Abstract
Background
To analyze the clinical outcomes after topography-guided femtosecond laser–assisted laser in situ keratomileusis (FS-LASIK) with Phorcides Analytic Engine (PAE) algorithm or Custom-Q FS-LASIK for myopia with nonastigmatic eyes.
Methods
In this retrospective study, a total of 90 eyes with myopia without manifest astigmatism (82 patients) were included. All surgeries were performed by topography-guided FS-LASIK planned with a PAE algorithm (42 eyes) or Custom-Q system (48 eyes). Refractive, visual outcomes and corneal aberrations were compared between the two groups.
Results
At 6 months postoperatively, the postoperative uncorrected distance visual acuity (UDVA) was 20/20 or better in 42 eyes (100%) in the PAE compared with 44 eyes (92%) in Custom-Q (P = .120). The postoperative UDVA of 20/16 or better was measured in 92% of eyes in the PAE group and 81% of eyes in the Custom Q group (P = .320). Postoperative corrected distance visual acuity, manifest refractive spherical equivalent and refractive astigmatism were similar between the two groups (P > .05). The postoperative optical path difference (OPD) and Strehl ratio (SR) were significantly better in the PAE group compared with the Custom Q group.
Conclusions
Topography-guided FS-LASIK with PAE algorithm or Custom Q demonstrated similar refractive efficacy and predictability. PAE for the patients with zero manifest astigmatism demonstrated better results in correcting corneal aberrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
With the prevalence of refractive surgery is increasing, femtosecond laser–assisted laser in situ keratomileusis (FS-LASIK) has been the preferred refractive surgery for correction of myopia and astigmatism [1]. Treatment of astigmatism in excimer laser ablation vision correction procedures is commonly done by two strategies: ocular refraction of the eye or the shape of the cornea [2]. Wavefront optimized ablation (WFO) treats the refractive astigmatism based on the manifest refraction with an additional ablation in the periphery to correct the positive spherical aberration [3, 4]. Custom-Q ablation algorithm is also based on the manifest refraction which can establish a target asphericity [4, 5]. Besides, topography-guided customized ablation treatment (TCAT) was originally developed as a method to regularize the uniform corneal surface and neutralize corneal irregularities [6, 7]. The two strategies rarely coincide in magnitude or axis [8]. This difference can be precisely described by ocular residual astigmatism (ORA), relevant to the corneal irregularity, lenticular astigmatism, and other nonoptical perceptive factors [2, 9,10,11,12]. The Phorcides Analytic Engine (PAE) algorithm combines the anterior corneal astigmatism, topographic irregularities, posterior corneal astigmatism and lenticular astigmatism when determining the optimal treatment of an eye with topography-guided FS-LASIK, which have been shown to be effective [13,14,15,16].
The aim of our study was to present the outcomes of topography-guided FS-LASIK for correcting myopia in patients planned by PAE. Previously, we compared the clinical outcomes in FS-LASIK planned with the PAE when the ORA was great or not [16]. Our attention was drawn to a specific patient cohort: those with myopia but no astigmatism. These individuals exhibited exceptional preoperative visual acuity, higher ORA, and responsive postoperative indicators. Thus, for this study, to simplify the astigmatism vector analysis, amplify the effects of PAE, and reduce interference factors, we retrospectively included patients with zero manifest astigmatism.
Moreover, we enrolled the Custom-Q cohort as a reference to facilitate comparison of visual outcomes. Our primary focus was on evaluating the outcomes and accuracy of the PAE algorithm in topography-guided FS-LASIK, rather than solely emphasizing the discrepancies in the effectiveness between the two ablation profiles. To the best of our knowledge, no previous studies have been conducted on patients without manifest astigmatism. In this study, we present the results based on the safety, efficacy, and accuracy of FS-LASIK at the 6-month follow-up in each group.
Patients and methods
Patients
In this retrospective study, 90 eyes with manifest myopia but without astigmatism that required refractive correction at Peking University Third Hospital from January 2018 to September 2022 were analyzed. All patients were sufficiently informed consent of the two algorithms, and the selection of the surgical approach was non-randomized based on preoperative examination and patient preference. Patients meeting the inclusion criteria were consecutively enrolled. The study received approval from the ethics committee of our institute(#KYC2023-363) and was conducted in accordance with the tenets of the Declaration of Helsinki. A written informed consent was obtained from each patient prior to the surgical procedure.
The inclusion criteria were age between 18 and 49 years, medically suitable for LASIK, having zero manifest astigmatism in noncycloplegic refraction and only myopia with corrected distance visual acuity (CDVA) no worse than 20/25, with a documented refractive stability for a minimum period of 1 year and discontinuation of soft contact lenses for at least 2 weeks. Exclusion criteria were: anisometropia greater than 1.50 D in the spherical equivalent, corneal thickness of less than 500 μm or predictive postoperative residual stromal bed less than 280 μm, topographic and/or tomographic evidence of corneal ectasia, previous ocular surgery, history of herpetic eye disease, corneal scarring, collagen vascular disease, pregnancy, and lactation.
Preoperative examinations
Preoperative evaluation included UDVA and CDVA, manifest cycloplegic and noncycloplegic refraction, slit-lamp biomicroscopy, dilated fundus evaluation, corneal thickness, combined corneal topography and tomography (Sirius; CSO, Florence, Italy), and corneal topography (Fig. 1, Vario Topolyzer, WaveLight; Alcon Laboratories, Inc., Fort Worth, TX). The total corneal aberrations and optic quality in a 6-mm zone were obtained from combined corneal topography and tomography. Parameters of corneal aberrations and optical quality included optical path difference (OPD); root mean square (RMS) of higher order aberrations (HOAs), corneal astigmatism, spherical aberration (SA), coma and Strehl ratio (SR). Corneal cylinder data was obtained from the Vario Topolyzer Placido-based topography.
Surgical procedures
All surgeries were performed by an experienced refractive surgeon (YC) under topical anesthesia. All flaps were created by the WaveLight FS200 femtosecond laser (Alcon Laboratories, Inc). The flap/canal/hinge parameters were: flap thickness of 110 μm; flap diameter of 8.5 to 9 mm; side-cut angle of 100°; hinge angle of 50°; and canal width of 1.5 mm. Following blunt dissection and flap lifting, the stromal bed was ablated with an excimer laser (EX500 WaveLight) using an optical zone of 6.5 mm with a 1.25-mm transition zone.
The software of PAE was offered by Dr. Mark Lobanoff. In the calculate table, after carefully checking and amending the transferred data, we input anterior and posterior corneal negative cylinder (power and axis) of 5 mm zone from Sirius combined topography and tomography [16]. Then, PAE uses geographic imaging software (GIS) to analyze the corneal topographic treatment image produced by the Contoura® Vision planning laptop. Each talus radius is measured, and its refractive effect is analyzed (Fig. 2). The refraction data (sphere, cylinder, and axis) used for all the PAE cases followed the calculated recommended results with PAE (Fig. 3).
The PAE uses geographic imaging software to analyze the corneal topographic treatment image. The talus from Fig. 2 is corresponding to the irregularities image from Fig. 1, and its refractive effect is analyzed
The PAE determines the lower-order astigmatic vector created by all sources (anterior cornea, posterior cornea and lenticular) and uses vector addition to calculate the final astigmatic vector. The figure (marked in red box) showing a manifest astigmatism is zero, but the anterior corneal cylinder and modified cylinder are not
The physiological Q-value provided preoperatively by the Topolyzer was taken as reference. The postoperative Q-target was programmed to be equal to the preoperative physiological Q. The ablation profile was centered on an estimated visual axis determined by the topographer (taking 75% of the pupil toward the corneal vertex [offset value]), which closely approximates the visual axis.
Infrared images of the iris were captured with the Topolyzer Vario topographer to control static and dynamic cyclotorsion during surgery. Patients were requested to focus on a pulsing green fixation light throughout the ablation. All treatments were centered on the pupil and all surgeries were programmed for emmetropia using the treatment nomogram provided by the manufacturer.
Postoperative care and follow-up
Postoperatively, all eyes received treatment with 0.1% fluorometholone (FML; Allergan pharmaceuticals, Dublin, Ireland) in tapering dose for 4 weeks, 0.5% levofloxacin (Cravit; Santen Pharmaceutical (China) Co. Ltd., Jiangsu, China) four times a day for 2 weeks, and lubricating drops four times a day for at least 4 weeks. The follow-up visits data had been obtained for stability at least 6 months. The follow-up examinations involved measurements of UDVA, slit-lamp examination, manifest refraction, CDVA, and combined corneal topography and tomography.
Statistical analysis
The Shapiro–Wilk test was used for confirming normality of data. Results were expressed as mean ± SD (range); normally distributed data were analyzed using t test between the two groups and the non-normally distributed data were analyzed using Wilcoxon signed-rank test preoperatively. The linear mixed model was applied considering the relevance between eyes in individual patients post operatively. The paired t-test was used to assess differences between preoperative and postoperative parameters under the assumption of normality, and Wilcoxon signed-rank test was used in case the data were not normally distributed. Comparison of the percentage of eyes between the preoperative and the postoperative used the chi-square test and Fisher exact test. Linear regression analyses were performed to compare achieved vs attempted outcomes. In corneal vector analysis, the keratometric refractive index (1.3375) was used. Data were analyzed using SPSS software (version 25.0; SPSS, Inc., Chicago, IL). A P value of less than 0.05 was considered statistically significant.
The results were based on the standard for reporting astigmatism outcomes of refractive surgery [17].
Results
This study included 90 eyes of 82 patients, among them 67 were women and 15 were men. Table 1 contained the two groups preoperative parameters. They were reasonably matched in Age, UDVA, sphere and CDVA. No significant differences were observed between the two groups.
Visual acuity, efficacy, and safety
The mean logMAR UDVA improved significantly 6 months postoperatively in both groups (P < 0.001) (Tables 1 and 2). No significant differences were observed in postoperative UDVA, Sphere, Cylinder, MRSE, CDVA between the two groups.
Figure 4A showed the distribution of monocular postoperative UDVA and preoperative CDVA. The preoperative CDVA was 20/20 or better in 41 eyes (98%) in the PAE compared with 47 eyes (98%) in Custom-Q, with no statistical significance (P > 0.99). The postoperative UDVA was 20/20 or better in 42 eyes (100%) in the PAE compared with 44 eyes (92%) in Custom-Q, with no statistical significance (P = 0.120). The preoperative CDVA was 20/16 or better in 37 eyes (88%) in the PAE compared with 43 eyes (90%) in Custom-Q, with no statistical significance (P = 0.823). The postoperative UDVA was 20/16 or better in 39 eyes (92%) and 39 eyes (81%) in the PAE and Custom-Q, respectively (P = 0.320).
Visual outcomes after PAE and Custom-Q FS-LASIK. A Cumulative 6-month postoperative UDVA and preoperative CDVA. Changes in the Snellen lines of postoperative UDVA (B) and CDVA (C), compared with preoperative CDVA. D The relationship between the attempted and achieved spherical equivalent (SEQ). E The accuracy of the SEQ compared with the intended target. F The comparative distribution of preoperative and 6-month postoperative astigmatism
Figure 4B showed the differences between preoperative CDVA and postoperative UDVA. At the 6-month follow-up, 3 eyes (7%) in the PAE (B1) and 6 eyes (13%) in the Custom-Q (B2) achieved 1 or more Snellen lines of UDVA compared with the preoperative CDVA. No significant differences were observed between the two groups (P = 0.157).
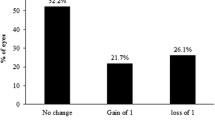
Figure 4C showed the changes in monocular CDVA. 11 eyes (26%) in the PAE (C1) and 13 eyes (27%) in the Custom-Q (C2) achieved 1 or more Snellen lines of postoperative CDVA compared with the preoperative CDVA. No significant differences were observed between the two groups (P = 0.828).
Accuracy
Figure 4D showed the relationship between the attempted and achieved SE. (coefficient of determination R2 = 0.997 in the PAE and R2 = 0.998 in the Custom-Q).
Figure 4E showed the accuracy of SE to the intended target. At the 6-month follow-up, 23 eyes (55%) in the PAE and 25 eyes (52%) in the Custom-Q achieved an SE between -0.13 D and + 0.13 D, respectively. No significant differences were observed between the two groups (P = 0.969).
Figure 4F showed the distribution of monocular preoperative & postoperative astigmatism. 33 eyes (79%) in the PAE and 41 eyes (85%) in Custom-Q achieved a postoperative refractive astigmatism of 0.25 D or less. No significant differences were observed between the two groups (P = 0.176).
Higher-order aberrations
Table 3 demonstrated the changes in corneal aberrations and Strehl ratio (SR) in 6-mm optical zone in two groups. No significant differences were observed between the two groups preoperatively (P = 0.122; P = 0.783; P = 0.075; P = 0.577; P = 0.743; P = 0.249). At postoperative 6 months, there were statistically significant differences in OPD (P = 0.044) and SR (P = 0.027).
The bottom row demonstrates the delta values between postoperatively and preoperatively in corneal aberrations in the two groups. There were significant different in OPD, AST RMS and SR in two groups.
Discussion
The discrepancy between corneal astigmatism and manifest astigmatism, which is crucial for determining the appropriate surgical treatment, has been a long-standing issue [8]. This difference may result in excess corneal astigmatism and resultant vision decreased [18]. We previously compared the clinical outcomes between TCAT LASIK and WFO LASIK in myopic patients [19]. The outcomes indicated that TCAT induced less corneal optical path difference, fewer higher order aberrations, and less coma than WFO LASIK. But it was not as accurate as WFO, especially in astigmatism correction. Therefore, a new alternative PAE combines the advantages of topography-guided LASIK while not being constrained solely by corneal astigmatism magnitude and axis as inputs to the ablation algorithm. This approach may thus provide an ablation pattern that is more responsive to corneal asymmetries.
The goal of this study was to determine whether using the PAE algorithm with topography-guided FS-LASIK improved clinical outcomes. It involved a retrospective evaluation of a specific patient cohort consisting of individuals with myopia but no manifest astigmatism. Although their manifest refraction might measure zero astigmatism, this could be artificially influenced by corneal high-order aberrations [20]. PAE, separating high-order aberrations from lower order aberrations to some extent improved the best corrected visual acuity after the refractive surgery. This clinical trial demonstrated for the first time the efficacy of correcting myopia with zero manifest astigmatism using topography-guided FS-LASIK.
ORA had been reported as a potentially limiting factor in the predictability of refractive correction [21,22,23]. A recently conducted large retrospective study [12] suggested that ORA in Chinese adults with myopia was affected by multiple ocular factors and the distribution was 0.74 ± 0.39 D, which was similar with the mean preoperative ORA in the PAE group(-0.87 ± 0.29) and the Custom-Q group(-0.79 ± 0.38). Previous studies found that using the PAE for topography-guided surgery planning and Custom-Q ablation treatments for the correction of myopia with astigmatism yielded excellent visual and refractive results [5, 13,14,15]. Lobanoff et al. noted that more than 94% of patients in the PAE group had 20/20 or better UDVA postoperatively, 62.5% had 20/16 or better UDVA [13]. Brunson et al. found that 60% of eyes had UDVA of 20/15 and 99% had UDVA of 20/20 using the PAE [14]. Alves EM et published that 87% in the Custom-Q group had a UDVA of 20/16 at the end of 12 months, and 100% in reached a UDVA of 20/25 or better [5]. Our study showed that 92% in Custom-Q and 100% in PAE had 20/20 or better UDVA postoperative. 74% of eyes had no change in CDVA in PAE and 71% in the Custom-Q. 26% achieved 1 or more Snellen lines of CDVA in PAE and 27% in the Custom-Q. The higher likelihood of 20/16 UDVA postoperative and relatively lower changes in the Snellen lines of postoperative CDVA compared with preoperative CDVA due to the difference in the relatively higher preoperative UDVA in our study.
The corneal aberrations and SR were found to be correlated with visual outcomes after refractive corneal surgery. Pre-existing HOAs can be exacerbated by LASIK due to the ablation process itself, as well as various other factors such as corneal dehydration and decentration [24, 25]. In this study, as the cases without astigmatism companied with better corneal aberrations preoperative, the postoperative parameters (RMSh, Coma RMS, SA RMS) in higher-order aberration failed to show superiority. However, the postoperative OPD and SR in PAE group is better than Custom-Q. SR, as a visual image quality metric, had been shown to be predictive of subjective best focus and well correlated with change in visual performance [26]. Additionally, the delta values between postoperative and preoperative corneal aberrations demonstrated significant differences in OPD, AST RMS, and SR between the two groups. This finding serves as a reminder that individuals with myopia but without astigmatism still require treatment for their corneal irregularities. Custom-Q ablation attempts to maintain the original shape of the cornea, while the TCAT aims to regularize the uniform corneal surface and neutralize corneal irregularities [5]. The better result may be attributed to the theoretical benefits of topography guided ablation with PAE algorithms, which is more responsive to asymmetry on the cornea and considered the topographic irregularities [13].
Previously, some studies had discussed the limitations of the PAE and attempted to demonstrate its inaccuracy [27,28,29]. They disagreed with Phorcides’ assumptions that astigmatism posterior to the anterior corneal surface is significant, requires treatment. In this study, we enrolled patients without manifest astigmatism, ensuring that the ORA equaled the astigmatism of the anterior corneal surface. The excellent postoperative visual acuity, along with comparable data in postoperative sphere, cylinder, and MRSE, suggested that the PAE was as accurate as Custom-Q, with the stable corneal aberrations. This demonstrated that in patients without manifest astigmatism, PAE not only addressed the limitations of previous studies on TCAT [19] but also achieved comparable refractive accuracy to conventional refraction algorithms while preserving its advantages to achieve better HOA outcomes. However, this conclusion is only based on patients without manifest astigmatism, which reduced the interference of noncycloplegic refraction error. However, achieving excellent clinical outcomes from theoretical calculations, particularly in terms of the accuracy of subjective examination, requires more comprehensive consideration. The concept of PAE was highly appealing, yet the precise treatment calculation demanded accurate preoperative measurements to meet its requirements effectively.
There were limitations to this study. The small sample size was due to the rare occurrence of myopia with zero manifest astigmatism. Furthermore, we only compared visual acuity, refraction, and corneal aberrations between the two groups; we did not have additional data such as subjective satisfaction. In addition, several flap related parameters could contribute to cylindrical changes. Lastly, this study had a retrospective design. For stronger evidence, a prospective, randomized, double-blind comparative study in normal contralateral eyes would be more convincing.
In conclusion, the results of visual acuity, efficacy, safety, accuracy were similar in the two groups. Topography guided ablation with PAE algorithms for patients with zero manifest astigmatism demonstrated better results in correcting corneal aberrations. Further understanding of topography guided with PAE algorithms to tolerate deviation and obtain more accurate refractive results was expected.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- FS-LASIK:
-
Femtosecond laser–assisted laser in situ keratomileusis
- PAE:
-
Phorcides Analytic Engine
- UDVA:
-
Uncorrected distance visual acuity
- AST RMS:
-
Root mean square of corneal astigmatism
- SR:
-
Strehl ratio
- WFO:
-
Wavefront optimized ablation
- TCAT:
-
Topography-guided customized ablation treatment
- ORA:
-
Ocular residual astigmatism
- CDVA:
-
Corrected distance visual acuity
- OPD:
-
Optical path difference
- RMS:
-
Root mean square
- HOAs:
-
Higher order aberrations
- SA:
-
Spherical aberration
- GIS:
-
Geographic imaging software
References
Kim TI, Barrio J, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. 2019;393(10185):2085–98.
Arbelaez MC, Alpins N, Verma S, Stamatelatos G, Arbelaez JG, Arba-Mosquera S. Clinical outcomes of laser in situ keratomileusis with an aberration-neutral profile centered on the corneal vertex comparing vector planning with manifest refraction planning for the treatment of myopic astigmatism. J Cataract Refract Surg. 2017;43(12):1504–14.
Manche E, Roe J. Recent advances in wavefront-guided LASIK. Curr Opin Ophthalmol. 2018;29(4):286–91.
Koller T, Iseli HP, Hafezi F, Mrochen M, Seiler T. Q-factor customized ablation profile for the correction of myopic astigmatism. J Cataract Refract Surg. 2006;32(4):584–9.
Alves EM, Lyra AF, Tenório M, Mesquita N, Bacelar C, Montenegro A, Alves L, Alves M. Femtosecond laser-assisted in situ keratomileusis with topography-guided or asphericity-adjusted derived data: a comparative contralateral eye study. BMC Ophthalmol. 2022;22(1):189.
De Stefano VS, Meister C, Ehlke GL, Krueger RR. Analysis of planning strategies in primary eyes gaining a line or more of visual acuity after topography-guided laser in situ keratomileusis. J Cataract Refract Surg. 2019;45(3):321–7.
Stulting RD, Fant BS, Bond W, Chotiner B, Durrie D, Gordon M, Milauskas A, Moore C, Slade S, Randleman JB, Stonecipher K. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. J Cataract Refract Surg. 2016;42(1):11–8.
Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg. 1997;23(1):65–75.
Wallerstein A, Gauvin M, Qi SR, Bashour M, Cohen M. Primary topography-guided LASIK: treating manifest refractive astigmatism versus topography-measured anterior corneal astigmatism. J Refract Surg. 2019;35(1):15–23.
Wallerstein A, Gauvin M, Cohen M. WaveLight(®) Contoura topography-guided planning: contribution of anterior corneal higher-order aberrations and posterior corneal astigmatism to manifest refractive astigmatism. Clin Ophthalmol. 2018;12:1423–6.
Read SA, Collins MJ, Carney LG. A review of astigmatism and its possible genesis. Clin Exp Optom. 2007;90(1):5–19.
Tang C, Du Z, Wu G, Tan X, Zang S, Yu H, Hu Y. The distribution pattern of ocular residual astigmatism in Chinese myopic patients. Front Med. 2022;9:763833.
Lobanoff M, Stonecipher K, Tooma T, Wexler S, Potvin R. Clinical outcomes after topography-guided LASIK: comparing results based on a new topography analysis algorithm with those based on manifest refraction. J Cataract Refract Surg. 2020;46(6):814–9.
Brunson PB, Mann Ii PM, Mann PM, Potvin R. Clinical outcomes after topography-guided refractive surgery in eyes with myopia and astigmatism - comparing results with new planning software to those obtained using the manifest refraction. Clin Ophthalmol. 2020;14:3975–82.
Stulting RD, Durrie DS, Potvin RJ, Linn SH, Krueger RR, Lobanoff MC, Moshirfar M, Motwani MV, Lindquist TP, Stonecipher KG. Topography-guided refractive astigmatism outcomes: predictions comparing three different programming methods. Clin Ophthalmol. 2020;14:1091–100.
Yuan Y, Zhang Y, Sun T, Sun X, Zhao X, Chen Y. Topography-guided FS-LASIK with PAE algorithm and sirius tomography data for correction of myopia and myopic astigmatism. J Refract Surg. 2022;38(4):235–42.
Reinstein DZ, Archer TJ, Randleman JB. JRS standard for reporting astigmatism outcomes of refractive surgery. J Refract Surg. 2014;30(10):654–9.
Wolffsohn JS, Bhogal G, Shah S. Effect of uncorrected astigmatism on vision. J Cataract Refract Surg. 2011;37(3):454–60.
Zhang Y, Chen Y. A randomized comparative study of topography-guided versus wavefront-optimized FS-LASIK for correcting myopia and myopic astigmatism. J Refract Surg. 2019;35(9):575–82.
Stulting RD, Lobanoff M, Mann PM 2nd, Wexler S, Stonecipher K, Potvin R. Clinical and refractive outcomes after topography-guided refractive surgery planned using phorcides surgery planning software. J Cataract Refract Surg. 2022;48:1010–5.
Kugler L, Cohen I, Haddad W, Wang MX. Efficacy of laser in situ keratomileusis in correcting anterior and non-anterior corneal astigmatism: comparative study. J Cataract Refract Surg. 2010;36(10):1745–52.
Qian Y-S, Huang J, Liu R, Chu R-Y, Xu Y, Zhou X-T, Hoffman MR. Influence of internal optical astigmatism on the correction of myopic astigmatism by LASIK. J Refract Surg. 2011;27(12):863–8.
Archer TJ, Reinstein DZ, Piñero DP, Gobbe M, Carp GI. Comparison of the predictability of refractive cylinder correction by laser in situ keratomileusis in eyes with low or high ocular residual astigmatism. J Cataract Refract Surg. 2015;41(7):1383–92.
Agarwal S, Thornell E, Hodge C, Sutton G, Hughes P. Visual outcomes and higher order aberrations following LASIK on eyes with low myopia and astigmatism. Open Ophthalmol J. 2018;12:84–93.
Jung HH, Ji YS, Oh HJ, Yoon KC. Higher order aberrations of the corneal surface after laser subepithelial keratomileusis. Korean J Ophthalmol. 2014;28(4):285–91.
Hastings GD, Marsack JD, Nguyen LC, Cheng H, Applegate RA. Is an objective refraction optimised using the visual Strehl ratio better than a subjective refraction? Ophthalmic Physiol Opt. 2017;37(3):317–25.
Wallerstein A, Gauvin M. Is phorcides more likely to give better vision than treating the manifest refraction? J Cataract Refract Surg. 2020;46(10):1451–2.
Motwani M. Predictions of residual astigmatism from surgical planning for topographic-guided LASIK based on anterior corneal astigmatism (LYRA Protocol) vs the phorcides analytic engine. Clin Ophthalmol. 2020;14:3227–36.
Motwani M. Clinical outcomes after topography-guided refractive surgery in eyes with myopia and astigmatism-comparing results with new planning software to those obtained using the manifest refraction [Letter]. Clin Ophthalmol. 2021;15:491–3.
Acknowledgements
Not applicable.
Funding
The authors have no financial relationships to disclose.
Author information
Authors and Affiliations
Contributions
XR, ZZ and RY was mainly to manage the patient, YF reviewed the patients’ records and wrote the manuscript. GY were involved in the process of diagnosis and management. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This present study was approved by Ethics Committee in Peking University Third Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, Y., Zhao, X., Dong, R. et al. Clinical outcomes after topography-guided FS-LASIK for myopia with nonastigmatic eyes. BMC Ophthalmol 24, 325 (2024). https://doi.org/10.1186/s12886-024-03593-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03593-5