.8), 10 μL of sample solution dissolved in ethanol at different concentrations, and 20 μL tyrosinase enzyme solution in buffer were mixed and incubated for 10 min at 37 °C, and 20 μL L-DOPA was added. After 10 min incubation at 37 °C in a 96-well microplate, the absorbances were measured at 475 nm. The findings were expressed as a percentage of enzyme inhibition (%) at a concentration of 200 μg/mL extracts.
2.6 Microbial Strains and Determination of Minimum Inhibitory Concentration (MIC)
The microorganisms used in this study are Chromobacterium violaceum CV12472, Chromobacterium violaceum CV026 and Pseudomonas aeruginosa PA01. MICs were determined by microtitre broth dilution method, as recommended by the Clinical and Laboratory Standards Institute [26]. The MIC was defined as the lowest plant extract concentration that yielded no visible growth. The test medium was Mueller–Hinton Broth (MHB) and the density of bacteria was 5 × 105 colony-forming units (CFU)/mL. Cell suspensions (100 μL) were inoculated into wells (96-well microtitre plates) in the presence of extracts with different final concentrations (2, 1, 0.5, 0.25, 0.125, 0.0625, 0.03125 mg/mL). The inoculated microplates were incubated at 37 °C for 24 h before being read.
2.7 Violacein Inhibition Assay Using C. violaceum CV12472
All extracts were subjected to qualitative analysis to find their QSI (Quorum-sensing inhibition) potentials against C. violaceum ATCC 12,472 [1]. Overnight culture (10 µL) of C. violaceum (adjusted to 0.4 OD at 600 nm) was added into sterile microtiter plates, containing 200 µL of LB broth and incubated in the presence and absence of sub-MIC concentrations of extracts and broth containing C. violaceum ATCC 12,472 was used as a positive control. These plates were incubated at 30 °C for 24 h and observed for the reduction in violacein pigment production. The absorbance was read at 585 nm. Each experiment was done in triplicate and the percentage of violacein inhibition was calculated via the following the formula:
2.8 Bioassay for Quorum-Sensing Inhibition (QSI) Activity Using C. violaceum CV026
The quorum sensing inhibition was evaluated as described elsewhere [27]. 5 mL of warm molten Soft Top Agar (1.3 g Agar agar, 2.0 g Tryptone, 1.0 g sodium chloride, 200 mL deionized water) was seeded with 100 µL of overnight CV026 culture and 20 µL of 100 µg/mL C6HSL was added as exogenous AHL (Acyl Homoserine Lactone) source. This preparation was softly mixed and poured immediately over the surface of a solidified Luria Bertani Agar (LBA) plate as an overlay. Wells of 5 mm in diameter were made on each plate after solidification of the overlay. Each well was filled with 50 µL of sub-MIC concentration filter-sterilized extracts. A white or cream-colored halo around this well against a purple lawn of activated CV026 bacteria indicated quorum-sensing inhibition (QSI). The activity detection limit was determined by applying serial dilutions of the EO (1:1 to 1:8, using LB broth as diluent) and the endpoints were estimated as the lowest dilution of extracts, leading to discernible inhibition of violacein synthesis. Each experiment was repeated three times. The assay plates were incubated at 30 °C for three days, and then the diameters of the quorum sensing inhibition zones were measured.
2.9 Swarming Motility Inhibition on P. aeruginosa PA01
The swarming motility inhibition assay was done as described previously [28]. Briefly, overnight cultures of P. aeruginosa PAO1 strain were point inoculated at the center of swarming plates consisting of 1% peptone, 0.5% NaCl, 0.5% agar and 0.5% of filter-sterilized d-glucose with various concentrations of extracts (50, 75, and 100 µg/mL). The plate without the extract was maintained as control and the plates were incubated at an appropriate temperature in an upright position for 18 h. The swarming migration was recorded by following swarm fronts of the bacterial cells.
3 Results
3.1 Phenolic Composition
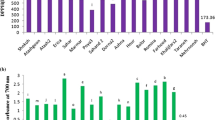
Phenolic compounds of extracts from C. zeylanicum and O. basilicum were determined as µg/g extract using by HPLC‐DAD and presented on Table 1. Totally, 26 pure standard phenolic compounds were used against which the ethanol extracts of both plants were analyzed and the chromatograms of detection are presented in Fig. 1. trans Cinnamic acid (179.90 ± 0.45 µg/g) and coumarin (84.50 ± 0.41 µg/g) were found to be major phenolic compounds of C. zeylanicum, while rosmarinic acid (360.40 ± 1.28 µg/g) and vanillic acid (36.30 ± 0.25 µg/g) were found in the extract of O. basilicum. Also, rutin (27.18 ± 0.53 µg/g), rosmarinic acid (15.65 ± 0.30 µg/g) and protocatechuic acid (11.65 ± 0.33 µg/g) were found as other predominant phenolic compounds of C. zeylanicum. Besides that, vanillic acid (36.30 ± 0.25 µg/g), pyrocatechol (16.48 ± 0.27 µg/g), chlorogenic acid (12.09 ± 0.18 µg/g) and rutin (12.08 ± 0.36 µg/g) have been identified as other major components of O. basilicum.
3.2 Anticholinesterase Activity
Alzheimer’s disease (AD) is a progressive neurological disorder, the most common type of dementia, characterized by memory loss and affects cognitive abilities. Inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) is the most common therapeutic strategy for treating AD [29, 30]. Ethanol extract of C. zeylanicum exhibited the strongest activity with 54.30 ± 0.97% and 66.43 ± 0.84% inhibitions against AChE and BChE, respectively at 200 µg/mL concentration. On the other hand, ethanol extract of O. basilicum showed moderate inhibition effect against AChE (23.43 ± 0.51%) and BChE (33.83 ± 0.75%).
3.3 Anti-urease Activity
Urease is nickel containing enzyme and involved in the hydrolysis of urea into carbon dioxide and ammonia. Some pathological disorders, such as gastric and peptic ulcers, are thought to be caused by urease enzyme [31]. New, safe and effective urease inhibitor agents from natural sources are needed to prevent that kind of serious health problems. Urease inhibitory activities of extracts are given in Table 2. Both of extracts, showed significant urease inhibitory activities with IC50 values of 19.21 ± 0.39 µg/mL (O. basilicum) and 25.80 ± 1.25 µg/mL (C. zeylanicum) when the compared with thiourea (IC50: 10.42 ± 0.75 µg/mL).
3.4 Anti-tyrosinase Activity
Tyrosinase is a copper-containing enzyme that is crucial for melanin pigment biosynthesis. Tyrosinase inhibitors can be used to treat skin disorders such as, hyperpigmentation and melanoma [32]. Extracts of C. zeylanicum and O. basilicum demonstrated 45.37 ± 0.93% and 37.51 ± 0.78% inhibitions at 200 µg/mL concentration respectively. Kojic acid which used as reference compound exhibited 81.51 ± 0.36% inhibition at the same concentration as shown on Table 2.
3.5 Violacein Inhibition on C. violaceum CV12472 and Quorum-Sensing Inhibition on C. violaceum CV026
O. basilicum and C. zeylanicum are food spices and flavoring agents in various cuisines of the world and they have been proven to posses medicinal properties notably, antibacterial activity. However, their effects on quorum-sensing (QS) effects on bacteria have not been elucidated. It should be noted that antibacterial agents involve in inhibition of bacterial growth without disrupting QS networks in bacteria are gradually falling out of use as they may encounter resistance from bacteria. The anti-QS activity of O. basilicum and C. zeylanicum was evaluated by two assays: violacein inhibition on C. violaceum CV12472 and QS inhibition on C. violaceum CV026.
Prior to the evaluation of violacein inhibition and anti-QS assay, the MIC values of both plant extracts were evaluated on CV12472 and CV026 and reported on Tables 3 and 4 respectively. On C. violaceum CV12472, C. zeylanicum was more active (MIC = 0.5 mg/mL) than O. basilicum (MIC = 2 mg/mL) meanwhile on C. violaceum CV026, O. basilicum had higher activity (MIC = 0.25 mg/mL) than C. zeylanicum (MIC = 1.25 mg/mL). Inhibition of violacein production and QS was then evaluated at MIC and sub-MIC as shown on Tables 3 and 4, so as to eliminate the hypothesis that these activities could be due to the death of bacteria. The violacein inhibition of O. basilicum was low as it showed only inhibition of 18.5 ± 0.9% at MIC and 11.2 ± 1.0% at MIC/2. Excellent inhibition of violacein synthesis in CV12472 was exhibited by C. zeylanicum with 100% inhibtion at MIC to MIC/4 and further inhibitions of 48.0 ± 2.0% (MIC.8) and 27.9 ± 1.2% (MIC/16). QS inhibition diameter zones on C. violaceum CV026 were 13.0 ± 1.0 mm at MIC and 9.5 ± 0.7 mm at MIC/2 for C. zeylanicum while O. basilicum showed QS inhibition on CV026 of 10.5 ± 1.0 mm only at MIC and could not inhibit QS at lower concentrations.
3.6 Swarming Motility Inhibition on P. aeruginosa PA01
Inhibition of microbial swarming motility was assayed using the flagellated P. aeruginosa PA01 at concentrations of 100–50 µg/mL and no inhibition was observed at the lowest concentration of 50 µg/mL as presented on Table 5. However, at 100 µg/mL, O. basilicum and C. zeylanicum showed swarming inhibition of 24.43 ± 1.09% and 13.62 ± 0.55%, respectively while at 75 µg/mL, swarming inhibitions were 13.01 ± 0.90% for O. basilicum against 03.48 ± 1.05% for C. zeylanicum. O. basilicum therefore showed higher swarming inhibition than C. zeylanicum as shown on Table 5.
4 Discussion
In the literature, many publications focused on isolation or pharmacological activities of cinnamic acid, due to its occurence in many edible and medicinal plants. These studies indicated that cinnamic acid has many biological activities such as, anti-diabetic, anti-inflammatory, anti-cancer, neuroprotective, antimicrobial, antioxidant [33, 34]. Coumarin is mostly found in plants. It has been reported that coumarin exhibited antibacterial, anticoagulant, anticancer, antioxidant, anti-inflammatory, antifungal, antiviral, antihypertensive, antitubercular, anticonvulsant, antihyperglycemic, and neuroprotective activities [35]. Rosmarinic acid, which is the major phenolic compound of O. basilicum, is especially common in Lamiaceae species. Rosmarinic acid has a wide range of biological activities such as, antioxidant, antibacterial, hepatoprotective, antimutagenic, anticholinesterase, antitumor, and antiviral [22, 36]. Till now, the chemical components of C. zeylanicum and O. basilicum have been reported, and our findings are consistent with previous research [20, 37,38,39,40].
Previously, anticholinesterase activities of C. zeylanicum from India reported and found that 40.83% and 51.53% inhibitions against AChE and BChE respectively, at 100 µg/mL concentration [41]. In another study, Arachchige (2017) reported 28.68% and 75.10% inhibitions of C. zeylanicum from Sri Lanka against AChE and BChE respectively [42]. Danış et al. (2014) demonstrated that methanol extract of O. basilicum exhibited AChE enzyme inhibitory activity with IC50 value of 5.76 mg/mL [43]. The methanol extract of O. basilicum showed with IC50 values of 4.84 ± 0.47 mg/mL (AChE) and 5.90 ± 0.81 mg/mL (BChE) [44]. The obtained results as shown on Table 2, support the previous studies about anticholinesterase activity of C. zeylanicum and O. basilicum. Previously, tyrosinase inhibitory activity of ethanol:water (1:1) extract of C. zeylanicum have been reported and found that 17% inhibition at 50 µg/mL [45]. Although, tyrosinase enzyme inhibition studies of O. basilicum mostly focused on its essential oil, some research is available concerning about extracts in literature. Lin et al. (2011) reported that aqueous aromatic extract of O. basilicum showed 39.2% tyrosinase inhibition [46].
Violacein pigment acts as an antioxidant protecting the bacterial membrane against oxidative stress through a QS-mediated process and C. violaceum CV12472 produces a violet coloration (violacein) which can be quantified easily as a QS mediated trait for quorum sensing activity of this bacterium [1, 47]. This implies that, the capacity of the plant extracts to inhibit the production of violacein corresponds to anti-QS activity reflected by inhibition of production of signal molecule. It should be noted that, CV12472 produces violacein while growing while the mutant strain CV026 cannot produce violacein except in the presence of an externally supplied acylhomoserine lactone hormone. In the QS assay in involving CV026, test plates were used and the appearance of a white or cream-colored ring around the well against a purple lawn of activated CV026 bacteria was an indication of QS inhibition (see Fig. 2) and the diameters of the cream colored rings is a measure of the QS inhibition [48]. In this assay, anti-QS activity reflects there the inhibition of signal molecule reception. In both assays, working at sub-MIC concentrations eliminates the hypothesis of bactericidal effect of extracts that occurs at high concentrations thereby giving way for proper understanding and confirmation of anti-QS potential of the tested plant extracts and this effect is desirable because it can avoid development of microbial resistance.
Bacteria use swarming movements to colonize surfaces and this step occurs prior to establishment of biofilms on surfaces, which is one of the major causes of microbial resistance [9, 28, 49]. Inhibition of swarming motility therefore could prevent surfaces and equipments from being colonized by pathogenic microbes and subsequently establishing sessile biofilms.
5 Conclusion
Various plants such as C. zeylanicum and O. basilicum are widely used by many cultures around the world since time immemorial as spices as well as medicines for some ailments and equally having added economic value. For these reasons, man has learned to cultivate them and also they have been subject of various forms of scientific research principally within the fields of food, agriculture, biology and chemistry. In this study, the ethanol extracts of both plants were shown to be rich in important bioactive phenolic compounds. These extracts inhibited cholinesterase enzymes thereby showing their potential use to remedy Alzheimer’s diseases. Equally, good anti-quorum sensing activity was detected by these plants and they could be used to overcome microbial resistance since pathogenic bacteria use QS mediated traits to develop resistance and prone severity of infections. Since these plants are food plants and are consumed by humans, it is advantageous that it guarantees their safety and will serve a double purpose as food and medicine.
Data Availability
All data obtained and analyzed during this research are provided and included within the manuscript data and the authors will make available any other related information supporting the findings upon reasonable request.
References
Tamfu AN, Ceylan O, Fru GC, Ozturk M, Duru ME, Shaheen F (2020) Antibiofilm, antiquorum sensing and antioxidant activity of secondary metabolites from seeds of Annona senegalensis. Persoon Microbial Pathogen 144:104191. https://doi.org/10.1016/j.micpath.2020.104191
Boudiba S, Tamfu AN, Berka B, Hanini K, Hioun S, Allaf K, Boudiba L, Ceylan O (2021) Anti-quorum sensing and antioxidant activity of essential oils extracted from juniperus species, growing spontaneously in Tebessa Region (East of Algeria). Nat Prod Commun 16(6):1–11. https://doi.org/10.1177/1934578X211024039
Ngenge AT, Ceylan O, Fru GC, Arab Y, Emin DM, Ozturk M (2021) Antimicrobial, antibiofilm, anti-quorum sensing and motility inhibition activities of essential oil from seeds of food spice Xylopia aethiopica (Dunal) A. Rich. on some pathogenic bacteria. Res J Biotechnol. 16(6):68–76
Owokotomo IA, Ekundayo O, Abayomi TG, Chukwuka AV (2015) In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol Rep 2:850–857. https://doi.org/10.1016/j.toxrep.2015.05.003
Tehrani MB, Rezaei Z, Asadi M, Behnammanesh H, Nadri H, Afsharirad F, Moradi A, Larijani B, Mohammadi-Khanaposhtani M, Mahdavi M (2019) Design, synthesis, and cholinesterase ınhibition assay of Coumarin-3-carboxamide-N-morpholine hybrids as new anti-Alzheimer agents. Chem Biodivers 16(7):e1900144. https://doi.org/10.1002/cbdv.201900144
Tamfu AN, Fotsing MT, Talla E, Ozturk M, Mbafor JT, Duru ME, Shaheen F (2019) Chemical composition and evaluation of anticholinesterase activity of essential oil from Cameroonian propolis. Issues Biol Sci Pharm Res 7(3):58–63. https://doi.org/10.15739/ibspr.19.007
Tamfu AN, Ceylan O, Kucukaydin S, Ozturk M, Duru ME, Dinica RM (2020) Antibiofilm and enzyme inhibitory potentials of two annonaceous food spices, African Pepper (Xylopia aethiopica) and African Nutmeg (Monodora myristica). Foods 9(12):1768. https://doi.org/10.3390/foods9121768
Jahangir MA, Shehzad A, Butt MS, Shahid M (2018) Influence of supercritical fluid extract of Cinnamomum zeylanicum bark on physical, bioactive and sensory properties of innovative cinnamaldehyde-enriched chocolates. Czech J Food Sci. 36:1–9. https://doi.org/10.17221/237/2016-CJFS
Tamfu AN, Ceylan O, Kucukaydin S, Duru ME (2020) HPLC-DAD phenolic profiles, antibiofilm, anti-quorum sensing and enzyme inhibitory potentials of Camellia sinensis (L.) O. Kuntze and Curcuma longa L. LWT Food Sci Technol 133:110150
Ranasinghe P, Pigera S, Premakumara GA, Galappaththy P, Constantine GR, Katulanda P (2013) Medicinal properties of “true” cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Comp Altern Med 13:275. https://doi.org/10.1186/1472-6882-13-275
Husain I, Ahmad R, Chandra A, Raza ST, Shukla Y, Mahdi F (2018) Phytochemical characterization and biological activity evaluation of ethanolic extract of Cinnamomum zeylanicum. J Ethnopharmacol 219:110–116. https://doi.org/10.1016/j.jep.2018.02.001
Muchuweti M, Kativu E, Mupure CH, Chidewe C, Ndhlala AR, Benhura MAN (2007) Phenolic composition and antioxidant properties of some spices. Am J Food Technol 2:414–420. https://doi.org/10.3923/ajft.2007.414.420
Kumar S, Kumari R, Mishra S (2019) Pharmacological properties and their medicinal uses of Cinnamomum: a review. J Pharm Pharmacol 71:1735–1761. https://doi.org/10.1111/jphp.13173
Wang J, Su B, Jiang H, Cui N, Yu Z, Yang Y, Sun Y (2020) Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): a review. Fitoterapia 146:104675. https://doi.org/10.1016/j.fitote.2020.104675
Balakrishnan P, Ramalingam PS, Purushothaman S, Balu R, Jolius G, Kumaran S (2018) A comprehensive review on Ocimum basilicum. J Natl Remed 18(3):71–85. https://doi.org/10.18311/jnr/2018/21324
Joshi RK (2014) Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Ancient Sci Life 33(3):151–156. https://doi.org/10.4103/0257-7941.144618
Vina A, Murillo E (2003) Essential oil composition from twelve varieties of basil (Ocimum spp.) grown in Colombia. J Braz Chem Soc 14:744–749. https://doi.org/10.1590/S0103-50532003000500008
Tangpao T, Chung HH, Sommano SR (2018) Aromatic profiles of essential oils from five commonly used Thai basils. Foods 7(11):175. https://doi.org/10.3390/foods7110175
Gebrehiwot H, Bachetti RK, Dekebo A (2015) Chemical composition and antimicrobial activities of leaves of sweet basil (Ocimum basilicum L.) herb. Int J Basic Clin Pharmacol 4(5):869–875. https://doi.org/10.18203/2319-2003.ijbcp20150858
Gülçin I, Elmastaş M, Aboul-Enein HY (2007) Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother Res. 21(4):354–361. https://doi.org/10.1002/ptr.2069
Barros L, Dueñas M, Ferreira ICFR, Baptista P, Santos-Buelga C (2009) Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem Toxicol 47:1076–1079. https://doi.org/10.1016/j.fct.2009.01.039
Çayan F, Deveci E, Tel-Çayan G, Duru ME (2020) Identifcation and quantifcation of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J Food Measur Charact 14:1690–1698. https://doi.org/10.1007/s11694-020-00417-0
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974. https://doi.org/10.1021/ac60252a045
Masuda T, Yamashita D, Takeda Y, Yonemori S (2005) Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci Biotechnol Biochem 69:197–201. https://doi.org/10.1271/bbb.69.197
CLSI (Clinical Laboratory Standards Institute) (2006) Quality control minimal inhibitory concentration (MIC) limits for broth dilution and MIC interpretative breakpoints (M27–s2). Wayne, Pennsylvania
Ceylan O, Tamfu AN, Doğaç Yİ, Teke M (2020) Antibiofilm and anti-quorum sensing activities of polyethylene imine coated magnetite and nickel ferrite nanoparticles. 3 Biotech. 10(12):513. https://doi.org/10.1007/s13205-020-02509-6
Kocak G, Tamfu AN, Bütün V, Ceylan O (2021) Synthesis of quaternary piperazine methacrylate homopolymers and their antibiofilm and anti-quorum sensing effects on pathogenic bacteria. J Appl Polym Sci 138:e50466. https://doi.org/10.1002/app.50466
Georgiev MI, Alipieva K, Orhan IE (2012) Cholinesterases inhibitory and antioxidant activities of Harpagophytum procumbens from in vitro systems. Phytother Res 26:313–316. https://doi.org/10.1002/ptr.3555
Wang ZY, Liu JG, Li H, Yang HM (2016) Pharmacological effects of active components of chinese herbal medicine in the treatment of Alzheimer’s disease: a Review. Am J Chin Med 44:1525–1541. https://doi.org/10.1142/S0192415X16500853
Amtul Z, Atta-ur-Rahman BSP, Siddiqui R, Choudhary M (2012) Chemistry and mechanism of urease inhibition. Curr Med Chem 9:1323–1348. https://doi.org/10.2174/0929867023369853
Parvez S, Kang M, Chung H, Bae H (2007) Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phyther Res 21:805–816. https://doi.org/10.1002/ptr.2184
Ruwizhi N, Aderibigbe BA (2020) Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci 21(16):5712. https://doi.org/10.3390/ijms21165712
Zhang WX, Wang H, Cui HR, GuoWB ZF, Cai DS, Xu B, Jia XH, Huang XM, Yang YQ (2019) Design, synthesis and biological evaluation of cinnamic acid derivatives with synergetic neuroprotection and angiogenesis effect. Eur J Med Chem 183:1–16. https://doi.org/10.1016/j.ejmech.2019.111695
Venugopala KN, Rashmi V, Odhav B (2013) Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res Int. https://doi.org/10.1155/2013/963248
Amoah SK, Sandjo LP, Kratz JM, Biavatti MW (2016) Rosmarinic acid-pharmaceutical and clinical aspects. Planta Med 82(5):388–406. https://doi.org/10.1055/s-0035-1568274
Vallverdú-Queralt A, Regueiro J, Martínez-Huélamo M, Rinaldi AJF, Leal LN, Lamuela-Raventos RM (2014) A comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem 1(154):299–307. https://doi.org/10.1016/j.foodchem.2013.12.106
Omoba OS, Olagunju AI, Salawu SO, Boligon AA (2019) HPLC-DAD phenolic profiling and ın vitro antioxidant activities of three prominent Nigerian spices. Prev Nutr Food Sci 24(2):179–186. https://doi.org/10.3746/pnf.2019.24.2.179
Elansary HO, Szopa A, Kubica P, Ekiert H, El-Ansary DO, Al-Mana FA, Mahmoud EA (2020) Saudi Rosmarinus officinalis and Ocimum basilicum L. Polyphen Biol Act Process 8:446. https://doi.org/10.3390/pr8040446
Wang Y, Harrington PB, Chen P (2020) Metabolomic profiling and comparison of major cinnamon species using UHPLC-HRMS. Ann Bioanal Chem 412(27):7669–7681. https://doi.org/10.1007/s00216-020-02904-1
Laha S, Sarkar D (2014) Screening of inhibitory effects on acetylcholinesterase and butyrylcholinesterase enzymes by some ındian medicinal plant’s extracts. Indian Res J Genet Biotechnol 6(2):406–411
Arachchige SPG, Abeysekera WPKM, Ratnasooriya WD (2017) Antiamylase, anticholinesterases, antiglycation, and glycation reversing potential of bark and leaf of ceylon cinnamon (Cinnamomum zeylanicum Blume) In Vitro. Evidence Based Comp Altern Med. https://doi.org/10.1155/2017/5076029
Danış Ö, Yuce-Dursun B, Çimen T, Demir S, Salan Ü, Yalçın G, Ogan A (2014) Evaluation of antioxidant, radical-scavenging and acetylcholinesterase ınhibitory activities of various culinary herbs cultivated in southern Turkey. J Food Biochem 38:602–611. https://doi.org/10.1111/jfbc.12095
Amat-ur-Rasool H, Symes F, Tooth D, Schaffert LN, Elmorsy E, Ahmed M, Hasnain S, Carter WG (2020) Potential nutraceutical properties of leaves from several commonly cultivated plants. Biomolecules 10:1556. https://doi.org/10.3390/biom10111556
Lianza M, Mandrone M, Chiocchio I, Tomasi P, Marincich L, Poli F (2020) Screening of ninety herbal products of commercial interest as potential ingredients for phytocosmetics. J Enzyme Inhib Med Chem 35(1):1287–1291. https://doi.org/10.1080/14756366.2020.1774571
Lin CC, Yang CH, Wu PS, Kwan CC, Chen YC (2011) Antimicrobial, anti-tyrosinase and antioxidant activities of aqueous aromatic extracts from forty-eight selected herbs. J Med Plants Res 5(26):6203–6209. https://doi.org/10.5897/JMPR.9000182
Kothari V, Sharma S, Padia D (2017) Recent research advances on Chromobacterium violaceum. Asian Pac J Trop Med 10(8):744–752. https://doi.org/10.1016/j.apjtm.2017.07.022
Alfred TN, Ceylan O, Kucukaydin S, Olmez OT, Godloves CF, Sylvain SK, Yeskaliyeva B, Duru ME, Ozturk M (2020) HPLC-DAD and GC-MS characterization of Cameroonian honey Samples and evaluation of their antibiofilm, anti-quorum sensing and antioxidant activities. Bull Environ Pharmacol Life Sci 9(10):132–142
Popova M, Gerginova D, Trusheva B, Simova S, Tamfu AN, Ceylan O, Clark K, Bankova V (2021) A Preliminary study of chemical profiles of honey, cerumen, and propolis of the african stingless bee Meliponula ferruginea. Foods 10:997. https://doi.org/10.3390/foods10050997
Acknowledgements
The authors are grateful to Department of Chemistry and the Ula Ali Kocman Vocational School, Mugla Sitki Kocman University for material support.
Funding
The authors received no financial support for the research and publication of this article.
Author information
Authors and Affiliations
Contributions
ANT, SK and OC did the conceptualization, performed the literature search, and devised the methods, carried out the experiments as well as the formal analysis. ANT and SK wrote the original draft. OC, MED and NS did the editing, provided materials and resources. OC and MED did the supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
About this article
Cite this article
Tamfu, A.N., Kucukaydin, S., Ceylan, O. et al. Phenolic Composition, Enzyme Inhibitory and Anti-quorum Sensing Activities of Cinnamon (Cinnamomum zeylanicum Blume) and Basil (Ocimum basilicum Linn). Chemistry Africa 4, 759–767 (2021). https://doi.org/10.1007/s42250-021-00265-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-021-00265-5