Abstract
Objective
We aimed to investigate the factors associated with UTI in patients with T2D whether being treated with SGLT-2i or not.
Methods
Adult patients with T2D, whose urine culture results were available, were analyzed retrospectively. Urine culture was obtained from mid-flow urine. Antibacterial treatment was given to the patients with UTI, which was defined by positive urine cultures and/or clinical findings. We grouped the patients as follows: Group A, those treated with SGLT-2i; and Group B, those not treated with SGLT-2i.
Results
A total of 101 patients were included. Median age was 56 (45–67), 56.4% (n = 57) of the patients were female. Urine culture was positive in 54.9% (n = 28) and 16% (n = 8) of Group A (n = 51) and Group B (n = 50), respectively. Of those for whom urine culture was positive, Escherichia coli was isolated in 83.3% (n = 30), and both Escherichia coli and Klebsiella pneumoniae (K.pneumoniae) were isolated in 16.7% (n = 6). Klebsiella pneumoniae was isolated only from Group A. The need for and duration of hospitalization were higher in Group A (p < 0.001). UTI was detected in 60 patients. ROC analysis showed that a HbA1c of > 5.8% was associated with UTI with good accuracy (AUC: 0.835, p < 0.001). In multiple logistic regression analysis, SGLT-2i use and glucosuria were positive predictors for UTI (p = 0.004, Odds Ratio: 1984.013; and p = 0.028, and Odds Ratio: 12.480, respectively).
Conclusion
Besides the association of HbA1c and BMI with UTI, SGLT-2i use and glucosuria predicted UTI. Urine culture is important with respect to the choice of antibacterial treatment, especially in those patients under SGLT-2i treatment. The effect of SGLT-2i on the development of UTI is independent of baseline BMI score or HbA1c.
Similar content being viewed by others
Introduction
Sodium glucose co-transporter 2 inhibitors (SGLT-2i) have been frequently used in the management of type 2 diabetes (T2D) for a decade [1]. They exert their effect via inhibition of sodium glucose transporter 2 (SGLT-2) in the proximal tubule of the kidney, independent of insulin, increasing urinary glucose excretion [2]. SGLT-2i were shown to be associated with an increased risk of genital mycotic infections, especially in those with a previous history of genital infections [3,4,5]. They also increased the risk of uncomplicated urinary tract infection (UTI), urosepsis or pyelonephritis [1, 3]. The findings indicating the association of UTI with SGLT-2i were not as consistent as that concerning the association of genital infections with SLGT-2i use [3]. Dapagliflozin and empagliflozin was shown to increase the risk of UTI in many studies including a DURATION-8 study [1, 3, 6, 7]. Some studies showed that SGLT-2i use was not associated with an increased risk of UTI [4, 5, 8,9,10,11,12,13,14,15]. In a recent meta-analysis of a considerable number of randomized trials analyzing SGLT-2i use, the risk of UTI was higher where higher doses of dapagliflozin were administered [16]. In one study, UTI incidence was shown to be 4–9%, and severe infection 0.4% after SGLT-2i use [17,18,19,20].
Although the mechanism underlying UTI associated with SGLT-2i has not been clearly defined, glucosuria, effect of SGLT-2i, may provide a microorganism-favoring environment [1, 21]. However, osmotic diuresis, by maintaining urinary flow, may counteract the microorganism-favoring milieu [22]. Adequate fluid intake was an in consistent factor, which might be associated with UTI [23].
The factors, which have a possible role in the development of UTI after SGLT-2i use in T2D, have been less studied. We aimed to investigate the clinical and laboratory factors associated with UTI, which occurred after SGLT-2i use in those patients with T2D.
Materials and methods
Study design
This retrospective study was conducted in SANKO University hospital, and approved by the local Ethics Committee of SANKO University (Date: 2021/11 Decision No: 02). Written informed consent was obtained from all of the participants.
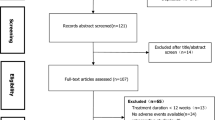
Adult patients diagnosed and followed-up with T2D in SANKO University hospital between January 2020 and December 2020, and whose urine culture results were available, were evaluated and analyzed retrospectively. The diagnosis and management of T2D was based on ADA guidelines [24, 25]. Those patients with any contraindications to treatment with SGLT-2i at the baseline were not given SGLT-2i [24, 25]. All patients underwent clinical and laboratory evaluation. Those patients with other types of diabetes mellitus, younger than 18 years of age, or those for whom data were missing were not included in the study. Benign prostate hypertrophy was known to be one of the causes of urinary tract infection in men. Therefore, male patients with a history of prostate hypertrophy were excluded. Those patients with genital infections or for whom a fungal agent was isolated before the study were excluded, because it might lead bias.
Data collection
Demographic parameters (age, sex, and body mass index [BMI]), smoking status, clinical parameters (symptoms [fever, dysuria, pollakuria, urgency, and nausea-vomiting], history of hypertension, need for hospitalization, duration of hospitalization [days]), and laboratory findings (complete blood count, CRP, fasting blood glucose [FBG], HbA1c, and urinalysis [pyuria, hematuria, bacteriuria, glucosuria, proteinuria, and nitrit positivity]) were recorded by using electronic and written patient files. Urine culture was obtained from mid-flow urine for each participant. Positivity of urine culture, species of bacteria isolated from urine culture, and antibacterial treatment were also recorded and analyzed. Urine culture was defined as positive if at least 105 colonies/mL was present.
Antibacterial treatment was given for 7–10 days to those patients in whom a UTI was detected. UTI was defined based both on the result of positive urine cultures and clinical signs and symptoms. To reveal a negative urine culture, a control urine culture was performed after antibacterial treatment in hospitalized patients.
Hospitalization was needed in unstable patients, those requiring intravenous antibacterial treatment, or with complicated UTIs. Pyelonephritis or urosepsis was not detected in any patients.
A complete blood count was studied with an automated analyzer. Urinalysis and FBG were measured by a glucose oxidase method with the Olympus AU-2700 analyzer. HbA1c was measured as NGSP (National Glycohemoglobin Standardization Program) units by the HPLC method (high purification liquid chromatography).
Patient groups
Patients were grouped based on SGLT-2i treatment: Group A, those treated with SGLT-2i; and Group B, those not treated with SGLT-2i. The participants in Groups A and B were given other antidiabetic medications according to the ADA guidelines, with the exception of insulin [25]. In those patients under SGLT-2i treatment, SGLT-2i had been used for at least 1 month. Empagliflozin or dapagliflozin at a dose of 10 mg/day was given in Group A. During the follow-up, mortality was also observed and analyzed. We compared demographic, clinical and laboratory parameters between the groups.
Statistical analysis
Data obtained in the study were analysed statistically using SPSS 26.0 (IBM Corporation, Armonk, New York, United States). The conformity of the data to normal distribution was evaluated using the Shapiro-Wilk francia test. When comparing two independent groups of quantitative data according to each other, we used the nonparametric Mann-Whitney U test with Monte Carlo results. When comparing categorical variables with each other, the Pearson Chi-square, Fisher’s exact and Fisher-Freeman-Holton tests with the Monte Carlo simulation technique were used. Comparison of column ratios with each other was expressed by Benjamini-Hochberg corrected p-values. ROC curve analysis was performed for quantitative parameters significant for urinary tract infection. To detect a cause-effect relationship for urinary tract infection, logistic regression analysis was performed with the Enter method. Quantitative variables were stated as mean (standard deviation), and median (minimum-maximum) values, and categorical variables as number (n) and percentage (%) in the tables. Variables were evaluated at a 95% confidence level, and a value of p < 0.05 was accepted as statistically significant.
Results
A total of 101 patients with T2D were included in the study. Group A consisted of 51 patients, and Group B 50 patients. Dapagliflozin was prescribed for 31 patients, and Empagliflozin for 20 patients in Group A. The median age was 56 (45–67), 56.4% (n = 57) of the patients were female. Hypertension was found in 56.4% (n = 57) of the patients. The BMI score was higher in Group A (p < 0.001). Fever, dysuria, pollakiuria, urgency, nausea-vomiting, pyuria, bacteriuria, and glucosuria were more frequent in Group A. Hematuria was not detected in any patients. Nitrite in urinalysis was positive in 27.5% (n = 14) of the patients in Group A, and 2% (n = 1) in Group B (p < 0.001).
Urine culture was positive in 35.6% (n = 36), 54.9% (n = 28), and 16% (n = 8) of the entire group, Group A and Group B, respectively. Among those for whom urine culture was positive, Escherichia coli was isolated in 83.3% (n = 30), and both Escherichia coli and Klebsiella pneumoniae were isolated in the same urine culture in 16.7% (n = 6) of the patients. Klebsiella pneumoniae was isolated only from Group A. 60 patients were treated with antibacterial medications. Antibacterial medications were similar in 2 groups. The need for hospitalization and the duration of hospitalization were higher in Group A (p < 0.001 and p < 0.001, respectively) (Table 1).
No patients died during the follow-up.
BMI scores and HbA1c were higher, and glucosuria and SGLT-2i treatment were more frequent in those patients with UTI (Table 2).
ROC analysis showed that a HbA1c of > 5.8% was associated with UTI with good accuracy (AUC: 0.835; p < 0.001; sensitivity: 93.3%; and specificity: 58.5%) (Fig. 1).
Multiple logistic regression analysis showed that SGLT-2i use and glucosuria were significant positive predictors for UTI (p = 0.004; Odds Ratio: 1984.013; p = 0.028; and Odds Ratio: 12.480; respectively) (Table 3).
Discussion
Clinical symptoms and laboratory signs of urinary tract infection (UTI) were more frequent in those patients treated with SGLT-2i. Of the patients treated with SGLT-2i, urine culture was positive in more than half, and one third were hospitalized. Besides the association of HbA1c and BMI with UTI, SGLT-2i use and glucosuria were seen to predict UTI.
SGLT-2i use was shown to be associated with UTI in previous studies [1, 3, 26,27,28,29]. But the association was not consistently detected, and some other studies did not find any association between SGLT-2 inhibitor use and UTI [4, 5, 8,9,10,11,12,13,14,15]. We concluded that SGLT-2i use predicted the development of UTI. The SGLT-2i class of medications exert their action by inhibiting the reabsorption of glucose in the proximal convoluted tubules by SGLT-2 channels, resulting in glucosuria [30]. Glucosuria has been proposed as a major mechanism facilitating the development of UTI associated with SGLT-2i use [1, 21, 29]. In T2D, the renal threshold for glucose excretion was increased via an over-expression of SGLT-2 channels [31]. SGLT-2i may result in a more enhanced glucosuria response in T2D than expected for a similar glycemic level in a non-diabetic subject. In our study, about 40% of the patients not treated with SGLT-2i did have glucosuria, although the ratio was less than in those treated with SGLT-2i. We showed that glucosuria predicted UTI in those patients with T2D independent of SGLT-2i use, and the predictive value of SGLT-2i for the development of UTI was higher than that of glucosuria. Therefore, not only glucosuria, but also other possible mechanisms seem to play a role in the development of UTI occurring after SGLT-2i use in those patients with T2D. We propose that poor hygiene, inadequate hydration or obesity itself may also contribute to an elevated risk of UTI in those patients with T2D. We showed that there is an association between BMI score and UTI. Besides, obesity may be associated with UTI as a result of associated difficulties in maintaining adequate hygiene standards.
Clinical signs and symptoms suggest UTI was associated with SGLT-2i use in our study. However, some symptoms such as pollakiuria might be a result of glucosuria-induced osmotic diuresis. In one study, pollakiuria due to canagliflozin was found to be associated with natriuresis rather than glucosuria, and shown to be transient [32]. Chronic complications, such as autonomic neuropathy, may be detected following a diagnosis of T2D. In our study, we did not analyze the duration of T2D. We did not perform objective genitourinary autonomic neuropathy tests, which may predispose to UTI or alter the clinical symptoms of UTI. In one study, new-onset nocturia and pollakiuria occurred less frequently after the initiation of SGLT-2i in those patients with T2D and autonomic neuropathy than in those without autonomic neuropathy [33]. Besides, hyperglycemia itself may lead to pollakiuria or nocturia as a result of osmotic diuresis in those patients with significant hyperglycemia. Therefore, rather than the symptoms alone, objective methods such as urinalysis and, more specifically, urine culture are essential to correctly reveal the association of the symptoms with UTI. We included over a hundred patients with T2D for whom a urine culture was performed.
A DURATION-8 study showed an increased risk of UTI after dapagliflozin use, and similar findings were also reported for empagliflozin [1, 3, 6, 26, 34]. Studies comparing different preparations of the SGLT-2i class in terms of UTI are scant. In a meta-analysis, the risk of UTI was found to be similar in different subgroups of the SGLT-2 inhibitor class, and was independent of the dose of SGLT-2i preparation with the exception of dapagliflozin [16]. In that study, high-dose dapagliflozin was associated with an increased risk of UTI. Another systemic meta-analysis suggested that empagliflozin was more likely associated with UTI than dapagliflozin [1]. We included the patients receiving empagliflozin or dapagliflozin, analyzed both together, but could not analyze the other preparations of the SGLT-2 inhibitor class. We treated the patients with the same dose of dapagliflozin or empagliflozin during the follow-up, and could not analyze the effect of the dose of SGLT-2i on the development of UTI.
SGLT-2i use was associated with not only uncomplicated UTI, but also with urosepsis and pyelonephritis in some reports (18–20). However, severe infection associated with SGLT-2i use is less likely, as shown in a previous study where there was a low incidence of severe UTI of 0.4% after SGLT-2i use [17,18,19,20]. In our study, one third of the patients receiving SGLT-2i were hospitalized due to the severity of the infection, and SGLT-2i use was associated with the need for hospitalization. We hospitalized the unstable patients, those requiring intravenous antibacterial treatment, or complicated UTI. Neither Pyelonephritis nor urosepsis was not detected in any patients. We also showed that a higher duration of hospitalization was associated with SGLT-2i use. In a meta-analysis, the risk of UTI was similarly greater in different subgroups of the SGLT-2i class, but the risk of upper urinary tract infection increased only where dapagliflozin 10 mg was used [29].
Klebsiella pneumoniae may be isolated from urine culture in those subjects with uncomplicated UTI, where the frequency is 1–2% (35). Klebsiella pneumoniae or Escherichia coli species may be resistant to antibacterial treatment, which may contribute to the need for hospitalization [35]. In our study, although the ratio of bacteria species isolated from the urine culture was similar to that in those patients treated with SGLT-2i or not, Klebsiella pneumoniae was isolated only in those patients treated with SGLT-2i. The frequency of acquired resistance is known to be > 30% for nitrofurantoin, and 10–20% for fluoroquinolones in Klebsiella pneumoniae [35]. About 25% of Klebsiella pneumoniae species produce extended-spectrum beta-lactamase [35]. Due to the reduced effect of fosfomycin or nitrofurantoin, oral treatment is more challenging for infection with ESBL-positive Klebsiella pneumoniae strains than ESBL-positive Escherichia coli strains [35]. Isolation of the Klebsiella pneumoniae strain in those patients treated with SGLT-2i might affect the need for hospitalization or the administration route of antibacterial treatment. Therefore, a urine culture study is so important in those patients with T2D, especially in those treated with SGLT-2i.
SGLT-2 inhibitors were also shown to be associated with an increased risk of genital mycotic infections especially in those patients with a history of genital mycotic infection and in uncircumcised males [3,4,5]. Genital infections may be co-existent with UTI in patients with T2D. We analyzed those patients with UTIs alone, and excluded those with any signs or symptoms of genital infections.
In our study, BMI score, FBG and HbA1c levels were higher in those patients treated with SGLT-2i than in those not treated with SGLT-2i. Actually, due to the known efficacy of SGLT-2 inhibitors on glycemic control in T2D, and the beneficial effects on body weight, we prescribed SGLT-2 inhibitors especially in those patients with a higher HbA1c or higher BMI score [6, 31]. Our findings suggested that a higher BMI score or HbA1c was associated with UTI. Among those patients in our study, who were treated with antidiabetic medications, the HbA1c cut-off for association with UTI was as low as 5.8%. According to our study, the predictive role of SGLT-2i use for UTI seems to be independent of BMI score or HbA1c.
Strength and limitations
We included an important number of patients with T2D for whom a urine culture was performed. We did not analyze the members of the SGLT-2i class other than empagliflozin or dapagliflozin. We analyzed those patients treated with empagliflozin or dapagliflozin together. We excluded patients with any signs or symptoms of genital infections, but could not analyze the presence of genitourinary autonomic dysfunction. We could not analyze the duration of T2D, or the duration between the emergence of UTI and the initiation of SGLT-2i.
Conclusion
Studies analyzing urine cultures in the investigation of the association of SGLT-2i use with UTI in T2D are limited. We found that SGLT-2i use and glucosuria predicted UTI in those patients with T2D. Our findings suggest that performing a urine culture is very important in choosing the type and administration route of antibacterial treatment, especially in those patients receiving SGLT-2i treatment. It seems that glucosuria is not the only mechanism underlying the development of UTI in patients treated with SGLT-2i. The effect of SGLT-2i on the development of UTI is independent of the baseline BMI score or HbA1c. Future studies, which include a large sample of subjects with T2D receiving SGLT-2i treatment, may indicate the other possible factors contributing to UTI.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The authors assure that this paper has not been published before nor has been submitted for publication to another scientific journal.
References
Pelletier R, Ng K, Alkabbani W, et al. Adverse events associated with sodium glucose co-transporter 2 inhibitors: an overview of quantitative systematic reviews. Ther Adv Drug Saf. 2021;26(12):2042098621989134. https://doi.org/10.1177/2042098621989134
Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their Basic and Clinical Pharmacology. Diabetes Ther. 2014;5(2):355–66. https://doi.org/10.1007/s13300-014-0089-4
McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Cardiol. 2019;15(124):45–52. https://doi.org/10.1016/j.amjcard.2019.10.029
Rådholm K, Wu JH, Wong MG, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular disease, death and safety outcomes in type 2 diabetes - A systematic review. Diabetes Res Clin Pract. 2018;140:118–28. https://doi.org/10.1016/j.diabres.2018.03.027
Zhang YJ, Han SL, Sun XF, et al. Efficacy and safety of empagliflozin for type 2 diabetes mellitus: Meta-analysis of randomized controlled trials. Med (Baltim). 2018;97(43):e12843. https://doi.org/10.1097/MD.0000000000012843
Xu B, Li S, Kang B, et al. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol. 2022;21(1):83. https://doi.org/10.1186/s12933-022-01512-w
Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):1004–16. https://doi.org/10.1016/S2213-8587(16)30267-4
Zhang L, Zhang M, Lv Q, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes and moderate renal function impairment: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;140:295–303. https://doi.org/10.1016/j.diabres.2018.03.047
Jabbour S, Seufert J, Scheen A, et al. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20(3):620–28. https://doi.org/10.1111/dom.13124
Li J, Gong Y, Li C, et al. Long-term efficacy and safety of sodium-glucose cotransporter-2 inhibitors as add-on to metformin treatment in the management of type 2 diabetes mellitus: a meta-analysis. Med (Baltim). 2017;96(27):e7201. https://doi.org/10.1097/MD.0000000000007201
Kohler S, Zeller C, Iliev H, et al. Safety and Tolerability of Empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. 2017;34(7):1707–26. https://doi.org/10.1007/s12325-017-0573-0
Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824. https://doi.org/10.1038/s41598-017-02733-w
Neal B, Perkovic V, Matthews DR. Canagliflozin and Cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. https://doi.org/10.1056/NEJMc1712572
Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME investigators. Empagliflozin, Cardiovascular Outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720
Baglioni P. Dapagliflozin and Cardiovascular Outcomes in type 2 diabetes. N Engl J Med. 2019;380(19):1880–1. https://doi.org/10.1056/NEJMc1902837
Donnan JR, Grandy CA, Chibrikov E, et al. Dose response of sodium glucose cotransporter-2 inhibitors in relation to urinary tract infections: a systematic review and network meta-analysis of randomized controlled trials. CMAJ Open. 2018;6(4):E594–E602. https://doi.org/10.9778/cmajo.20180111
https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious#:~text=Safety%20and%20Availability-,FDA%20revises%20labels%20of%20SGLT2%20inhibitors%20for%20diabetes%20to%20include,and%20serious%20urinary%20tract%20infections&text=To%20lessen%20the%20risk%20of,information%20for%20SGLT2%20inhibitor%20medicines. (Access Date: 20 August 2022).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204042s011lbl.pdf (Access Date: 20 August 2022).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202293s020lbl.pdf (Access Date: 20 August 2022).
https://. docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/Patient%20Info/Jardiance%20Medication%20Guide?DMW_FORMAT=pdf (Access Date: 20 August 2022).
Chandrashekar M, Philip S, Nesbitt A, et al. Sodium glucose-linked transport protein 2 inhibitors: an overview of genitourinary and perioperative implications. Int J Urol. 2021;28(10):984–90. https://doi.org/10.1111/iju.14624
Kinduryte Schorling O, Clark D, Zwiener I, et al. Pooled safety and tolerability analysis of Empagliflozin in patients with type 2 diabetes Mellitus. Adv Ther. 2020;37(8):3463–84. https://doi.org/10.1007/s12325-020-01329-7
Fasugba O, Mitchell BG, McInnes E, et al. Increased fluid intake for the prevention of urinary tract infection in adults and children in all settings: a systematic review. J Hosp Infect. 2020;104(1):68–77. https://doi.org/10.1016/j.jhin.2019.08.016
American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(1):17–38. https://doi.org/10.2337/dc22-S002
American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 9. Pharmacologic approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(1):125–43. https://doi.org/10.2337/dc22-S009
Devi R, Mali G, Chakraborty I, et al. Efficacy and safety of empagliflozin in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Postgrad Med. 2017;129(3):382–92. https://doi.org/10.1080/00325481.2017.1259544
Qiu R, Balis D, Xie J, et al. Longer-term safety and tolerability of canagliflozin in patients with type 2 diabetes: a pooled analysis. Curr Med Res Opin. 2017;33(3):553–62. https://doi.org/10.1080/03007995.2016.1271780
Storgaard H, Gluud LL, Bennett C, et al. Benefits and Harms of Sodium-Glucose co-transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and Meta-analysis. PLoS ONE. 2016;11(11):e0166125. https://doi.org/10.1371/journal.pone.0166125
Zaccardi F, Webb DR, Htike ZZ, et al. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18(8):783–94. https://doi.org/10.1111/dom.12670
Wright EM. SGLT2 inhibitors: physiology and pharmacology. Kidney360. 2021;2(12):2027–37. https://doi.org/10.34067/KID.0002772021
Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73–9. https://doi.org/10.1097/MED.0000000000000311
Tanaka H, Takano K, Iijima H, et al. Factors affecting Canagliflozin-Induced transient urine volume increase in patients with type 2 diabetes Mellitus. Adv Ther. 2017;34(2):436–51. https://doi.org/10.1007/s12325-016-0457-8
Chilelli NC, Bax G, Bonaldo G, et al. Lower urinary tract symptoms (LUTS) in males with type 2 diabetes recently treated with SGLT2 inhibitors-overlooked and overwhelming? A retrospective case series. Endocrine. 2018;59(3):690–3. https://doi.org/10.1007/s12020-017-1301-x
Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36(12):4015–21. https://doi.org/10.2337/dc13-0663
Thomas A, Russo JR, Johnson. Diseases Caused by Gram-Negative Enteric Bacilli. In Harrison’s Principles of Internal Medicine, 21st Edition McGraw Hill, 2022, New York.
Acknowledgements
We would like to express our special thanks to all patients that participate in our study.
Funding
No funding was taken during the whole process of study, writing of the article or preparation of manuscript.
Author information
Authors and Affiliations
Contributions
M.T.N., M.B., M.T.K. and H.D.: wrote the main manuscript text and MTN, MB, HD, AU, GT and MTK: literature search. AU: collected the data. The first draft of the manuscript was written by MTN and co-author commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and approved by The Medical Research Ethics Committee of Sanko University (Date: 2021/11, Decision No: 02). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tanrıverdi, M., Baştemir, M., Demirbakan, H. et al. Association of SGLT-2 inhibitors with bacterial urinary tract infection in type 2 diabetes. BMC Endocr Disord 23, 211 (2023). https://doi.org/10.1186/s12902-023-01464-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01464-6