Abstract
Background
The activity and number of immune cells in the tumor microenvironment are closely related to the overall survival of patients with hepatocellular carcinoma (HCC). The sex-determining region Y-box 4 (SOX4) gene is abnormally expressed in various tumor tissues and is critical for tumor development. However, the correlation between SOX4 expression in HCC and tumor immunity is unclear.
Methods
SOX4 expression was explored using data from The Cancer Genome Atlas, and UALCAN databases. Real-time reverse transcription quantitative and western blotting were used to analyze SOX4 expression in several liver cancer cell lines. Additionally, correlations among SOX4 expression, cancer immune characteristics, and infiltrated immune cell gene marker sets in patients with HCC were analyzed using data from the Tumor Immune Estimation Resource, Gene Expression Profiling Interactive Analysis, and Tumor-Immune System Interactions databases. Moreover, we evaluated SOX4 expression in HCC tissues and the correlation of SOX4 expression with survival rate. Subsequently, noncoding RNAs (ncRNAs) responsible for SOX4 overexpression were identified using expression, correlation, and survival analyses.
Results
SOX4 expression was significantly upregulated in HCC and correlated with a poor prognosis. Additionally, SOX4 upregulation in HCC positively correlated with immune cell infiltration, several biomarkers of immune cells, and immune checkpoint expression. Finally, the MCM3AP-AS1/hsa-miR-204-5p axis was identified as the most likely upstream ncRNA-related pathway for SOX4 in HCC. These results indicated that ncRNA-mediated upregulation of SOX4 correlated with the immune infiltration level and poor prognosis in HCC. Our findings provide new directions for the development of novel immunotherapeutic targets for HCC.
Similar content being viewed by others
Introduction
According to the latest global cancer burden statistics from the World Health Organization, hepatocellular carcinoma (HCC) is the most common form of primary liver cancer, and liver cancer is the sixth leading cause of cancer-related deaths worldwide [1]. Liver cirrhosis, chronic hepatitis B or C virus infection, alcoholic fatty liver disease, and nonalcoholic fatty liver disease are the main risk factors for HCC [2,3,4]. In China, poor prognosis and low overall survival (OS) rate of patients with HCC [5] is likely due to late-stage diagnosis and limited treatment options for advanced disease [4, 5]. Owing to the success of immune checkpoint blockers in various cancers, immunotherapy strategies based on checkpoint inhibitors could soon become the first-line treatment for advanced HCC [6,7,8]. Therefore, a deeper understanding of the roles of tumor immunity in the pathogenesis of HCC is essential for identifying prospective biomarkers and new immune-related therapeutic targets in patients with HCC.
The sex-determining region Y-box (SOX) developmental transcription factor family regulates cell fate [9]. In addition, two SOX factors, SOX9 and SOX4, are strongly associated with cancer [10]. Interestingly, transcription profiling of more than 3,700 human cancers revealed that SOX4 was the only upregulated universal “cancer signal” among 64 genes [9]. SOX4 is abnormally expressed in colorectal cancer [11], breast cancer [12], glioblastoma [13], and other tumor tissues and participates in the development of malignant tumors by affecting cell proliferation, cell cycle progression, apoptosis, and other processes [10]. In addition, SOX4 inhibits the production of myeloid differentiation primary response gene 88 and most Toll-like receptors by binding to its promoter to attenuate gene transcription and suppress nuclear factor kappa-B and interferon regulatory factor 3/7 expression by promoting protein degradation to enhance viral copy number [14]. Bagati et al. [15] confirmed that the SOX4 transcription factor confers triple-negative breast cancer cell resistance to T cell-mediated cytotoxicity, and drives immune escape. These findings suggest that SOX4 may play key roles in the regulation of innate and tumor immunity. However, the association between SOX4 expression and tumor immunity in HCC and the related mechanisms remain unclear.
Accordingly, in this study, we aimed to evaluate SOX4 expression in multiple types of human cancers; analyze the relationships among SOX4 expression and OS in patients with HCC; and evaluate the associations of SOX4 with tumor-infiltrating immune cells, immune cell biomarkers, and immune checkpoints in HCC. We also aimed to determine the mechanisms through which noncoding RNAs (ncRNAs) regulate SOX4 in HCC.
Materials and methods
Reagents
Antibodies against SOX4 (ab70598) were purchased from Abcam (Cambridge, MA, USA). Anti-GAPDH (GB11002) was purchased from Servicebio (Wuhan, China). Horseradish peroxidase-conjugated secondary antibodies (SA00001-1) were purchased from Proteintech. The SPlink Detection Kit was purchased from ZSGB-BIO Technology (Beijing, China).
Liver cancer orthotopic modeling
This animal study strictly adhered to internationally recognized ethical guidelines for experimental animals. Eight-week-old pregnant female C57BL/6J mice were purchased from Guangdong Medical Laboratory Animal Center, with the license number: SYXK (GD) 2022-0002. All mice were housed in SPF-grade conditions, ensuring a stable temperature range of 21–25 °C, humidity at 40–60%, and a standard diurnal light-dark cycle. The mice were provided with standard feed, with ad libitum access to water and food. This research was formally approved by the Animal Welfare and Ethics Committee of China Science Industries Holdings (Shenzhen) Co., Ltd. (approval number: 202300127). During the experiment, we utilized these pregnant mice to breed 2-week-old male pups, which were then randomly divided into two experimental groups (n = 6 each): control and model groups. To induce liver cancer, the model group mice intraperitoneally injected with 25 mg/kg N-nitrosodiethylamine (DEN) (Sigma-Aldrich, MO, USA). After long-term observation (38 weeks), we anesthetized the mice using isoflurane and preserved the serum and liver tissue samples at -80 °C. To further evaluate pathological changes in the liver, we fixed the mice livers using 4% paraformaldehyde (PFA). Subsequently, we processed the tissue using an ethanol (100%, 100%, 95%, 80%, 70%, and 50%) gradient dehydration method, with each dehydration step lasting 10 min. The samples were then cleared in xylene, embedded in paraffin, and sectioned into 4 μm-thick paraffin slices. Following the standard hematoxylin and eosin (H&E) staining protocol, we stained the tissue sections and mounted them with neutral resin. Finally, we conducted detailed pathological observations of the liver tissue using an optical microscope and recorded and preserved the image data using a slide scanning system.
Cell culture and treatment
The human hepatoma cell line Hep3B cells was purchased from Shanghai Institutes for Biological Sciences (Shanghai, China), and Huh7 cells were purchased from Suyan Biotechnology Co., Ltd. HCCLM3 cells were purchased from the China Center for Type Culture Collection. HepG2 cells were purchased from the Cell Bank of the Chinese Academy of Sciences. HCCLM3, Hep3B, HepG2 and Huh7 cells were grown in DMEM/high-glucose medium (Gibco, Carlsbad, CA, USA), supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.), at 37 °C and 5% CO2. Miha cells were purchased from Fenghui Biotechnology Co., Ltd and grown in RPMI-1640 medium (Gibco, Carlsbad, CA, USA), supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.), at 37 °C and 5% CO2.
TIMER database analysis
TIMER (https://cistrome.shinyapps.io/timer/) is a public website that systematically analyzes the immune infiltration of different cancer types. We used the TIMER website to evaluate the relationship between SOX4 gene expression and six tumor-infiltrating immune cells (B cells, neutrophils, macrophages, CD4 + T cells, CD8 + T cells, and dendritic cells) in patients with HCC. We showed the correlation between SOX4 gene expression and HCC purity and its association with specific marker genes of tumor-infiltrating immune cells. Moreover, the mRNA levels of SOX4 in several cancers, including HCC, were identified using the TIMER2.0 database [16, 17].
UALCAN database analysis
We used the “Expression” module of the UALCAN database, investigated SOX4, hsa-mir-204-5p, and MCM3AP-AS1 gene expression in HCC. P < 0.05 was considered statistically significant. In addition, we used the “Survival” module of the UALCAN database to analyze the prognostic significance of high or low SOX4 expression in different clinical characteristics of liver hepatocellular carcinoma (LIHC) [18].
Kaplan–Meier analysis
According to the median SOX4 expression, patient data was divided into two groups: SOX4 high expression and SOX4 low expression. SOX4 expression levels affecting the clinical outcomes of patients with HCC were estimated using Kaplan–Meier survival curves (http://kmplot.com/analysis/) [19].
GEPIA database and TISIDB analysis
We used GEPIA data to verify the association between immune cell marker genes and SOX4 expression in the TIMER network. We used the “immunostimulators” and “immunoinhibitor” modules in the TISIDB platform to evaluate the relationship between SOX4 expression and immune checkpoints. P < 0.05 was considered statistically significant.
StarBase and MiRWalk analysis
StarBase was used to predict candidate lncRNAs that could potentially bind to hsa-mir-204-5p. In addition, expression correlation analysis for lncRNA MCM3AP-AS1 and hsa-mir-204-5p or SOX4 in HCC was performed using StarBase. MiRWalk (http://mirwalk.umm.uni-heidelberg.de/) is a comprehensive bioinformatics resource that allows for in-depth analysis of microRNAs (miRNAs). It generates predicted and validated miRNA-target gene interactions. In this study, MiRWalk was utilized to predict miRNAs upstream of SOX4 that potentially bind to and regulate the expression of the gene. To ensure the reliability, specificity, and a high degree of confidence in the predictions, the dataset was filtered by selecting only those miRNA-binding sites located in the 3’ untranslated region (3’UTR) of SOX4, and only those predictions that were also present in the miRDB database ( that scored “1”) were considered.
Statistical analysis
Pearson correlation analysis was performed to analyze the similarity of the samples, and Spearman correlation analysis was performed to analyze correlations. Each experiment was repeated independently at least thrice. One-way analysis of variance or paired t-tests were used to compare the two groups. Statistical significance was set at P < 0.05. All analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
SOX4 is highly expressed in HCC
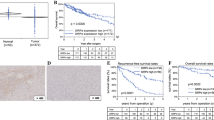
To explore the possible roles of SOX4 in carcinogenesis, we analyzed TCGA RNA-seq data via the TIMER database. Compared with that in corresponding normal tissues, SOX4 expression was significantly upregulated in tissues from several types of tumors, including bladder urothelial carcinoma, breast invasive carcinoma, cholangiocarcinoma, colon adenocarcinoma, esophageal carcinoma, glioblastoma multiforme, head and neck cancer, kidney renal papillary carcinoma, liver hepatocellular carcinoma (LIHC), lung adenocarcinoma, lung squamous cell carcinoma, pheochromocytoma and paraganglioma, prostate adenocarcinoma, rectal adenocarcinoma, stomach adenocarcinoma, thyroid carcinoma, and uterine corpus endometrial carcinoma (Fig. 1A). Correspondingly, the UALCAN analysis showed that SOX4 expression in HCC tissues was significantly higher than that in normal tissues (Fig. 1B).
Expression of SOX4 mRNA in cancers. (A) Differential expression of sex-determining region Y-box (SOX4) mRNA in different human cancers and specific cancer subtypes from The Cancer Genome Atlas (TCGA) database in Tumor Immune Estimation Resource (TIMER). (B) High or low SOX4 expression in HCC and normal tissues in the UALCAN database. (C–F) SOX4 expression in HCC and adjacent noncancer tissues from the GSE36776, GSE60502, GSE84402, and GSE62232 datasets from the Gene Expression Omnibus database. (G) Representative images of liver in a liver cancer model in mice; Representative images of HE staining of liver tissue from mice; Immunohistochemical staining for SOX4 of liver tissue from mice. (H) SOX4 mRNA levels in different liver cancer cell lines. (I, J) Protein expression level of SOX4 in different liver cancer cell lines by Western blotting. After transferring the proteins and confirming the quality of the blots, we performed a precise cutting of the nitrocellulose membranes by comparing the molecular weights and electrophoretic migration rates of known proteins, allowing for subsequent quantitative analysis. Each experiment was repeated independently at least thrice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS: not significant
Validation using the gene expression omnibus (GEO) database and cell and animal specimens
To further validate SOX4 expression in HCC, SOX4 transcript levels in cancer tissues and adjacent tissues from patients with HCC were analyzed using four GEO datasets (i.e., GSE36776, GSE60502, GSE62232, and GSE84402). SOX4 transcript levels were significantly higher in HCC tissues than in noncancerous adjacent tissues in all four HCC datasets (P < 0.05; Fig. 1C–F). The results of this comparison were validated in a liver cancer mouse model and several liver cancer cells (Fig. 1G–J).
Prognostic significance of SOX4 expression levels in HCC
The prognostic significance of SOX4 expression in HCC was analyzed using the Kaplan–Meier plotter database. Increased SOX4 expression was associated with OS, progression-free survival, disease-specific survival, and relapse-free survival (Fig. 2A–D). Correspondingly, the UALCAN “survival analysis” module was used to analyze the prognostic significance of different expression levels of SOX4 in HCC. This study revealed that increased SOX4 expression was inversely associated with OS, progression-free survival, disease-specific survival, and relapse-free survival (Fig. 2A–E). Next, we used the same module to analyze the prognostic significance of SOX4 expression for different clinical characteristics of LIHC. In patients with high SOX4 expression, OS was higher in individuals with obesity than in overweight or normal weight individuals (Fig. 2F). Similarly, in patients with low SOX4 expression, OS was higher in overweight than in normal individuals. In, patients with low SOX4 expression, tumor grade is associated with SOX4 levels (Fig. 2G). Furthermore, in patients with LIHC with high SOX4 expression, low OS was associated with sex and race (Fig. 2H, I).
Prognostic significance of SOX4 expression in different tumor types. (A–D) Survival curves showing overall survival (OS), progression-free survival (PFS), disease-specific survival (DSS), and relapse-free survival (RFS) in hepatocellular carcinoma (HCC) from the Kaplan–Meier database. (E) UALCAN database estimates of OS in patients with liver hepatocellular carcinoma (LIHC) from TCGA database. (F–I) Subgroup analysis for body weight (F), tumor grade (G), sex (H), and race (I) in the UALCAN database “survival analysis” module. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NC: not significant
Correlation between SOX4 expression and infiltrating immune cells in HCC
Previous analyses have suggested that the activity and quantity of tumor-infiltrating immune cells greatly influence the survival times of patients with various types of cancer [20]. Therefore, we utilized the ‘immunedeconv’ package in R software to analyze the score distribution of SOX4 expression in HCC. TIMER scores in B cells, CD4+ T cells, neutrophils, macrophages, and dendritic cells were significantly increased in HCC tissues, showing high SOX4 expression (Fig. 3A). Next, we used the TIMER website to analyze the associations between SOX4 expression and infiltrating levels of eight tumor-immune cells (CD8+ T cells, dendritic cells, CD4+ T cells, B cells, macrophages, regulatory T cells, natural killer [NK] cells, and neutrophils) in HCC. We found that SOX4 expression was significantly positively associated with six infiltrating immune cells (B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils, dendritic cells, and NK cells) in HCC (Fig. 3B, C). Together, these results suggest that SOX4 expression in HCC correlates with the degree of B cell, CD4 + T cell, neutrophil, macrophage, and dendritic cell infiltration.
Correlation between SOX4 expression and infiltrating immune cells in hepatocellular carcinoma (HCC). (A) TIMER scores of various immune cells in patients with HCC with high or low SOX4 expression. (B, C) Correlation of SOX4 expression with B cells, CD8 + T cells, CD4 + T cells, regulatory T cells (Tregs), macrophages, neutrophils, dendritic cells, and natural killer (NK) cells in HCC
Relationship between SOX4 expression and markers of different subsets immune cells
To explore the possible role of SOX4 in the infiltration of different immune cell types in HCC, we used the TIMER database to analyze the relationship between the expression of SOX4 in HCC and markers from different subsets of tumor-infiltrating immune cells. The results confirmed that SOX4 expression in HCC was associated with more than half of the immune cell marker sets (see Supplementary Table S1). Specifically, SOX4 expression significantly correlated with the expression of infiltrated specific immune cell gene markers such as tumor-associated macrophages (TAM), CCL2, CD68, IL10, regulatory T (Treg) markers, CCR8, STAT5B, IL2RA, Tfh markers, BCL6, ICOS, CXCR5, and T cell markers in HCC, namely PDCD1, CTLA-4, LAG3, and HAVCR2 (Supplementary Table S1). In addition, we used the GEPIA website to evaluate the relationship between SOX4 and marker genes of macrophages, neutrophils, T helper 1 (Th1), Th2, NK cells, TAM, and Treg marker genes in HCC. Consistent with the TIMER results, we found that in addition to Treg cells, the immune sets marked by monocytes, neutrophil, Th1, Th2, NK, TAM, and T-cell depletion correlated strongly with SOX4 expression in HCC. These results suggest that SOX4 expression is a significant factor in immune cell infiltration in HCC (Fig. 4A–T).
Relationships between SOX4 expression and markers of different subsets immune cells in GEPIA. (A–G) The plots show the association between SOX4 expression in hepatocellular carcinoma (HCC) and markers from tumor-infiltrating monocytes (CD86) (A), neutrophils (CCR7 and ITGAM) (B, C), Th2 cells (STAT6) (D), natural killer (NK) cells (KIR3DL1, XCL1, CD7) (E–G), tumor-associated macrophages (TAMs; IL-10 and CD68) (H, I), Tregs (CCR8, STAT5B, and TGFB1) (J–L), Th1 cells (STAT1, IFNG, TBX21, and TNF) (M-P), and exhausted T cells (LAG3, PDCD1, CTLA4, and HAVCR2) (Q-T) in HCC. TAMs, NK cells, Th1 cells, follicular helper T (Tfh) cells, and Tregs. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Relationship between immune checkpoint and expression of SOX4 in HCC
Given the importance of immune checkpoints in immunotherapy, we further analyzed the relationship between SOX4 expression and immune checkpoints in many types of cancers via the TISIDB database (Fig. 5A, F). Interestingly, there was significant positive correlation between SOX4 and immunoinhibitor expression: transforming growth factor (TGF)B1, LGALS9, VTCN1, and CTLA4 (Fig. 5B–E). Moreover, we identified a close correlation between SOX4 expression and several immunostimulators. Four immunostimulators (IL6R, ICOSLG, PVR, and CD40) negatively correlated with SOX4 expression (Fig. 5G–J). Hence, these findings further suggest that SOX4 expression is significantly related to HCC-induced immune responses.
Correlation analysis between immune checkpoints and SOX4 expression in hepatocellular carcinoma (HCC). (A) Heatmap analysis of the correlation between SOX4 expression and immunoinhibitors across human cancers. (B–E) SOX4 expression in HCC positively correlated with TGFB1, LGALS9, VTCN1, and CTLA4. (F) Heatmap analysis of the correlation between SOX4 and immunostimulators across human cancers. (G–J) SOX4 expression in HCC was negatively correlated with IL6R, ICOSLG, PVR, and CD40
Prediction and analysis of upstream miRNAs of SOX4
ncRNAs are involved in gene regulation. To further elucidate the underlying molecular mechanism of the role of SOX4 in HCC, we constructed an mRNA–miRNA–lncRNA interaction network. We first hypothesized that upstream miRNAs might bind to SOX4; 23 miRNAs were identified (Fig. 6A). Based on the mechanism by which miRNA recognizes target mRNA through complementary base pairing and guides the silencing complex to degrade target mRNA or repress target mRNA according to the degree of complementarity, a negative correlation between miRNA and SOX4 was hypothesized. Therefore, an expression correlation analysis was performed. SOX4 significantly negatively correlated with hsa-miR-204-5p, hsa-mir-6730, and hsa-mir-5139 in HCC (Fig. 6B). Therefore, we further evaluated the expression of these miRNAs in HCC and found that the expression of hsa-miR-204-5p was significantly lower than that in the control group and negatively correlated with the status of lymph node metastasis (Fig. 6C–H). Further analysis showed that patients with HCC with high hsa-mir-204-5p levels had better OS (Fig. 6I). These findings suggest that hsa-mir-204-5p may be the most likely regulatory miRNA for SOX4 in HCC.
Identification of hsa-miR-204-5p as a potential miRNA upstream of SOX4 in hepatocellular carcinoma (HCC). (A) The miRNA/SOX4 regulatory network. (B) Correlation of predicted miRNA expression and SOX4 expression in HCC, analyzed using the R software package ‘ggstatsplot’ with a dataset comprised of mRNA-seq data for HCC from The Cancer Genome Atlas. (C–E) Expression of hsa-miR-5139 (C), hsa-miR-6730 (D), and hsa-miR-204-5p (E) in HCC and control normal samples, determined using the UALCAN database. (F–H) Expression of hsa-miR-6730 (F), hsa-miR-5139 (G), and hsa-miR-204-5p (H) in normal tissues and cancers with nodal metastasis status or nodal metastasis status, as determined using the UALCAN database. (I) The prognostic significance of high or low hsa-miR-204 expression in HCC was analyzed using the Kaplan–Meier plotter database. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NC: not significant
Prediction and analysis of upstream lncRNAs of hsa-mir-204-5p
Next, we predicted the upstream lncRNAs of hsa-miR-204-5p using data from starBase, and found 29 possible lncRNAs (see Supplementary Table S2). Then, the expression levels of these lncRNAs in HCC were determined using the UALCAN database. We found that, among the 29 lncRNAs, LINC00472, MCM3AP-AS1, NEAT1, KCNQ1OT1, and MALAT1 were significantly upregulated in HCC (Fig. 7A–E). Subsequently, expression correlation analysis of five lncRNAs and SOX4 in HCC was performed. As shown in Fig. 7F, SOX4 expression positively correlated with that of five lncRNAs, with the strongest correlation observed for KCNQ1OT1 and MCM3AP-AS1. Simultaneously, the prognostic value of five lncRNAs in HCC was further evaluated (Fig. 7G–K). It was found that only patients with HCC with higher MCM3AP-AS1 expression had poorer OS (Fig. 7G). Next, we examined the expression correlation between lncRNA MCM3AP-AS1 and hsa-miR-204-5p in HCC using starBase. As shown, lncRNA MCM3AP-AS1 inversely correlated with hsa-miR-204-5p expression (Fig. 7L). According to the ceRNA hypothesis, lncRNAs increase mRNA expression by competitively binding to shared miRNAs; therefore, there should be a negative or positive correlation between lncRNAs and miRNAs. Thus, MCM3AP-AS1 is a potential lncRNA for the hsa-miR-204-5p/SOX4 axis in HCC.
Identification of hsa-miR-204-5p as a potential miRNA upstream of SOX4 in hepatocellular carcinoma (HCC). (A–E) Expression of LINC00472 (A), MCM3AP-AS1 (B), NEAT1 (C), KCNQ1OT1 (D), and MALAT1 (E) in HCC and control samples, as determined using the UALCAN database. (F) Correlation of predicted lncRNA expression and SOX4 expression in HCC, as analyzed using the R software package ‘ggstatsplot’ with a dataset comprised of mRNA-seq data for HCC from The Cancer Genome Atlas. (G–K) The prognostic significance of high or low MCM3AP-AS1, NEAT1, KCNQ1OT1, MALAT1, and LINC00472 expression in HCC, as analyzed using starBase. (L) Correlation between predicted MCM3AP-AS1 expression and hsa-miR-204-5p expression in HCC, as analyzed using starBase. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NC: not significant
Discussion
HCC is associated with a high incidence and poor prognosis. Elucidating the molecular mechanisms of hepatocarcinogenesis may provide key insights into the development of effective therapeutic targets and allow for the identification of promising prognostic biomarkers. Accumulating evidence suggests that SOX4 plays a key role in the initiation and progression of various human cancers, including liver cancer [9]. However, our current understanding of the roles of SOX4 in liver cancer remains insufficient.
SOX4 is an established stem/ancestor transcription factor and SRY-related HMG-box family member of cancer-related transcription factors [9]. Moreover, SOX4 has a highly conserved HMG-box domain that can bind directly to DNA to induce DNA bending or chromatin structure changes, contributing to the assembly of the transcription complex [9]. Additionally, SOX4 is abnormally expressed in many types of tumor tissues, such as colorectal cancer [11], breast cancer [ [12], and glioblastoma [13], and is closely correlated with poor prognosis and disease progression in patients. In this study, we first performed a pan-cancer analysis of SOX4 expression using TCGA data, and found that SOX4 expression is upregulated in most cancers. We then further validated SOX4 overexpression using the UALCAN database and GEO datasets. Survival analysis indicated that patients with HCC showing high SOX4 expression had poor prognosis. Li et al. [21] proposed that SOX4 activates TGF-β-mediated pathways, leading to HCC metastasis. The results of our analysis are consistent with that of Li et al., supporting that SOX4 plays an oncogenic role in the pathogenesis of HCC. Some studies suggest a correlation between high body mass index and longer OS in hepatocellular carcinoma patients undergoing treatments like the T + A regimen. Our analysis revealed that, among patients with high SOX4 gene expression, obese individuals exhibited significantly better OS compared to those who were overweight or of normal weight. Conversely, among patients with low SOX4 expression, those who were overweight had a better OS compared to those with normal weight. These findings imply that while SOX4 expression influences HCC biology, its direct impact on the body weight-OS relationship in HCC may be limited [22].
The activity and number of immune cells in the tumor microenvironment play key roles in HCC pathogenesis [13]. HCC is a typical inflammation-related cancer. Approximately 90% of HCC burden is related to long-term inflammation caused by viral hepatitis, excessive alcohol intake, nonalcoholic fatty liver disease, or nonalcoholic steatohepatitis [13]. Multiple immune cell types, including neutrophils, macrophages, and different lymphocyte subtypes, in the chronically inflamed liver interact with liver cells to promote the occurrence of liver cancer [23]. Many studies have illustrated that immune-related molecules, such as interleukin-6 [24, 25], lymphotoxin-α [26], and tumor necrosis factor [27, 28], can accelerate the occurrence of liver cancer and affect the aggressiveness of tumors; however, the immune response also limits the progression of liver cancer. The liver contains many immune cells and maintains a unique and tolerant immune state, allowing for continuous inflammatory signals from the intestine [23]. Given the complex interactions between malignant tumor cells and immune cells in HCC, it is imperative to understand the mechanisms regulating the immune system in HCC. Recent studies have demonstrated that SOX4 also plays vital roles in regulating innate and tumor immunities [9, 15]. However, the potential relationships between SOX4 and tumor immunity in HCC have not yet been clarified. Therefore, in this study, we also aimed to investigate the relationships between SOX4 expression and immune cell infiltration in HCC. TIMER and GEPIA analyses showed a moderate relationship between SOX4 expression and the infiltration levels of B cells, macrophages, neutrophils, CD4+ T cells, CD8+ T cells, and dendritic cells in HCC. Thus, these results indicated that SOX4 may influence the prognosis in HCC by altering tumor immunity.
The status of tumor-infiltrating immune cells is closely related to immune marker genes [29, 30]. We used the TIMER and GEPIA websites to evaluate the correlations between SOX4 expression in HCC and gene markers of infiltrating immune cells. We examined the correlations between SOX4 expression and the expression of the monocyte marker CD86; the tumor-associated macrophage markers CCL2, CD68, and interleukin-10; and the M2 macrophage markers CD163, ARG1, and MRC1. These results indicate that SOX4 regulates the infiltration levels and activities of monocytes and tumor-associated macrophages. In addition, T-cell exhaustion markers (e.g., PDCD1, CTLA4, LAG3, and HAVCR2) are key immune checkpoint inhibitor proteins [30,31,32], and their expression is positively correlated with SOX4 expression. In addition, we used the TISIDB database to analyze the relationships between SOX4 and the expression of immune checkpoints. We revealed that SOX4 positively correlates with some immunoinhibitors and negatively correlates with other immunostimulators. Most malignant tumors, including liver cancer, exhibit upregulation of inhibitory ligands, and this alteration escapes the immune response by suppressing T-cell function, thereby leading to cancer development [33, 34]. We speculate that the overexpression of inhibitory immune checkpoint proteins in HCC may be related to SOX4 upregulation. Changes in these mechanisms can alter the antitumor functions of T cells, leading to a poor prognosis in HCC. Further studies are required to evaluate this hypothesis. Collectively, these findings indicate that tumor immune infiltration may partially account for the oncogenic roles of SOX4 in HCC.
ncRNAs, including miRNAs, lncRNAs, and circulating RNAs (circRNAs), communicate with each other through the ceRNA mechanism and are involved in the regulation of gene expression [35]. To explore the possible mechanisms through which SOX4 mediates HCC, we identified the important regulatory miRNAs upstream of SOX4 in HCC. After correlation, expression, and survival analyses, hsa-miR-204-5pP was selected as the most likely potential miRNA upstream of SOX4 in HCC. Previous studies have reported that hsa-miR-204-5p plays an inhibitory role in regulating HCC proliferation and migration, targeting NUAK1 to suppress drug resistance and metastasis in HCC and inhibit the growth of HCC endothelial cells [36,37,38,39]. These results and our analyses showed that the hsa-miR-204/SOX4 axis may play an important role in the progression of HCC.
According to the ceRNA hypothesis, the potential lncRNAs involved in the hsa-miR-204/SOX4 axis in liver cancer may also be oncogenic. Furthermore, lncRNAs upstream of the hsa-miR-204/SOX4 axis were also predicted, and 29 possible lncRNAs were found. Through expression, survival, and correlation analyses, we identified MCM3AP-AS1 as one of the most highly upregulated lncRNAs. The lncRNA MCM3AP-AS1 has been reported to function as an oncogene in various malignancies, including liver cancer [40]. For example, MCM3AP-AS1 inhibits cell proliferation in cervical squamous cell carcinoma by downregulating miR-93 [41] and promotes HCC growth by targeting the miR-194-5p/FOXA1 axis [42]. MCM3AP-AS1 also enhances HCC metastasis by regulating epidermal growth factor receptor and autophagy [43]. Thus, the MCM3AP-AS1/hsa-miR-204-5p/SOX4 axis may be a potential regulatory pathway in HCC.
Conclusions
In this study, we revealed that SOX4 is highly expressed in various types of human tumors, including liver cancer, and positively correlates with poor prognosis in liver cancer. Our current findings also suggested that SOX4 may exert its oncogenic role by increasing tumor immune cell infiltration and immune checkpoint expression. Additionally, we identified a possible potential upstream regulatory mechanism of SOX4 in HCC; that is MCM3AP-AS1/hsa-miR-204-5p/SOX4 axis regulation. This study had some limitations. First, our analysis was limited to previously reported data and various databases. Second, platform differences and data heterogeneity may have affected the outcomes. Third, animal experiments have not been conducted to verify the functions of MCM3AP-AS1/hsa-miR-204/SOX4 in HCC development. In the future work, using immunohistochemical staining or flow cytometry to detect proteins and functional analysis of the roles of the MCM3AP-AS1/hsa-miR-204-5p/SOX4 axis in regulating immune cell infiltration in HCC, we aim to verify the results of the present study. We hope that these analyses will provide insights into the use of immunotherapy to treat HCC.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019; (10):589–604.
Villanueva A, Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–62.
Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544.
Zhu ZX, Huang JW, Liao MH, Zeng Y. Treatment strategy for hepatocellular carcinoma in China: radiofrequency ablation versus liver resection. Jpn J Clin Oncol. 2016;46(12):1075–80.
Liu X, Qin S. Immune checkpoint inhibitors in Hepatocellular Carcinoma: opportunities and challenges. Oncologist. 2019;24(Suppl 1):S3–10.
Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37(1):110.
Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70(1):204–14.
Hanieh H, Ahmed EA, Vishnubalaji R, Alajez NM. SOX4: epigenetic regulation and role in tumorigenesis. Semin Cancer Biol. 2020;67(Pt 1):91–104.
Moreno CS. SOX4: the unappreciated oncogene. Semin Cancer Biol. 2020;67(Pt 1):57–64.
Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;17(1):106.
Zhang J, Xiao C, Feng Z, Gong Y, Sun B, Li Z, et al. SOX4 promotes the growth and metastasis of breast cancer. Cancer Cell Int. 2020;29(20):468.
Yang J, Smith DK, Ni H, Wu K, Huang D, Pan S, et al. SOX4-mediated repression of specific tRNAs inhibits proliferation of human glioblastoma cells. Proc Natl Acad Sci U S A. 2020;117(11):5782–90.
Shang J, Zheng Y, Mo J, Wang W, Luo Z, Li Y, et al. Sox4 represses host innate immunity to facilitate pathogen infection by hijacking the TLR signaling networks. Virulence. 2021;12(1):704–22.
Bagati A, Kumar S, Jiang P, Pyrdol J, Zou AE, Godicelj A, et al. Integrin αvβ6-TGFβ-SOX4 pathway drives Immune Evasion in Triple-negative breast Cancer. Cancer Cell. 2021;39(1):54–e679.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–14.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: a web server for Comprehensive Analysis of Tumor-infiltrating Immune cells. Cancer Res. 2017;77(21):e108–10.
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, et al. UALCAN: a portal for facilitating Tumor Subgroup Gene expression and survival analyses. Neoplasia. 2017;19(8):649–58.
Menyhárt O, Nagy Á, Győrffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R Soc Open Sci. 2018;5(12):181006.
Geukes Foppen MH, Donia M, Svane IM, Haanen JB. Tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Mol Oncol. 2015;9(10):1918–35.
Li L, Liu J, Xue H, Li C, Liu Q, Zhou Y, et al. A TGF-β-MTA1-SOX4-EZH2 signaling axis drives epithelial-mesenchymal transition in tumor metastasis. Oncogene. 2020;39(10):2125–39.
Shuichiro S, Hitoshi M, Maki T, Tatsuya Y. Obesity and non-alcoholic steatohepatitis in immunotherapy for hepatocellular carcinoma. Hepatol Int. 2023;4:17.
Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. 2018;36:247–77.
Heymann F, Tacke F. Immunology in the liver — from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110.
Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64(6):1403–15.
Wolf MJ, Seleznik GM, Zeller N, Heikenwalder M. The unexpected role of lymphotoxin beta receptor signaling in carcinogenesis: from lymphoid tissue formation to liver and prostate cancer development. Oncogene. 2010;29(36):5006–18.
Liedtke C, Trautwein C. The role of TNF and Fas dependent signaling in animal models of inflammatory liver injury and liver cancer. Eur J Cell Biol. 2012;91(6–7):582–9.
Koelzer VH, Sirinukunwattana K, Rittscher J, Mertz KD. Precision immunoprofiling by image analysis and artificial intelligence. Virchows Arch. 2019;474(4):511–22.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–51.
Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–9.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18.
Tanaka A, Sakaguchi S. Targeting Treg cells in cancer immunotherapy. Eur J Immunol. 2019;49(8):1140–6.
McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and Cancer. Annu Rev Immunol. 2019;37:457–95.
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9.
Zhao R, Fu J, Zhu L, Chen Y, Liu B. Designing strategies of small-molecule compounds for modulating non-coding RNAs in cancer therapy. J Hematol Oncol. 2022;15(1):14.
Jiang G, Wen L, Zheng H, Jian Z, Deng W. Mir-204-5p targeting SIRT1 regulates hepatocellular carcinoma progression. Cell Biochem Funct. 2016;34(7):505–10.
Luo YH, Tang W, Zhang X, Tan Z, Guo WL, Zhao N, et al. Promising significance of the association of mir-204-5p expression with clinicopathological features of hepatocellular carcinoma. Med (Baltim). 2017;96(30):e7545.
Cui ZH, Shen SQ, Chen ZB, Hu C. Growth inhibition of hepatocellular carcinoma tumor endothelial cells by mir-204-3p and underlying mechanism. World J Gastroenterol. 2014;20(18):5493–504.
Yu Y, Wang Y, Xiao X, Cheng W, Hu L, Yao W, et al. MiR-204 inhibits hepatocellular cancer drug resistance and metastasis through targeting NUAK1. Biochem Cell Biol. 2019;97(5):563–70.
Yu X, Zheng Q, Zhang Q, Zhang S, He Y, Guo W. MCM3AP-AS1: an indispensable Cancer-related LncRNA. Front Cell Dev Biol. 2021;9:752718.
Lan L, Liang Z, Zhao Y, Mo Y. LncRNA MCM3AP-AS1 inhibits cell proliferation in cervical squamous cell carcinoma by down-regulating miRNA-93. Biosci Rep. 2020;40(2):BSR20193794.
Wang Y, Yang L, Chen T, et al. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol Cancer. 2019;18:28.
Zhang H, Luo C, Zhang G. LncRNA MCM3AP-AS1 regulates epidermal growth factor receptor and autophagy to promote Hepatocellular Carcinoma Metastasis by interacting with miR-455. DNA Cell Biol. 2019;38(8):857–64.
Acknowledgements
The authors thank Editage (www.editage.com) for English language editing.
Funding
Thanks to the grants from the Shenzhen Science and Technology Project (NO. JCYJ20210324120405015); Scientific Research Project of Traditional Chinese Medicine Bureau Of Guangdong Province (NO.20231302).
Author information
Authors and Affiliations
Contributions
Xiaozhou Zhou contributed to the study conception and design. Jing Li, Minling Lv, Xinfeng Sun, and Qi Huang acquired, analyzed, and interpreted the data. Jing Li, Minling Lv, and Qi Huang drafted and revised the manuscript. Rui Hu and Xin Zhong performed data analysis. Wei Zhang, MengQing Ma, and Xinfeng Sun assisted with specimen collection. Zhiyi Han, and Wenxing Feng analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by the Animal Welfare and Ethics Committee of China Science Industries Holdings (Shenzhen) Co., Ltd. (approval number: 202300127).
Consent for publication
Not applicable.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Sun, X., Lv, M. et al. ncRNA-mediated SOX4 overexpression correlates with unfavorable outcomes and immune infiltration in hepatocellular carcinoma. BMC Gastroenterol 24, 265 (2024). https://doi.org/10.1186/s12876-024-03346-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03346-0