Abstract
Purpose
To evaluate the clinical results of primary topography-guided femtosecond laser-assisted in situ keratomileusis (TG-FS-LASIK) using the Pentacam-measured anterior corneal astigmatism axis (ACA) or manifest refractive astigmatism axis (MRA).
Methods
In this prospective cohort study, all eyes were treated with primary TG-FS-LASIK using the manifest cylinder. Thirty-two right eyes were treated using ACA with axis disparity > 5° in the experimental group, and 32 right eyes were treated using MRA with axis disparity ≤ 5° in the control group. Visual, refractive outcomes, and corneal higher-order aberrations were evaluated. Vector analysis of astigmatism was performed using Alpins method with the ASSORT software.
Results
The mean logMAR UDVA in the experimental group was − 0.12 ± 0.06, − 0.05 ± 0.08, and − 0.08 ± 0.08 at 1-week, 1-month, and 3-month follow-up, whereas − 0.05 ± 0.06, − 0.12 ± 0.06, and − 0.14 ± 0.06 in the control group (p = 0.017, p < 0.001, and p = 0.003). At 3-month follow-up, 79% eyes achieved a UDVA of 20/16 or better, 9% gained one line of corrected distance visual acuity, the mean manifest cylinder was − 0.375 ± 0.254 D, 84% showed a manifest cylinder within ± 0.50 D, the mean magnitude of difference vector was 0.41 ± 0.35 D, the mean absolute angle of error (AE) was 7.36 ± 6.21°, and 41% exhibited an arithmetic AE within ± 5° in the experimental group, whereas 94%, 19%, − 0.203 ± 0.148 D, 100%, 0.21 ± 0.15 D, 3.39 ± 3.68°, and 69% in the control group (all p < 0.05). The postoperative and preoperative corneal spherical aberrations were comparable in both groups (p > 0.05).
Conclusion
Primary topography-guided FS-LASIK using Pentacam-measured anterior corneal astigmatism axis exhibited slightly inferior clinical results to that using the manifest refractive astigmatism axis. Both methods achieved comparable postoperative and preoperative corneal spherical aberrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Manifest refractive astigmatism is widely used in refractive surgery plan. It represents the astigmatism related to the anterior corneal higher-order aberrations (HOAs), the anterior and posterior cornea, internal optics, and cortical perception [1]. The primary topography-guided excimer ablation is designed to eliminate the anterior corneal HOAs for the virgin eye to get good visual quality. However, the contribution of corneal HOAs to manifest refractive astigmatism is not fully understood. That equivalent astigmatism should be subtracted from the manifest refractive astigmatism when planning topography-guided excimer ablation to get good refractive predictability. Since astigmatism is a vector, how to accurately design the astigmatism magnitude and axis for surgery is the biggest challenge for refractive surgeons.
The confirmed FDA protocol for primary topography-guided excimer ablation has strict patient inclusion criteria. Not all virgin eyes meet this protocol [2]. Several other planning algorithms, including topography-modified refraction (TMR) [3], the Layer Yolked Reduction of Astigmatism protocol [4], the Phorcides Analytic Engine [5], and mutual comparative analysis [6], have been developed. Currently, there is still no gold-standard protocol for this type of surgery design.
With axis disparity of manifest astigmatism and anterior corneal astigmatism, the anterior corneal astigmatism magnitude and axis are used under TMR protocol. But astigmatism is often overcorrected because of the ocular residual astigmatism (ORA) compensation [7]. Wallerstein et al. [8] analysed the patients undergoing primary topography-guided laser in situ keratomileusis (LASIK) treated using refractive astigmatism magnitude. One group was treated with WaveLight Contoura-measured anterior corneal astigmatism axis (ACA), and the other group was treated with manifest refractive astigmatism axis. He found that no matter with small axis discrepancy (5° < axis disparity < 20°) or with large axis discrepancy (21° < axis disparity < 45°), the primary topography-guided LASIK treated using Contoura-measured ACA showed inferior refractive and visual outcomes. So, we tried to use manifest cylinder and Pentacam-measured ACA to perform primary topography-guided LASIK for patients with small axis disparity. The visual, refractive outcomes, and astigmatism correction were compared with patients treated using FDA protocol. The changes in corneal HOAs were also analysed and compared.
Patients and methods
Patient selection and grouping
This prospective cohort study enrolled 64 patients undergoing primary topography-guided femtosecond laser-assisted in situ keratomileusis (FS-LASIK) at Shenyang Aier Eye Hospital. Of these, thirty-two patients were enrolled in the experimental group, and 32 were enrolled in the control group. Only right eyes were selected for the analysis. All patients’ eyes exhibited a preoperative corrected distance visual acuity (CDVA) of 20/20 or better. The manifest cylinder was used for treatment in all eyes. In the experimental group, the anterior corneal astigmatism axis measured by Pentacam (PentacamHR, Oculus Optikgeräte GmbH, Wetzlar, Germany) was used for surgery design. The enrolled patients exhibited an astigmatic axis disparity of 6° or more between the Pentacam-measured anterior corneal astigmatism and manifest refractive astigmatism. The normal manifest refraction was performed first. And then with the same magnitude of sphere, the manifest cylinder and Pentacam-measured ACA were used to do the refraction again. If patients can get the same CDVA as that measured by manifest refraction, then they will be enrolled in the experimental group. In the control group, the manifest refraction was used for surgery design. These patients exhibited an astigmatic axis disparity of no more than 5° between the Pentacam-measured anterior corneal astigmatism and manifest refractive astigmatism.
The study protocol was approved by the Institutional Review Board/Ethics Committee. The study followed the tenets of the Declaration of Helsinki. All patients provided written informed consent for the study. Patients with a manifest cylinder less than 0.75 D and anterior corneal astigmatism magnitude less than manifest cylinder were excluded. The standard inclusion criteria for FS-LASIK were also required: age older than 18 years, stable refraction for at least 2 years, central corneal thickness of more than 480 µm, a calculated postoperative residual stromal bed thickness of more than 280 µm, no established keratoconus or forme fruste keratoconus as evidenced by corneal topography, no significant dry eye, and no previous history of ocular disease or surgery and no autoimmune diseases or medications that could affect wound healing.
Preoperative examinations
Preoperative examinations included uncorrected distance visual acuity (UDVA) measurement, CDVA measurement, pupil size evaluation, intraocular pressure measurement, manifest/cycloplegic manifest refraction, corneal pachymetry, slit-lamp examination, dilated fundoscopy, corneal tomography (PentacamHR), and topographic analysis with the Topolyzer Vario (Alcon Laboratories, Inc., Fort Worth, TX ).
Surgical planning
The Contoura images were acquired with the WaveLight Topolyzer VARIO (Alcon Laboratories), as described previously [9]. By zeroing out the sphere and cylinder correction in the Contoura treatment planning software, the HOA ablation pattern was verified to be consistent with the Pentacam anterior elevation map, and the maximum corneal HOA ablation depth (HOA-AD) was recorded. After entering the manifest cylinder and designed cylinder axis, the spherical refraction was then adjusted according to the equalization of C4 and C12 as well as the nomogram recommendation (A_LI_D1_Nomogramm STD_10_2007 Rev.0 Mar 2011) provided by Alcon Laboratories. All surgeries were performed by the same surgeon with the aim of plano correction for all eyes. The flap was created using the FS200 Femtosecond Laser (FS200, Alcon Laboratories), with a thickness and diameter of 110 μm and 8.5 mm, respectively. The hinge position was superior. Myopic ablation was accomplished using the EX500 Excimer Laser (WaveLight EX500, Alcon Laboratories). A 6.5-mm optical zone and 1.25-mm transition zone were used in all eyes. The antibiotics and steroids used postoperatively were the same as those described previously [10].
Postoperative examinations
Routine postoperative follow-up examinations were scheduled at 1 day, 1 week, 1 month, and 3 months. These included UDVA measurement, CDVA measurement, autorefractometry, non-contact tonometry, manifest refraction, slit-lamp microscopy, and measurement of corneal HOAs using Pentacam. All follow-up examinations were performed by the same technician.
Data and statistical analysis
Safety and predictability were evaluated. Astigmatism was analysed with vector analysis using the Alpin’s method [11] incorporated in the Assort software (Version 5.64, ASSORT Pty. Ltd). The ocular residual astigmatism was calculated. The vectors evaluated for astigmatic corrections were target-induced astigmatism (TIA), surgically induced astigmatism (SIA), angle of error (AE), difference vector (DV), and correction index (CI). The central 6-mm corneal total HOA, spherical aberration, vertical coma, and horizontal coma were analysed and compared.
SPSS for Windows (version 23; IBM, Armonk, NY) was used for all statistical analyses. The data were expressed as mean ± standard deviation. Snellen visual acuity values were converted to the logarithm of the minimum angle of resolution units (logMAR). The Kolmogorov–Smirnov test was used to evaluate data normality. Student’s t test or the Mann–Whitney U test was used according to the normality of parameters. The χ2 test was used to compare proportions. Correlation analyses were performed using Pearson’s method. A p value of less than 0.05 was considered statistically significant.
Results
Of the 64 enrolled patients, thirty-two patients were enrolled in the experimental group, and 32 patients were enrolled in the control group. Only the right eye was selected for the analysis. The preoperative patient information is shown in Table 1.
Visual acuity and safety
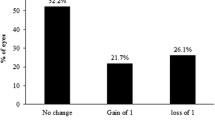
At 3 months postoperatively, 91% of the eyes achieved a UDVA of 20/20 or better, 79% achieved a UDVA of at least 20/16, and 16% achieved a UDVA of at least 20/12.5 in the experimental group, as compared to 100%, 94%, and 47% in the control group, respectively. There was a significant difference in the constituent ratio of UDVA between the groups (p = 0.007, F = 10.061) (Fig. 1A). None of the patients’ eyes lost one or more lines of CDVA, and 9% of the eyes in the experimental group and 19% in the control group gained one line of CDVA. The difference between the groups was significant (p = 0.042, F = 4.153) (Fig. 1B).
Refractive and visual outcomes at the 3-month follow-up in the experimental group and control group. (A) Cumulative Snellen UDVA at the 3-month postoperative follow-up. (B) Changes in lines of CDVA at the 3-month postoperative follow-up (safety). (C) Distribution of postoperative MRSE (predictability) at the 3-month postoperative follow-up. (D) Changes in UDVA after surgery. (E) Attempted versus achieved MRSE at the 3-month postoperative follow-up in the experimental group. (F) Attempted versus achieved MRSE at the 3-month postoperative follow-up in the control group. VA visual acuity, CDVA corrected distance visual acuity, D dioptre, MRSE manifest refraction spherical equivalent, UDVA uncorrected distance visual acuity, PCA group stands for the experimental group, MRA group stands for the control group. * statistical significance
From the 1-week follow-up to the 3-month follow-up, the UDVA increased gradually in both groups. However, the UDVA in the control group was better than that in the experimental group at each follow-up (all p < 0.05 by Mann–Whitney U test) (Fig. 1D). The constituent ratio of UDVA at each follow-up in both groups is shown in Table 2.
Predictability
At 3 months postoperatively, the mean manifest refractive spherical equivalent (MRSE) was 0.43 ± 0.33 D, and an MRSE within ± 0.50 D was achieved in 72% of the patients in the experimental group, whereas the corresponding values were 0.25 ± 0.33 D and 81% in the control group. There was a significant difference in the mean MRSE between the groups (p = 0.033), but not in the constituent ratio of MRSE between the groups (p = 0.133, F = 2.253) (Fig. 1C). There was a positive correlation between the achieved MRSE and the attempted MRSE in both groups (Fig. 1E, F).
Astigmatism correction
There was no significant difference in the constituent ratio of preoperative astigmatism between the groups (p = 0.804, F = 2.318) (Fig. 2A, B). There was a positive correlation between SIA and TIA in both groups (experimental group: p < 0.001, r = 0.631; control group: p < 0.001, r = 0.867) (Fig. 2C, D). At 3 months postoperatively in the experimental group, the mean manifest cylinder was − 0.375 ± 0.254 D, the mean absolute AE was 7.36 ± 6.21° (Fig. 2E), the mean magnitude of DV was 0.41 ± 0.35 D, the mean CI was 1.12 ± 0.38, 84% of the patients exhibited a manifest cylinder within ± 0.50 D (Fig. 2A), and 41% of the patients exhibited arithmetic AE within ± 5° (Fig. 2E). In the control group, the mean manifest cylinder was − 0.203 ± 0.148 D, the mean absolute AE was 3.39 ± 3.68° (Fig. 2F), the mean magnitude of DV was 0.21 ± 0.15 D, the mean CI was 1.04 ± 0.16, 100% of the patients showed a manifest cylinder within ± 0.50 D (Fig. 2B), and 69% of the patients exhibited arithmetic AE within ± 5° (Fig. 2F) (p = 0.002; p = 0.003; p = 0.003; p = 0.289; p < 0.001, F = 17.304; p < 0.001, F = 15.838, respectively).
Refractive outcomes and the results of the vector analysis of astigmatism correction at the 3-month follow-up in the experimental group and control group. (A) Distribution of preoperative and postoperative astigmatisms at the 3-month postoperative follow-up in the experimental group. (B) Distribution of preoperative and postoperative astigmatisms at the 3-month postoperative follow-up in the control group. (C) Target-induced astigmatism (TIA) versus surgically induced astigmatism (SIA) at the 3-month postoperative follow-up in the experimental group. (D) Target-induced astigmatism (TIA) versus surgically induced astigmatism (SIA) at the 3-month postoperative follow-up in the control group. (E) Distribution of postoperative angle of error at the 3-month postoperative follow-up in the experimental group. (F) Distribution of postoperative angle of error at the 3-month postoperative follow-up in the control group. D dioptre, TIA target-induced astigmatism, SIA surgically induced astigmatism. PCA group stands for the experimental group; MRA group stands for the control group
Corneal HOAs
There were no significant differences in preoperative and postoperative corneal total HOA, spherical aberration, vertical coma, and horizontal coma between the groups (p > 0.05) (Table 3). At the 3-month follow-up, there was almost no change between postoperative and preoperative corneal spherical aberrations in both groups (p > 0.05) (Table 3). However, the postoperative corneal total HOA, vertical coma, and horizontal coma increased in both groups (p < 0.05).
Discussion
In the current study, the manifest cylinder and Pentacam-measured anterior corneal astigmatism axis was used for the primary topography-guided FS-LASIK. The safety, predictability, and results of the vector analysis of astigmatic correction and corneal HOAs were analysed. The clinical results were compared with patients treated using manifest refractive astigmatism. We found that the UDVA values at the 1-week, 1-month, and 3-month follow-ups in the experimental group were inferior to those in the control group. At 3 months postoperatively, the proportion of patients with a UDVA of at least 20/16, mean MRSE, mean manifest cylinder, ratio of manifest cylinder less than 0.50 D, mean magnitude of DV, mean absolute AE, and ratio of absolute AE within ± 5° in the experimental group were all inferior to those in the control group. These differences were statistically significant. In both groups, the postoperative corneal spherical aberration did not increase significantly when compared to the preoperative corneal spherical aberration.
The clinical results at the 3-month follow-up were used for comparison. Studies have reported that wound healing tends to be stable at 3 months after corneal refractive surgery [12]. No significant epithelial remodelling was observed in the corneal central zone between 3 and 6 months postoperatively [13].
The magnitude of the anterior corneal astigmatism was once used for primary topography-guided excimer ablation. However, astigmatism was often overcorrected after the surgery [7]. This protocol does not take into account the intraocular astigmatism. Wallerstein et al. [14] analysed the relationship between manifest refractive astigmatism and anterior corneal astigmatism. They found that in 87% of eyes with with-the-rule corneal astigmatism, the manifest refractive astigmatism magnitude was less than the anterior corneal astigmatism magnitude, and in 89% of eyes with against-the-rule corneal astigmatism, the manifest refractive astigmatism magnitude was greater than the anterior corneal astigmatism magnitude. Koch et al. [15] reported that 86.6% of corneas exhibited against-the-rule posterior corneal astigmatism. This also explains the decreased manifest cylinder in most eyes with with-the-rule corneal astigmatism and the increased manifest cylinder in most eyes with against-the-rule corneal astigmatism. They found that 9% of patients’ eyes exhibited posterior corneal astigmatism more than 0.5 D. It may explain why astigmatism was overcorrected by the TMR protocol.
The method used to design the astigmatism axis is also important in primary topography-guided excimer ablation. It has been estimated that the cylindrical power will be undercorrected by 3.3% for every degree of off-axis correction [16]. In the current study, the anterior corneal astigmatic axis measured by Pentacam was used for surgical planning. The reasons for this were as follows: first, the pupil size changes at different light conditions. The astigmatic axis varies at different optical zones. It is easy to identify the anterior corneal astigmatic axis at different areas within the central corneal 8 mm by Pentacam refractive maps. It helps to guarantee the accuracy of the astigmatic axis correction, especially for patients with irregular corneal astigmatism. The area of Contoura-measured anterior corneal astigmatism is limited, ranging from central corneal 5 to 7 mm, with 0.50 mm at interval. Second, if the patient can achieve the best-corrected distant visual acuity using the anterior corneal astigmatic axis measured by Pentacam, surgeons can easily identify the laser ablation map with the anterior corneal elevation map. Third, it is convenient to check the magnitude and axis of posterior corneal astigmatism and its relation with anterior corneal astigmatism by Pentacam refractive maps. It helps to prevent astigmatic overcorrection.
Wallerstein et al. [8] analysed 369 patients undergoing WaveLight Contoura LASIK using the manifest cylinder and Contoura-measured anterior corneal astigmatism axis. They divided the patients into those with a small axis discrepancy (axis disparity of 5–20°) and those with a large axis discrepancy (axis disparity of 21–45°) based on the relationship between manifest refractive astigmatism and Contoura-measured anterior corneal astigmatism. We refined the grouping based on Wallerstein’s study [8]. The basic idea for the surgery design is that with comparable preoperative manifest astigmatism and comparable ocular residual astigmatism, whether the anterior corneal higher-order aberrations ablation using this surgery plan will cause too much ocular residual astigmatism compensation. And the lenticular astigmatism of the enrolled patients is very small and similar in both groups. The roughly calculated values were 0.35 ± 0.22D in the experiment group and 0.29 ± 0.18D in the control group. There was no statistical difference between the two groups (p = 0.277). We assumed that the dynamic changes of the small lenticular astigmatism would not affect the postoperative astigmatism. We tried to use manifest astigmatism magnitude and Pentacam-measured anterior corneal astigmatism axis for surgery design. The axis disparity between Pentacam-measured anterior corneal astigmatism and manifest refractive astigmatism is small (within 23°) in the experimental group. We tried to find whether this surgery design could get as good visual, refractive outcomes, and astigmatism correction as that treated with FDA protocol. The clinical results in the experimental group were slightly inferior to those treated with FDA protocol. With comparable postoperative corneal higher-order aberrations in both groups, the visual and refractive outcome differences may mainly come from not so accurate astigmatism correction in the experimental group. First, the astigmatism magnitude we used for surgery plan is not so precise. In order to reduce the astigmatism overcorrection under TMR protocol, the manifest cylinder was used for surgery plan. The anterior corneal higher-order aberrations will induce certain magnitude of astigmatism [17, 18]. We ignored that for this kind of patients in the experimental group. The ignored part will be compensated by the ocular residual astigmatism. Second, the astigmatism axis we tried to use for surgery plan is not so accurate. The absolute AE is higher in the experimental group than that in the control group. That will also add the errors for astigmatism correction.
The limitation of the current study is that patients exhibiting lenticular astigmatism were excluded. This needs to be further studied for primary Contoura topography-guided corneal surgery. In addition, the impact of corneal HOAs on the disparity in manifest cylinder was ignored. Although the method used in the current study is convenient, it is not accurate in primary topography-guided corneal refractive surgery planning.
In conclusion, with axis disparity between the Pentacam-measured anterior corneal astigmatism and manifest refractive astigmatism, primary topography-guided FS-LASIK using the Pentacam-measured anterior corneal astigmatism axis with a disparity > 5° exhibited slightly inferior clinical results to that performed using the manifest refractive astigmatism axis with a disparity ≤ 5°. The postoperative and preoperative anterior corneal spherical aberrations were similar 3 months after surgery in both groups. For complex corneal total HOAs, complex intraocular astigmatism, and their interactions with each other, as well as their interactions with lower-order aberrations, a more precise algorithm for primary topography-guided excimer ablation is still needed for best clinical results.
Data availability
Data and material are available upon reasonable request.
Code availability
Not applicable.
References
Alpins N (2017) Practical Astigmatism: Planning and Analysis. Slack Incorporated, New Jersey
Wallerstein A, Gauvin M, Qi SR, Cohen M (2020) Effect of the vectorial difference between manifest refractive astigmatism and anterior corneal astigmatism on topography-guided LASIK outcomes. J Refract Surg 36:449–458. https://doi.org/10.3928/1081597X-20200609-01
Kanellopoulos AJ (2016) Topography-modified refraction (TMR): adjustment of treated cylinder amount and axis to the topography versus standard clinical refraction in myopic topography-guided LASIK. Clin Ophthalmol 10:2213–2221. https://doi.org/10.2147/OPTH.S122345
Motwani M (2017) The use of WaveLight® Contoura to create a uniform cornea: the LYRA Protocol. Part 3: the results of 50 treated eyes. Clin Ophthalmol 11:915–921. https://doi.org/10.2147/OPTH.S133841
Lobanoff M, Stonecipher K, Tooma T, Wexler S, Potvin R (2020) Clinical outcomes after topography-guided LASIK: comparing results based on a new topography analysis algorithm with those based on manifest refraction. J Cataract Refract Surg 46:814–819. https://doi.org/10.1097/j.jcrs.0000000000000176
Cao K, Liu L, Zhang T, Liu T, Bai J (2020) Mutual comparative analysis: a new topography-guided custom ablation protocol referencing subjective refraction to modify corneal topographic data. Eye Vis (Lond) 7:36. https://doi.org/10.1186/s40662-020-00201-7
Kim J, Choi SH, Lim DH, Yoon GJ, Chung TY (2020) Comparison of outcomes after topography-modified refraction versus wavefront-optimized versus manifest topography-guided LASIK. BMC Ophthalmol 20:192. https://doi.org/10.1186/s12886-020-01459-0
Wallerstein A, Gauvin M, Qi SR, Bashour M, Cohen M (2019) Primary topography-guided LASIK: treating manifest refractive astigmatism versus topography-measured anterior corneal astigmatism. J Refract Surg 35:15–23. https://doi.org/10.3928/1081597X-20181113-01
Wallerstein A, Caron-Cantin M, Gauvin M, Adiguzel E, Cohen M (2019) Primary topography-guided LASIK: refractive, visual, and subjective quality of vision outcomes for astigmatism ⩾2.00 diopters. J Refract Surg 35:78–86. https://doi.org/10.3928/1081597X-20181210-01
Zhang Y, Chen Y (2019) A randomized comparative study of topography-guided versus wavefront-optimized FS-LASIK for correcting myopia and myopic astigmatism. J Refract Surg 35:575–582. https://doi.org/10.3928/1081597X-20190819-01
Alpins N (2001) Astigmatism analysis by the Alpins method. J Cataract Refract Surg 27:31–49. https://doi.org/10.1016/S0886-3350(00)00798-7
Shetty R, Francis M, Shroff R, Pahuja N, Khamar P, Girrish M, Nuijts RMMA, Sinha Roy A (2017) Corneal biomechanical changes and tissue remodeling after SMILE and LASIK. Invest Ophthalmol Vis Sci 58:5703–5712. https://doi.org/10.1167/iovs.17-22864
Fan L, Xiong L, Zhang B, Wang Z (2019) Longitudinal and regional non-uniform remodeling of corneal epithelium after topography-guided FS-LASIK. J Refract Surg 35:88–95. https://doi.org/10.3928/1081597X-20190104-01
Wallerstein A, Gauvin M, Cohen M (2018) WaveLight® Contoura topography-guided planning: contribution of anterior corneal higher-order aberrations and posterior corneal astigmatism to manifest refractive astigmatism. Clin Ophthalmol 12:1423–1426. https://doi.org/10.2147/OPTH.S169812
Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L (2012) Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg 38:2080–2087. https://doi.org/10.1016/j.jcrs.2012.08.036
Novis C (2000) Astigmatism and toric intraocular lenses. Curr Opin Ophthalmol 11:47–50. https://doi.org/10.1097/00055735-200002000-00007
Zhou W, Stojanovic A, Utheim TP (2016) Assessment of refractive astigmatism and simulated therapeutic refractive surgery strategies in coma-like-aberrations-dominant corneal optics. Eye Vis (Lond) 3:13. https://doi.org/10.1186/s40662-016-0044-8
Motwani M (2017) The use of WaveLight® Contoura to create a uniform cornea: the LYRA Protocol. Part 1: the effect of higher-order corneal aberrations on refractive astigmatism. Clin Ophthalmol 11:897–905. https://doi.org/10.2147/OPTH.S133839
Acknowledgements
We thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81670884 and No. 81873684).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (the Ethics Committee of Shenyang Aier Eye Hospital) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Luo, T., Fang, X. et al. Clinical results of topography-guided laser-assisted in situ keratomileusis using the anterior corneal astigmatism axis and manifest refractive astigmatism axis. Graefes Arch Clin Exp Ophthalmol 261, 247–256 (2023). https://doi.org/10.1007/s00417-022-05775-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05775-7