Abstract
Background
The bronchial suction has been applied in speeding lung collapse. Low suction pressure may not speed lung collapse, but high pressure causes occult lung injury. The aim of the study was to explore efficacy and safety of different suction pressure for speeding lung collapse.
Methods
Eighty-four subjects undergoing uniport video-assisted thoracoscopic surgery (VATS) were randomly assigned for non-suction (Group 0), -10 cmH2O suction pressure (Group − 10), and − 30 cmH2O suction pressure (Group − 30). The primary outcome were the lung collapse scores (LCS) at 0 min (T0) after the visualization of the lung using a 10-point visual analogue scale and area under the curve (AUC) of LCS over time. The secondary outcomes included disconnection from the ventilator, the assessment of occult lung injury using NOS-3 expression, histologic scores of lung injury, and lung W/D weight ratio, intraoperative hypoxemia, the incidence of perioperative pulmonary complications.
Results
Both the LCS at T0 and AUC analysis showed that compared with Group 0, Group − 10 and Group − 30 significantly achieved good lung collapse (P < 0.05), but no difference between Group − 10 and Group − 30. Four patients in Group 0 were treated with disconnection maneuver. The assessment of occult lung injury showed no differences.
Conclusions
Applying − 10 cmH2O suction pressure for 1 min when pleural incision is a relatively safe method to promote lung collapse without the occurrence of occult lung injury.
Trial registration
Chinese Clinical Trial Registry number, ChiCTR2200062991. Registered on 26/08/2022.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Bronchial blockers (BB) and double lumen tube (DLT) are two common devices used for lung isolation in video-assisted thoracoscopic surgery (VATS) [1]. Compared to DLT, BB has the advantages of simple operation and fewer complication of throat pain [2]. However, BB’s lumen is narrow and longer; and the residual gas in the non-ventilated lung (NVL) is removed slowly [3].
There are many methods used to speed lung collapse in clinical, such as suction technique, disconnection technique, inhalation of oxygen (O2) and nitro oxide (N2O) gas mixture. Disconnection for a long time, the patient is at risk of hypoxia [4]. N2O is rarely used in clinical because of its clinical limitation [5]. Suction is one of the commonly used methods to speed lung collapse, however, the suction pressure is controversial. The pressure used in each study varies, and the conclusions of speeding lung collapse are inconsistent [6,7,8]. Low suction pressure may not promote the quality of lung collapse [6]. High suction pressure promotes lung collapse without increased risk of pulmonary complications, but occult lung injury may be present [8]. In addition, previous studies on bronchial suction are mostly based on triple-port VATS. With the improvement of thoracic techniques, uniport VATS has been gradually replaced triple-port VATS because of its advantages of less surgical trauma, less postoperative pain and faster recovery [9]. The triple-port VATS takes approximately 10 min from the skin incision to the completion of the three ports [8], however, uniport VATS only requires approximately 90 s [7]. The time point of operation is advanced, and more attention is given to whether lung collapse can be completed early in the opening of the pleura [4].
The primary aim of this prospective, single-center, randomized study was to investigate efficacy and safety of different suction pressure for speeding non-ventilated lung collapse during uniport VATS.
Materials and methods
Study population
This study protocol was approved by the ethics committee of The First People’s Hospital of Kunshan (approval No. 2022-03-014-K01). Written informed consent was obtained from all participants. Clinical trial registration was performed at http://www.chictr.org.cn (identifier: ChiCTR2200062991). Patients with pulmonary nodules scheduled to receive uniport video-assisted thoracoscopic wedge resection under general anesthesia between September 1, 2022, and September 1, 2023, at the First People’s Hospital of Kunshan were enrolled. The manuscript adheres to the applicable Consolidated Standards Of Reporting Trials (CONSORT) guidelines.
The inclusion criteria were as follows: patients with a persistent (with at least a three-month follow-up) pulmonary nodule, have an American Society of Anesthesiologists (ASA) physical status I-III and were aged 18–75 years. The exclusion criteria were as follows: forced vital capacity (FVC) were less than 50% of predicted values, Body Mass Index (BMI) more than 35 kg·m− 2; previous history of thoracic surgery; abnormalities of bronchial anatomy (tracheal bronchus) and pleural adhesion [7].
Randomization and concealment of group assignments
Patients were randomly assigned for non-suction (Group 0), -10 cmH2O suction pressure (Group − 10), and − 30 cmH2O suction pressure (Group − 30) with a 1:1:1 allocation. The randomization was performed by a resident who was not involved in the study. The participants were randomized using a random sequence generated by SPSS version 26.0 (SPSS Inc. Chicago, IL, USA). Each participant was given a number between 0 and 1 randomly generated by the SPSS software. The software will generate two split points to randomly divide the patients into three groups. The allocation sequence is concealed using opaque envelopes. As soon as the patient arrived in the operating room, the attending anesthesiologist received opaque envelopes [10]. The group situation was known to a resident who was not involved in the study. the outcome assessors were unaware of the allocation of the study [5].
Anesthesia protocol
SpO2 and ECG were employed in all patients. Radial artery catheterization for invasive blood pressure monitoring under local anesthesia. After preoxygenation, general anesthesia was induced with sufentanil 0.5 μg/kg, propofol 2.5 mg/kg, succinylcholine 1.5 mg/kg. Patients were intubated with single-lumen endotracheal tube (male: 8.0 mm, female: 7.5 mm). Anesthesia was maintained using propofol 2 ∼ 4 mg·kg− 1·h− 1, remifentanil 0.05 ∼ 0.4 μg·kg− 1·min− 1 to maintain BIS 40 ∼ 60, and intermittent boluses cisatracurium 0.05 mg/kg [10]. The dosage of anesthetic drugs was adjusted according to hemodynamics. Two-lung ventilation was instituted with FiO2 = 100%, tidal volume 6–8 mL/kg, and respiratory rate adjusted to achieve an end-tidal carbon dioxide pressure within 35–45 mmHg [11]. After lateral position, the BB (Hangzhou Tappa Medical Technology CO, Hangzhou, China) was inserted in the endotracheal tube, the position of the blocker was confirmed by fiberoptic bronchoscopy (FOB) and the blocker cuff was inflated with 4 mL to 6mL of air, which was defined as the start of one lung ventilation (OLV). The lumen of the BB remains closed until the pleura incision. OLV was instituted with FiO2 = 50%, tidal volume 4–6 mL/kg. During the surgery, ephedrine was injected with 6 mg when the decrease of mean arterial pressure (MAP) was more than 30% before induction, and a continuous infusion of phenylephrine (0.2–0.4 μg·kg− 1·min− 1) was started when the ephedrine was injected more than 5 times [7]. After the chest drainage tube was inserted, all patients were given alveolar recruitment maneuver. Then, the BB was removed. After surgery, all patients were admitted to the post anesthesia care unit (PACU), and endotracheal tube was extubated.
Experimental protocol
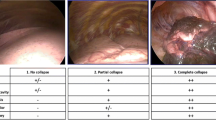
The lumen of the BB was opened, and the pressure-limiting valve and suction device were connected to the lumen of the BB using two suction tubes (Fig. 1A) at the time point of pleura incision. The gap between the suction catheter and the lumen of the BB was closed using an adhesive membrane (Fig. 1B). The suction pressure is regulated through the pressure limiting valve and lasts one minute. In Group 0, the suction device was only used to make noise to keep the surgeon blinded. After pleurotomy, the trocar was placed (the skin incision is in the fifth intercostal space along the midaxillary line). The 2D video thoracoscopic camera inserted into the chest directed to the upper edge of the thoracic cavity, the surgeon starts recording the video. All surgeries were performed by the same surgeons who was blinded to group allocation. To not interfere with the progress of the operation, the lung collapse was evaluated by video after the operation. Two surgeons who performed the surgeries evaluated the LCS by video recordings at 0 min (defined as T0) and 10 min (defined as T10) after the visualization of the lung using a 10-point visual analogue scale.10-point visual analogue scale is one of the most commonly methods for evaluating the quality of lung collapse, and a score of 0 indicates the lungs are fully inflated and a score of 10 indicates complete lung collapse [8, 12].
The disconnection technique was implemented to facilitate lung collapse, if the surgeon proposed that the lung collapse is extremely poor and inoperable. The ventilator was turned off and an adjustable pressure-limiting valve was fully opened, deflation of the cuff of the BB, and the ventilator was turned on allowing only dependent-lung ventilation after 1 min [1].
After the resection of the diseased tissue, the lung tissue away from the tumor was cut out immediately and was frozen at -80 ℃ for analysis of the nitric oxide synthase type 3 (NOS-3) expression, histologic scores of lung injury, and lung wet/dry (W/D) weight ratio.
Expression of NOS-3 level was evaluated using Quantitative Real-Time PCR (qPCR). RNA was extracted using Trizol (Solarbio) according to manufacturer’s instructions. The concentration and purity of RNA were determined by ultramicrospectrophotometer (Allsheng, Nano-300). cDNA was synthesized with 2000 ng of RNA samples using the Superscript First strand synthesis system for RT-PCR (Biometra GmbH, TAdvanced). qPCR was performed with the Real Time PCR system (BIOER, FQD-96 A). Primers were generated and purchased from Sangon Biotech. Primer sequences are as follows: NOS-3, f 5′ TCT CAC CTT CTT CCT GGA CAT CAC 3′, r 5′ AAC CAC TTC CAC TCC TCG TAG C 3′, GAPDH (HUMEN), f 5′ CAG GAG GCA TTG CTG ATG AT 3′, r 5′ GAA GGC TGG GGC TCA TTT 3′. The relative change in gene expression was calculated by the ΔΔCt method from triplicate determinations using GAPDH (HUMEN) as a housekeeping gene [13].
Part of the preserved lung tissue was fixed in 10% formalin, and lung tissue was embedded in paraffin, sectioned, and stained them with hematoxylin-eosin. A pathologist unaware of the assignment analyzed the severity of lung injury. Lung injury scores were determined by a blinded pathologist using 10 random fields at 100 times field of view. Lung injury was quantitatively graded on a scale of 0 (no injury), 1 (less than 25% involved), 2 (25–50% involved), or 3 (more than 50% involved) based on the presence of alveolar fibrin/edema, alveolar hemorrhage, septal thickening, and cellular infiltration [14].
After rewarming, lungs placed in a tared microcentrifuge tube and weighed. Lungs were then desiccated at 70℃ until the weight is constant and weighed again. The wet lung mass was divided by the dry lung mass to give the wet-dry ratio [15].
Outcomes
The primary endpoint was the LCS at T0 and area under the curve (AUC) of lung collapse scores (LCS) over time. The secondary outcomes included the disconnection from the breathing circuit, NOS-3 expression, histologic scores of lung injury, and lung W/D weight ratio, intraoperative hypoxemia (defined as SpO2 < 90%) that need CPAP or recruitment maneuvers, the incidence of perioperative pulmonary complications such as pulmonary oedema, respiratory failure. Furthermore, preemptive OLV time (defined as the time from the beginning of OLV to pleural incision), the time of skin incision (defined as the time from the skin incision to the pleural incision), the time of trocar placement (defined as the time from pleural incision to thoracoscopic entry into the chest), the operation time (defined as the time from the thoracoscopic entry into the chest to the placement of the chest drainage tube), histology of tumor, tumor stage, tumor size were recorded.
Statistical analysis
The sample size calculation of this pilot study was performed on the basis of pretest data. In the preliminary trial that we conducted, the mean of LCS in 4 patients who accepted non-suction was 3.5, the standard deviation (SD) was 1.118; the mean of LCS in 4 patients who accepted − 10 cmH2O pressure was 4.125, the SD was 0.892; the mean of LCS in 4 patients who accepted − 30 cmH2O pressure was 4.5, the SD was 0.612. As calculated from these data by analysis of variance (ANOVA) using the software package PASS version 11 (NCSS LLC, Kaysville, UT), assuming the SD was 1.118 (the highest within 3 group SD in the pretests), effect size = 0.369, a randomization ratio of 1:1:1, a type I error (α) probability of 0.05 [8], a power (1-β) of 80% [6], and 10% of drop-out, the sample size was determined as 28 subjects in each group [16].
Statistical analyses were performed using SPSS version 26.0 (SPSS Inc. Chicago, IL, USA) and the statistical package SAS, version 9.4 (SAS Institute Inc, Cary, NC, USA). Kolmogorov–Smirnov test was used to test normality, and Levene’s test was used to test homogeneity of variance. Continuous variables with normal distribution are presented as mean (SD) or mean (standard error [SE]), analyzed among groups using 1-way ANOVA. Non-normal variables are presented as median [interquartile range (IQR)] and are compared using the Kruskal-Wallis’s test. If the Kruskal-Wallis’s test results were significant, pairwise group comparisons were performed by Mann–Whitney U tests. The differences in medians were reported as Hodges–Lehmann estimators with 95% confidence intervals (CIs) [16]. Categorical data are presented as percentages (%), and the comparison between groups is performed using the χ2 test. P value < 0.05 was considered statistically significant.
Results
Eighty-four patients were recruited for the study and were subsequently randomly assigned to 3 groups. Four patients were excluded because of lung adhesions and one patient was excluded because of an early take-off of the right upper lobe bronchus. Accordingly, data were analyzed from seventy-nine patients (Fig. 2).
Patient demographics and other preoperative data are listed in Table 1. The groups were similar with regard to age, sex, weight, BMI, height, VATS side, location of lung nodule, ASA, preemptive OLV time, skin incision time, trocar placement time, and the operation time, histology of tumor, tumor stage, tumor size.
Kruskal-Wallis’s test of the LCS at T0 (Fig. 3) show that differences among three groups (median [quartiles]: Group 0 = 3.0 [2.0–5.0], Group − 10 = 5.0 [4.0-5.5], Group − 30 = 4.5 [4–5.0], P < 0.01). The results of the pairwise comparison revealed group differences (reported as the Hodges–Lehmann estimator [99% CI], P values) of Group 0 vs. Group − 10 (− 1.5 [− 2.5 to − 0.5], P < 0.01); Group 0 vs. Group − 30 (− 1.5 [− 2.0 to 0.5], P < 0.05), but not for Group − 10 vs. Group − 30 (0.5 [0 to 1.0], P > 0.05).
Lung collapse scores of T0 on a 10-point visual analogue scale
NOTE: The data are presented as medians, interquartile ranges (25th–75th percentile), and ranges. Comparison of lung collapse scores of T0 in 3 groups analyzed by using with Kruskal-Wallis test, the results of the pairwise comparison analyzed by the Mann–Whitney U test. Abbreviations: *: P < 0.05; **: P < 0.01
AUC analysis (Fig. 4) indicated differences (mean [SD], P values) between LCS of Group 0 and Group − 10 (55.48 [8.703] vs. 65.00 [5.911], P < 0.01), Group 0 and Group − 30 (55.48 [8.703] vs. 65.48 [5.792], P < 0.01), but no difference for Group − 10 and Group − 30 (65.00 [5.911] vs. 65.48 [5.792], P > 0.05).
AUC analysis for Lung collapse scores
NOTE: A, AUC for LCS over time. B, AUC for LCS in 3 groups. The area is presented as mean (standard deviation). Comparison of area in 3 groups analyzed by using with Kruskal-Wallis test, the results of the pairwise comparison analyzed by the Mann–Whitney U test. Abbreviations: **: P < 0.01, AUC: Area under the curve
Four patients in Group 0 were treated with disconnection technique to accelerate lung collapse due to poor lung collapse. No disconnection technique was taken in Group − 10 and Group − 30. There were differences among the three groups (P < 0.05).
Intraoperative hypoxemia that needs CPAP or recruitment maneuvers and perioperative pulmonary complications did not occur in any patient. In the assessment of NOS-3 expression, histologic evaluation and lung W/D weight ratio, no statistically significant difference was found between the Group 0 and Group − 30 (P > 0.05) (Fig. 5).
Assessment of NOS-3 expression, histologic scores of lung injury and lung W/D weight ratio in the Group 0 and Group − 30
NOTE: A, NOS-3 relative expression. B, Histologic scores of lung injury. C, Lung W/D weight ratio
Data are presented as means (standard error) (n = 4 per group, P > 0.05, t test). D, Photomicrographs of lung histology (hematoxylin-eosin, ×200). (a): Photomicrograph of a pulmonary section from the Group 0. (b): Photomicrograph of a lung section from the Group − 30. (Abbreviations: ns: no significance, NOS-3: nitric oxide synthase type 3, W/D: wet / dry)
Discussion
BB is a common OLV device for lung isolation in VATS. However, its exhaust pipe is narrow, and the residual gas in the non-ventilated lung is slowly expelled [4]. Good lung collapse is the key for the success of VATS. It is crucial to speed lung collapse in improving safety of patients’ surgery when using a BB as a OLV device.
It has been reported that suction is one of the common methods to speed lung collapse, but the optimal suction pressure suction pressure is not clear. El-Tahan et al. [7]. found that Arndt BB combined with a suction pressure of -30 cmH2O could significantly increase the quality of lung collapse. Narayanaswamy et al. [6]. reported that − 20 cmH2O suction pressure applied to the Cohen BB significantly increased the LCS. Our study showed that Group − 10 and Group − 30 significantly achieved good lung collapse than Group 0 without the occurrence of occult lung injury. However, there is no difference in LCS between Group − 10 and Group − 30. It is considered that increase of suction pressure has the potential risk of increasing lung injury. Therefore, a suction pressure of -10 cmH2O for 60 s after pleural incision is recommended in uniport VATS to speed lung collapse.
Due to the advantages of less surgical trauma, less postoperative pain, and faster recovery, uniport VATS techniques have been increasingly applied to the treatment of lung cancer patients [9]. At present, the application of suction in uniport VATS has not been reported. Uniport VATS takes approximately 90 s from the skin incision to the completion of the uniport instead of 10 min for triple-port VATS [7, 8]. The time point of operation is advanced, and more attention is given to whether lung collapse can be completed early when the thoracoscope enters the chest cavity. Therefore, unlike previous studies on suction, our primary outcome was LCS when the thoracoscope enters the chest cavity. In addition, we also assessed the AUC for the LCS over time.
The manipulation and mechanism of suction to speed lung collapse are similar to that of artificial airway suctioning. Excessive pressure for artificial airway suction lead to lung injury [17]. It has been reported that ulcers and ulcers with necrosis were observed in the tracheal tissues due to artificial airway suction with a pressure of -272 cmH2O for 160 s [18]. Although the suction pressure to speed lung collapse was far less than the pressure recommended by artificial airway suction guidelines and no intraoperative hypoxemia and perioperative pulmonary complications were reported in previous studies of speed lung collapse [17], whether the suction cause the risk of occult lung injury has always been a hot topic of concern for anesthesiologists [8]. Most of the suction pressure reported to speed lung collapse below − 30 cmH2O, so the maximum suction pressure of -30 cmH2O was applied in our experiment [7, 8]. The trial chose to evaluate the occurrence of occult lung injury and lung injury by assessment of NOS-3 expression, histologic evaluation, and lung W/D weight ratio. NOS-3 holds significant importance as a form of human nitric oxide synthase [19]. The upregulation of the NOS-3 expression significantly contributes to the generation of nitric oxide (NO), and the augmentation of NO assumes a significant role in mitigating pulmonary vascular tension and ameliorating lung injury [19]. The lung W/D weight ratio is the gold standard for the evaluation of occult lung injury [15]. Lung histologic evaluation can observe whether there is many red blood cells aggregation and edema fluid in alveolar space [14].
No study has clarified that the suction time and why a particular time to be selected to perform suction. Previous study performed suction after the application of surgical drapes until the assessing surgeon determined that total lung collapse had been achieved [7]. Narayanaswamy et al. [6]. applied the suction at the start of lung isolation. Some scholars have suggested 60 s of suction immediately at the time point of pleural cavity opening [9]. The author approve this viewpoint because the time of suction is consistent with the occurrence of elastic recoil of the NVL [20]. The trial found that the average time from pleural incision to thoracoscopic entry into the chest was 72 s, and that’s enough to complete the suction.
The scholars has proposed that the suction can either be through the FOB after deflation of the bronchial cuff and cessation of ventilation [7]. The maneuver and mechanism of bronchial suction technique are similar to artificial airway suction used in BB. In current guidelines of artificial airway suction, for adult patients, it is recommended to keep the suction pressure below − 272 cmH2O, and the time should be applied for a maximum of 15 s per suctioning procedure [17]. Further research is needed to prove whether maneuver of artificial airway suction after the deflation of cuff (keep the suction pressure below − 272 cmH2O and continued for 15 s) is more appropriate for patients.
There is 1 patient observing tracheal bronchus (right upper lobe bronchial opening variation) under FOB. The right upper lobe opening was located above upper edge of the carina, and the cuff of BB could not completely block the non-ventilated lung, so the patient was excluded [21]. Furthermore, when the distance between the right upper lobe opening and carina is short, the right upper lobe opening is blocked by the cuff of BB, and the residual gas in the right upper lung is not expelling by suction [4]. Therefore, DLT or BB combined disconnection technique may be more appropriate for patients whose lesion is in the right upper lung. There are 4 patients observing severe pleural adhesions when pleural incision. The first phase of lung collapse depends on passive elastic recoil of the lung, so the patients were excluded [8].
The Tappa BB (Hangzhou Tappa Medical Technology CO, Hangzhou, China) was used in this study. Its structure is basically consistent with that of Coopdech BB (Coopdech, Osaka, Japan). Both BB’s have an outer diameter (OD) of 3 mm. It is reported that the size of Fuji Uniblocker (Vitaid Ltd., Williamsville, New York, USA), Cohen BB (Cook Critical Care, Bloomington, Indiana, USA), Arndt BB (Cook Critical Care, Bloomington, Indiana, USA) is 9 F [22], and the OD is about 3 mm (OD = 0.33 × F), which is similar to Tappa BB and Coopdech BB. However, the internal diameters (center channel) of these BB are slightly different. Whether the conclusion of suction accelerating lung collapse can be extended to other types of BB deserves further study.
The present study had several limitations. Firstly, the method used for assessing lung collapse, which lacks objectivity and may introduce bias into the evaluation process [23]. Scholars have proposed using the distance of lung collapse away from the chest wall to assess the degree of lung collapse. It is objective, but the size of the patient’s thorax varies with different patients, which could influence the measurement and is less clinically relevant [7]. The methods for assessing lung collapse need to be further explored. Secondly, only patients with normal pulmonary function tests and BMI were included. Further investigation is needed for evaluating efficacy of the suction in such patients. Thirdly, Further limitation might be that the study was a single-center study with short-term follow-up. The findings still need to be confirmed by further large-scale and multi-center studies.
Conclusion
A suction pressure of -10 cmH2O for 60 s after pleural incision is recommended in uniport VATS to speed lung collapse without the occurrence of occult lung injury.
Data availability
Data is provided within the manuscript.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- AUC:
-
The area under the curve
- BB:
-
Bronchial blocker
- BIS:
-
Bispectral Index
- BMI:
-
Body Mass Index
- COPD:
-
Chronic obstructive pulmonary disease
- DLT:
-
Double-lumen endotracheal tube
- FiO2 :
-
Fraction of inspiration O2
- FOB:
-
Fiberoptic bronchoscopy
- FVC:
-
Forced vital capacity
- LCS:
-
Lung collapse scores
- MAP:
-
Mean arterial pressure
- NO:
-
Nitric oxide
- NOS-3:
-
Nitric oxide synthase type 3
- NVL:
-
Nonventilated lung
- OD:
-
Outer diameter
- OLV:
-
One lung ventilation
- PACU:
-
Post anesthesia care unit
- SD:
-
Standard deviation
- SE:
-
Standard error
- SpO2 :
-
Peripheral capillary oxygen saturation
- VATS:
-
Video-assisted thoracoscopic thoracic surgery
- W/D:
-
Wet-dry
References
Yoo JY, Kim DH, Choi H, Kim K, Chae YJ, Park SY. Disconnection technique with a bronchial blocker for improving lung deflation: a comparison with a double-lumen tube and bronchial blocker without disconnection. J Cardiothorac Vasc Anesth. 2014;28:904–7.
Xiang YY, Chen Q, Tang XX, Cao L. Comparison of the effect of double-lumen endotracheal tubes and bronchial blockers on lung collapse in video-assisted thoracoscopic surgery: a systematic review and meta-analysis. BMC Anesthesiol. 2022;22:330.
Palaczynski P, Szarpak L, Pruc M, Misiolek H, Szarpak L, Smereka J, et al. Systematic review and meta-analysis of efficiency and safety of double-lumen tube and bronchial blocker for one-lung ventilation. J Clin Med. 2023;12.
Li YL, Hang LH. Recommendations and considerations for speeding the collapse of the non-ventilated lung during single-lung ventilation in thoracoscopic surgery: a literature review. Minerva Anestesiol. 2023;89:792–803.
Yang W, Liu Z, Yang C, Liu S, Guo M, Wen W, et al. Combination of nitrous oxide and the modified inflation-deflation method for identifying the intersegmental plane in segmentectomy: a randomized controlled trial. Thorac Cancer. 2021;12:1398–406.
Narayanaswamy M, McRae K, Slinger P, Dugas G, Kanellakos GW, Roscoe A, et al. Choosing a lung isolation device for thoracic surgery: a randomized trial of three bronchial blockers versus double-lumen tubes. Anesth Analg. 2009;108:1097–101.
El-Tahan MR. A comparison of the disconnection technique with continuous bronchial suction for lung deflation when using the Arndt endobronchial blocker during video-assisted thoracoscopy: a randomised trial. Eur J Anaesthesiol. 2015;32:411–7.
Quan X, Yi J, Huang Y, Zhang X, Shen L, Li S. Bronchial suction does not facilitate lung collapse when using a double-lumen tube during video-assisted thoracoscopic surgery: a randomized controlled trial. J Thorac Dis. 2017;9:5244–8.
Wang L, Liu D, Lu J, Zhang S, Yang X. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer. 2017;17:75.
Risse J, Szeder K, Schubert AK, Wiesmann T, Dinges HC, Feldmann C, et al. Comparison of left double lumen tube and y-shaped and double-ended bronchial blocker for one lung ventilation in thoracic surgery-a randomised controlled clinical trial. BMC Anesthesiol. 2022;22:92.
Lu Y, Dai W, Zong Z, Xiao Y, Wu D, Liu X, et al. Bronchial blocker versus left double-lumen endotracheal tube for one-lung ventilation in right video-assisted thoracoscopic surgery. J Cardiothorac Vasc Anesth. 2018;32:297–301.
Liang C, Lv Y, Shi Y, Cang J, Miao C. The fraction of nitrous oxide in oxygen for facilitating lung collapse during one-lung ventilation with double lumen tube. BMC Anesthesiol. 2020;20:180.
Amatullah H, Maron-Gutierrez T, Shan Y, Gupta S, Tsoporis JN, Varkouhi AK, et al. Protective function of DJ-1/PARK7 in lipopolysaccharide and ventilator-induced acute lung injury. Redox Biol. 2021;38:101796.
Xu L, Bao HG, Si YN, Han L, Zhang R, Cai MM, et al. Effects of adiponectin on acute lung injury in cecal ligation and puncture-induced sepsis rats. J Surg Res. 2013;183:752–9.
Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–41.
Funcke S, Pinnschmidt HO, Wesseler S, Brinkmann C, Beyer B, Jazbutyte V, et al. Guiding opioid administration by 3 different analgesia nociception monitoring indices during general anesthesia alters intraoperative sufentanil consumption and stress hormone release: a randomized controlled pilot study. Anesth Analg. 2020;130:1264–73.
Blakeman TC, Scott JB, Yoder MA, Capellari E, Strickland SL. AARC clinical practice guidelines: artificial airway suctioning. Respir Care. 2022;67:258–71.
Czarnik RE, Stone KS, Everhart CC Jr, Preusser BA. Differential effects of continuous versus intermittent suction on tracheal tissue. Heart Lung. 1991;20(2):144–51.
Ware LB, Summar M. Understanding the role of NOS-3 in ventilator-induced lung injury: don’t take NO for an answer. Am J Physiol Lung Cell Mol Physiol. 2010;299:L147–9.
Pfitzner J, Peacock MJ, McAleer PT. Gas movement in the nonventilated lung at the onset of single-lung ventilation for video-assisted thoracoscopy. Anaesthesia. 1999;54:437–43.
Cheng L, Liu S, Qi W, Dong Y. The incidence of Tracheal Bronchus in thoracic surgery patients and its implication for lung isolation: a retrospective cohort study. J Cardiothorac Vasc Anesth. 2020;34:3068–72.
Campos JH. Which device should be considered the best for lung isolation: double-lumen endotracheal tube versus bronchial blockers. Curr Opin Anaesthesiol. 2007;20:27–31.
Kawanishi R, Kakuta N, Sakai Y, Hari Y, Sasaki H, Sekiguchi R, et al. Desflurane improves lung collapse more than propofol during one-lung ventilation and reduces operation time in lobectomy by video-assisted thoracic surgery: a randomized controlled trial. BMC Anesthesiol. 2022;22:125.
Acknowledgements
Jia-Jun JU conducted the data acquisition and performed the clinical studies. Shi-Yu ZHANG performed statistical analysis of the data. We thank all the participants and study staffs involved in this study for their great contributions.
Funding
This research was funded by Medical Scientific Research Project of Jiangsu Provincial Health Commission in 2021 (M2021072), Kunshan High-level Medical Talents Project (Ksgccrc2004), Medical Health Science and Technology Innovation Project of Kunshan First People’s Hospital (KSKFQYLWS2023005).
Author information
Authors and Affiliations
Contributions
Yulin Li wrote the manuscript, Haihui Huang prepared the manuscript and acquired the data. Lihua Hang performed the validation. All authors read and approved the final version of the manuscript. Yulin Li and Haihui Huang contributed to the work equally and should be regarded as co-first authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was approved by the ethics committee of The First People’s Hospital of Kunshan (approval No. 2022-03-014-K01). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Huang, H. & Hang, L. Comparison of efficacy and safety of different suction pressure for speeding non-ventilated lung collapse in uniport video-assisted thoracoscopic surgery: a randomized-controlled trial. BMC Surg 24, 247 (2024). https://doi.org/10.1186/s12893-024-02539-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-024-02539-4