Abstract—
The role of astroglial cells is much more complicated than the notion of an “elastic framework,” which provides structural and metabolic support for brain structures. Astrocytes can affect synaptic processes by participating in the transmission of information by releasing or modulating the activity of neurotransmitters. In vitro and in situ studies revealed a large spectrum of molecules secreted by neuroglial cells. Analysis of the patterns that accompany the secretory mission of astrocytes led to the term “gliotransmission,” which gave rise to a new understanding of plastic regulation of the synaptic function and organization of the neural network, memory, and cognitive processes. The concept of the integrating mission of astroglia also opens up new opportunities for understanding the mechanisms of neurodegenerative diseases and identifying new targets for therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Contrary to the conventional dogma of astroglia as a system of support cells of the brain, a different concept has formed as a result of studies of the last twenty years. In this concept, the role of astrocytes is more important than its functioning as a flexible frame that provides structural and metabolic support for neurons. Modern studies combining highly sensitive electron microscopy with biochemical, computer, and neurophysiological techniques provided information about the special role of astroglial cells in the modulation of synaptic processes and the formation of neuronal domains.
In view of above, studies of the role of astrocytes in the orchestration of the synaptic process, the network consolidation of neurons, the organization of information fields, and the effect on cognitive processes seem unique. This work takes into account the vast body of modern information in an attempt to generalize and systematize such data and to present a perspective view of the unique mission of astroglial cells.
ASTROGLIA: A GENERAL VIEW
Astrocytes are involved in the developmental processes of the central nervous system. They provide metabolic support for neurons and affect their survival, differentiation, synaptogenesis, etc. Astrocytes are involved in a wide range of synaptic processes, such as the formation, maturation, and elimination (pruning) of synapses, activation of specific receptors, release of neurotransmitters, and modulation of synaptic plasticity (Araque et al., 2014; Dallérac and Rouach, 2016).
New methodological approaches indicate the topographical peculiarities of astrocyte populations in different regions of the brain (Khakh and Sofroniew, 2015). The plasma astrocytes, which are located in close proximity to the nerve cell body, are often referred to as its satellites. The morphology and, correspondingly, the functions of astrocytes in different brain regions are different. Moreover, certain subclasses of astrocytes can exist within one brain region. Comparative studies show that the ratio of the number of astrocytes to the number of neurons substantially increases in the evolutionary line of the brain (Bass et al., 1971). Human astrocytes are structurally more sophisticated, and their intercellular signaling, which is realized with the involvement of glial neurotransmitters and accessory proteins, becomes more diverse (Oberheim et al., 2012).
Astrocytes are differently organized cells with a small soma (diameter <10 μm) and branched processes extending over distances up to 100 μm. A characteristic feature of astrocytes is the formation of numerous contacts with nerve cells with both presynaptic and postsynaptic structures.
Neurons and astrocytes are sequentially formed from the pool of neural stem cells. Neurogenesis (transformation of progenitor cells) is accompanied via regulated reprogramming between neurons and astrocytes. These processes are crucial in the formation of a balanced number of astrocytes.
Human astrocytes exhibit a greater morphological diversity in the cerebral cortex layers, which indicates a high degree of specialization and, hence, opportunities to solve complex problems. Based on comparative studies, it was concluded that the morphology and number of human and rodent astrocytes considerably differ. This finding may be associated with the conclusion about the role of astroglia in performing more advanced physiological functions (Vasile et al., 2017).
A characteristic feature of astrocytes is the change in their morphology in contact with neurons, blood vessels, and other astrocytes. They form a neuronal network of interconnected elements that form individual domains. It was found that, within one domain, the astrocyte can form junctions with a great number of synapses (reaching tens of thousands). This means that the contact between astrocytes and neurons is a highly dynamic mechanism that is important for the organization of information association in the brain (Bushong et al., 2002; Halassa et al., 2007). Astrocytes are extremely sensitive to changes in the intracellular Ca2+ concentration and respond by releasing signaling molecules with different functions. The most common agents in this list are glutamate, ATP, GABA, D‑serine, and taurine. Astrocytes are also involved in the release of interleukin-1, tumor necrosis factor (TNF-α), neurotrophins (BDNF), and neuropeptide Y.
ASTROCYTES AS SYNAPTIC MODULATORS
Cajal was the first to assume that the nervous tissue consists of elementary units (neurons), which have an independent value in the anatomical, genetic, functional, trophic, pathological, and behavioral aspects. This listing is the essence of the positions expressed by Cajal himself (Cajal, 1937). Developing this conjecture, Sherrington assumed that there are stops (synapses) in the neuronal network, where the signal “makes a decision” (Sherrington, 1906).
New data made it possible to develop the idea of the synapse as the main tool in the mediator-regulated transfer of the information signal. High-resolution laser microscopy reveals spines, bulb-like microstructures protruding from the neuronal processes (dendrites). Their main function is to receive and amplify the excitatory signals produced in synapses and to transduce them to hundreds of similar microformations in the neuronal network. Spine genesis patterns indicating regulated rearrangements in response to external stimuli were recorded. It is important to note that the changes in the shape and density of spines are associated with learning and memory. An incorrect structure of spines and a decrease in their number hamper information signal transduction and, moreover, are associated with mental and neurodegenerative diseases (De Roo et al., 2008; Bourne and Harris, 2011). Laser confocal microscopy studies made it possible to obtain unique patterns of synaptic organization. Using three-dimensional reconstructions, the interaction of dendritic spines with astroglial cells was demonstrated via analysis of the neuropil volume in the hippocampal zones. The formation of presynaptic buds (multiple synapses) on dendritic spines in CA1 and CA3 hippocampal zones was shown (Popov et al., 2003).
Modern studies with in situ and in vivo imaging made it possible to obtain the patterns of changes in the shape of astrocytes contacting with neurons. Chemical activation caused changes in the structure and mobility of astroglial cells; the astrocyte interacted with synapses by forming leaflets or open-work coverlets. The stimulation of leaflet mobility facilitated these contacts, and the degree to which the astrocyte covered the synapse depended on the activity of regulatory molecules (Allen and Barres, 2009).
These results allowed the formulation of the concept of a tripartite synapse (Araque et al., 1999), which considers astrocytes to be direct participants of the signal function of neurons. According to the tripartite synapse concept, astrocytes form a space in which synaptic, extrasynaptic, and perisynaptic mechanisms function; they are involved in the implementation of integrative processes of the neuronal network. This relationship between the neuroglia and neurons is realized via gliotransmitters—mediators secreted by astrocytes.
The ability of one astrocyte to contact with a large number of synapses suggests the possibility of a simultaneous activation of entire neuron populations. Such effects were demonstrated in the hippocampus, where the expression of astrocytes and the release of glutamate led to the synchronous excitation of entire clusters of pyramidal cells (Angulo et al., 2004). Structural redefinitions of astrocytes were observed under these conditions; they formed special units in the form of microdomains with a branched network (Nedergaard et al., 2003). Within the structural microdomains, astrocytes interact with synapses and form fixed contacts with membranes of neurons and blood vessels (Halassa et al., 2007). The shape, velocity, and degree to which the astrocyte covers the neuron are regulated by additional chemical influences, which affect the efficiency and nature of synaptic transmission. These modulated contacts are created due to the diffuse influence of neurotransmitters on different cellular targets.
GLIOTRANSMISSION: RELEASE OF CHEMICAL TRANSMITTERS FROM ASTROCYTES
The doctrine developed over 100 years ago considered the neuronal network as the only instrument of the brain. Cytobiochemical studies performed in the last decade gave evidence that the special synaptic mission of astroglia is to provide an astroglial cradle that shields the neuron from the extrasynaptic signals, thus ensuring the dialectic constancy and plasticity of synaptic contacts (Nedergaard and Verkhratsky, 2012).
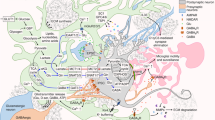
There is evidence of changes in the calcium concentration in astrocytes that are required to initiate the synaptic signal. The bidirectional nature of intracellular oscillations of Ca2+ was established, when the communication between neurons and astrocytes is ensured with the involvement of other astroglial molecules (Pasti et al., 1997; Perea and Araque, 2005; Wu et al., 2014; Guerra-Gomes et al., 2017) (Fig. 1). Genomic analysis documented the changes in the intracellular Ca2+ concentration due to the coding signaling proteins RYR3, MRVI1, and RGN, which promote the expression of transporters of the neurotransmitter glutamate and the activation of receptors for other neurotransmitters (Genoud et al., 2006; Henneberger and Rusakov, 2010).
Schematic representation of the tripartite synapse. Changes in the calcium ion concentration in astrocytes cause synaptic signal initiation. The bidirectional nature of intracellular Ca2+ oscillations determines the interactions between neurons and astrocytes with the involvement of glutamate (Glu) and other mediator molecules (gliotransmitters).
Experiments performed on single astrocytes showed that Ca2+ changes stimulate a cascade of biochemical processes to release mediators called gliotransmitters (Bezzi and Volterra, 2001). This new concept is logically associated with the tripartite synapse structure as a mechanism of information processing in the interaction between astrocytes and neurons. The regulated order of the bidirectional communication is defined as labile deviations of signaling (transmitter) molecules. Astrocyte activation leads to expression of their receptors associated with G proteins, which interact with the neurotransmitters released from the synapse upon its activation. A rapid change in the Ca2+ concentration in the cytosol stimulates the release of gliotransmitters, which, in turn, can interact with the synaptic elements (Santello et al., 2012).
Some gliotransmitters show great variability in affecting neuronal targets. The role of glutamate should be specially mentioned: it excites the presynaptic NMDARs in the dentate gyrus of the hippocampus, activates mGluRs in the CA1–CA3 hippocampal structures, or functions as an inhibitor of presynaptic kainate receptors. D-serine, which is synthesized in astroglial cells, functions as an endogenous coagonist of NMDARs (Oliet and Mothet, 2009). Purinergic (transmitted by ATP and adenosine) signaling molecules also play an important role in synaptic transmission (Fellin et al., 2006b). However, in addition to participation in the initiation of neural excitation, astrocytes, by activating NMDARs, are involved in the generation of apoptotic signals and the elimination of damaged neurons (Loane et al., 2012). The general principle of such polyphony is that one gliotransmitter can stimulate various reactions due to interaction with neurons of different phenotypes. By functioning at the presynaptic and postsynaptic levels, gliotransmitters affect neurotransmission by both excitatory and inhibitory synapses.
The use of D-aspartic acid as a false gliotransmitter made it possible to determine the degree of local excitation of neurons during the release of substances from the astrocytes in the ventrobasal thalamic structures, hippocampal CA1 cells, and the somatosensory cortex. These results demonstrate that astrocytes function as integrative nodes that can affect neurons in different brain regions. The obtained results also suggest that an excessive increase in the glutamate level may lead to unregulated expression of other gliotransmitters and cause desynchronization of neurons and development of pathological states (Pirttimaki et al., 2017).
It was shown that astrocytes secrete proteins that are involved in the tone regulation of synaptic activity. Transforming growth factor beta (TGF-β1) promoted the formation of new synapses in the brain cortex by influencing the activity of astrocytic D-serine (Diniz et al., 2012). Astrocytes also secrete neuropeptide Y (NPY): the NPY gene expression in cultures of rat and human astrocytes was demonstrated (Barnea et al., 1998). The secretion of this coregulator from astrocytic granules is associated with increased levels of cytosolic Ca2+ and mGluR activation (Ramamoorthy and Whim, 2008).
Thus, by secreting vesicular gliotransmitters, astrocytes are involved in the reorganization of the synaptic process. The complex of gliotransmitter molecules creates a specific neurotransmission mechanism that ensures the network lability. It was shown in vivo with a three-dimensional reconstruction model that astrocytes in the hippocampus, as well as in the cerebral cortex, occupy nonoverlapping areas. Experiments with immunofluorescent markers made it possible to establish that one astrocyte can form contacts with numerous neurons. Based on these studies, it was hypothesized that entire synaptic islets, which are modulated by the gliotransmitter environment, may exist within the network areas (Halassa et al., 2007).
The generalization of this information led to the development of a concept that sheds new light on the role of astroglia (Araque et al., 2014). Certain logic can be traced in the understanding of the influence of astrocytes on synapses and, as a logical extension, on the functional properties of the neuronal network. Apparently, astrocytes transmit signals primarily through the high-affinity receptors by performing spatiotemporal network integration of cells via glioneuronal transmission. The following sequence of events that made it possible to characterize the role of astroglia in the brain can be constructed.
(1) The primacy of biochemical changes in astrocytes.
Monitoring via modern techniques showed that the initial cause of astrocyte activation is the Ca2+ concentration in the cytosol rather than the electrical changes in the membrane. The high biochemical and morphological dynamics of astrocytes is the basis for the creation of a single network that ensures polyvariance of neurotransmission processes.
(2) Astrocytes as functional elements of synaptic neurotransmission.
Changes in the Ca2+ level in astrocytes stimulate the release of glutamate, ATP, D-serine, and other substances that regulate neuronal excitability and synaptic transmission. The tripartite synapse structure includes the effects of various gliotransmitter molecules on the presynaptic and postsynaptic neuronal structures. Due to this specificity, astrocytes structurally and functionally integrate the extracellular space and are thus are directly involved in the information transmission over the neural network.
(3) Astrocytes as a regulatory component of an integrated neuronal network.
The interaction of an individual astrocyte with a large number of synapses allows the simultaneous activation of different neuronal populations. This effect was demonstrated in the hippocampus, where an increase in Ca2+ concentration and subsequent glutamate release led to a synchronous excitation of entire neuronal clusters. The gliotransmitter mission variability provides a great complexity of the physiological effects at the level of the tetrad: astroglia → synapse → neuron → network domains.
INFLUENCE OF ASTROCYTES ON SYNAPTIC PLASTICITY
In modern neurophysiology, it is believed that the key processes for the assessment of synaptic plasticity are long-term potentiation (LTP) and long-term inhibition (LTD), which demonstrate the strengthening or weakening of junctions between adjacent cells. Synaptic plasticity is associated with changes in the neuron structure, the distribution of dendritic spines in LTR and, conversely, their limitation in LTD. Recent studies suggest that the integrated morphofunctional construction “astrocyte–signal transmitters—synapse—neuron–synaptic potentiation/synaptic inhibition” is the basis for the interaction of nerve cells, their organization into functionally active domains, and the recording of information that is important for the reproduction of memory and cognitive processes.
The appearance of a new participant in these events, astrocytes, and the related polyphony of cytological and molecular events marks a new stage in brain research. Studies by many authors suggest that astrocytes contribute to short-term or long-term potentiation observed for individual synapses (Perea and Araque, 2007; Henneberger et al., 2010; Sibille et al., 2015). Data obtained on hippocampal sections also indicate the involvement of astrocytes in hippocampal neuronal synchronization. The activity of CA3 hippocampal cell correlated with changes in the Ca2+ level in astrocytes. It was shown that Ca2+ blockade with an injection of a chelating agent reduced the synaptic transmission in neighboring pyramidal cells, leading to desynchronization of neuronal networks (Fellin et al., 2004; Sasaki et al., 2014).
The involvement of astroglia in synaptic plasticity is based on the following cascade: Ca2+ → glutamate → NMDARs → D-serine. Knockout blockade of D-serine or the disruption of exocytosis in an individual astrocyte blocks the LTP. Thus, the Ca2+ -dependent release of D-serine regulates the NMDAR-associated plasticity, and this effect may extend to other excitatory synapses (Henneberger et al., 2010). At the same time, excitatory synaptic activity causes new expression of Ca2+ in astrocytes as a feedback response, thereby stimulating the activation of metabotropic glutamate receptors (mGluRs). The cyclicity of these processes shows that the Ca2+ waves induced in astrocytes stimulate the expression of gliotransmitters, which potentiate or inhibit synaptic transmission. With respect to ATP as a gliotransmitter, it was found that its release from astrocytes affect the excitability of neurons in a concentration-dependent manner. Using mutant mice with disrupted ATP release, it was shown that synaptic potentiation evoked weak responses. Conversely, an increased ATP release from astrocytes increased the synaptic activity of hippocampal neurons (Lee et al., 2013).
Regulated activation and the release of gliotransmitters may be important for the regulation of synchronous polarization of neuronal groups. It is a question of deviant processes of potentiation or suppression of synaptic transmission. Due to the mosaic scheme of the action of gliotransmitter, astrocytes ensure a balanced state of excitation and inhibition of the neuronal system. However, there are many instances when an altered activity of astrocytes and a shift in the equilibrium of synaptic processes lead to disturbances, including neurodegeneration, epilepsy, schizophrenia, and cognitive dysfunction, which may develop as a result of hyper- or hypoactivation of associated neurons (Elsayed and Magistretti, 2015; Sajja et al., 2016; Khaspekov and Frumkina, 2017; Neal and Richardson, 2018).
According to modern neurophysiology data, synapses are regarded as specific structures that are involved in processing and storing diverse information. New ideas about the role of astrocytes in the tone regulation of synapses allowed the proposal of the concept of heterosynaptic plasticity. Spatial binding to neurons forms tight contacts of glial branches of astrocytes through synaptic connections. By forming astrocytic networks, gliotransmitters (apparently due to a coordinated release of glutamate, ATP, D-serine, and endocannabinoids) become factors of heterosynaptic plasticity, which are involved in balancing the neuronal activity processes (Letellier et al., 2016). Therefore, it can be concluded that the dynamic interactions of astrocytes and neurons lead to changes in the synaptic network properties. Obviously, the phenomenon of heterosynaptic plasticity reflects the special role of astroglia as an integrator of the spatiotemporal determinants of the brain due to the influence on the formation of conjugated network units.
ASTROCYTE INVOLVEMENT IN THE ORGANIZATION OF THE BRAIN SYNAPTIC NETWORK
There is a substantiated assumption that the special function of astrocytes is not limited to the formation of a local tripartite synapse but may manifest itself in other, fairly remote synapses. Due to the lateral regulation of synaptic transmission, astrocytes serve as a bridge of synaptic interaction without a direct neural connection, preventing crosstalk interventions between synapses. This new concept postulates that the neuronal network function is determined by the cooperative interaction of astrocytes and neurons (Covelo and Araque, 2016). The understanding that the complex structure of brain astrocytes affects their interaction with other astrocytes, oligodendrocytes, neurons, and vascular cells greatly changes the notion of the organization of information processes in the brain. An important observation is that active astrocytes may occupy a considerable space. A working hypothesis that the astrocytes that form operational microdomains determine the plastic changes in the neuronal network can be formulated (Pasti et al., 1997; Araque and Navarrete, 2010).
Entirely new provisions on the functional mission of astrocytes were formulated with allowance for the principle of heterosynaptic plasticity (Halassa et al., 2009).
(1) Astrocytes are involved in synaptic integration, bringing together different neuronal phenotypes.
(2) Astrocytes realize a flexible scheme of diverse information processes as integrating units, in contrast to the processes that are typical for the conventional postulates about neural circuits.
(3) Integration of the neural network, which is implemented by astrocytes, may include extrasynaptic signals from vascular, immune, and other cells, which provides the optimal reprogramming of junctions in accordance with the surrounding environment.
THE NEW MISSION OF ASTROCYTES: PROLOGUE TO REGULATION OF MEMORY MECHANISMS
The sum and logics of the described results were combined to propose a revolutionary concept, according to which the special mission of astroglia contributes to the organization of the mechanisms of information functions in the brain—memory, cognitive processes, and behavior. As mentioned above, this concept was objectively substantiated in studies of the last decade and is discussed in a number of reviews (Bruel-Jungerman et al., 2007; Morris et al., 2013; Ota et al., 2013).
According to the branched network organization, astrocytes are spatially conjugated with various brain neurons. This architecture is strategically convenient for the reception and transmission of information signals, which are then transformed into the memory function. This is a question of the global scaling of neuronal networks with the involvement of the morphobiochemical complex created by astroglial cells (Stellwagen and Malenka, 2006). Additional arguments illustrate the role of astrocytes as coordinators of neuronal activity in the organization of information-storing microdomains. In addition, astrocytes are involved in memory fixation processes via integration of the neurons included in separate subastrocytic domains (Kang et al., 2005). Based on the specific morphology of astrocytes and the variability of gliotransmission factors, it was assumed that astrocytes exhibit selectivity in implementing cognitive tasks: distant communication (interlayer astroglia), integrating cooperation (protoplasmic astroglia), and metabolic support (fibrous astroglia) (Fellin et al., 2006a). It was established with electrophysiology and visual microscopy methods that astrocytes may affect the inhibitory sensory processes in the thalamic brain region (Copeland et al., 2017). This implies the assumption that the reduction in the electrical activity of the thalamus due to astrocytic expression of the mGlu2 receptor reflects the control of the sensory function as a component of learning and memory (Copeland et al., 2017).
The involvement of astroglia in the realization of contextual memory was established by computer simulation. A structure in which the astrocyte affects the induction of CA1 and CA3 hippocampal neurons, which leads to delta-rhythm tuning, was simulated. This model is significantly associated with the electrophysiological pattern of memory formation recorded in neurons (Small et al., 2001; Tewari and Parpura, 2013). In transgenic mice with vesicular protein blockade, it was shown that the inhibition of gliotransmission in astrocytes caused desynchronization of theta-oscillations in neurons at the level of the dorsal hippocampus and prefrontal cortex. Such mice had significant cognitive disorders. The addition of the gliotransmitter D-serine restored theta-synchronization and the spatial and long-term memory indices. With this elegant study as an example, the sequence of transmission processes in astrocytes and in the neuronal network with direct consequences for the cognitive function can be traced (Sardinha et al., 2017).
The chemical blockade of gliotransmitter release led to a disturbance of cognitive abilities in awake mice. A series of tests was used to illustrate the memory deficit that occurs as a result of disruption of astroglial vesicles, which secrete transmitters. These effects were accompanied by changes in the EEG. In addition, the chemical restriction of gliotransmission, which was studied on hippocampal sections, caused changes in gamma oscillations as evidence of altered synaptic plasticity. Taken together, these experimental data substantiate the role of astroglia in the control of the cognitive function (Lee et al., 2014).
The role of astrocytes in the neurochemical regulation of a complicated behavioral pattern such as fear was studied on the excitatory synapses of the central amygdala. The expression of astrocytes, adenosine secretion, and bidirectional modulation of A1 and A2A adenosine receptors reduced impulses in the amygdala neurons and, most importantly, neutralized the fear response in animals. Thus, astrocytes as transmitter signal donors are involved in the control of this behavioral pattern by selectively affecting the activity of synapses (Martin-Fernandez et al., 2017).
Thus, in addition to the generally accepted idea that astroglia functions as an elastic framework of the brain, a new ideology is substantiated. It provides evidence that astrocytes, the dominant glial cells, are an important component of the synaptic network. The notion of the role of astrocytes as gliotransmitters encourage us to revisit the conventional scheme of organization of synaptic mechanisms. The establishment of the importance of astrocytes as interneuronal integration factors testifies to its unique mission when the delicate structures of astrocytes are involved in the information field in time and space of brain structures, which leads to an understanding of the cognitive and behavioral processes of the highest order.
Two decades ago, participants of the International Program “Decade of the Brain” formulated the goal to elucidate how individual nerve cells in the brain by means of their collective interaction generate human intelligence (Jones and Mendell, 1999). At that time, it looked like a task for science fiction. The sequence synapses → neurons → information signal is still regarded as a universal mechanism for the integration of neuronal structures. However, this mechanism, which was well studied in the last century, remains insufficient for an understanding of the essence of intelligence. Astrocytes amplify the opportunity to interact with several synapses and several neurons at once in the polyphony of regulatory instruments (gliotransmitter and related signaling proteins), in the organization of information-carrying neural domains, and in the integration of the network spaces required for the reception, storage, and reproduction of information as the content base of the cognitive function. The new mission represents a significant addition to the mechanism of information communication of brain cells and, apparently, provides new opportunities for an understanding of the enigma of intelligence. A particular problem is the identification of new targets of neurodestructive disorders and traumatic and age disorders, as well as the use of means for their therapeutic correction.
CONCLUSIONS
The data obtained in cytobiochemical and physiological studies confirm the assumption that astrocytes are integral components of neural networks. A fundamentally new concept defining the mechanisms of the brain was discussed. It estimates the neuroglial system as a special organized service. Sequentially listed provisions summarize the current understanding of the role of astrocytes as the key element of the glial brain tissue.
(1) Astrocytes structurally and functionally form the extracellular space of the brain.
(2) Astrocytes function as switching cells.
(3) Astrocytes directly interacting with neurons form the tripartite synapse structure.
(4) Astrocytes form network neuronal domains—carriers of information necessary for the memory function.
(5) Disturbance or insufficiency of the gliotransmitter function affects brain activity in the form of age-related, neurodestructive, neuropsychiatric, and other diseases of the brain that are associated with memory, adaptive responses, and disorders of cognitive capacities.
The role of gliotransmitter as regulatory mediators involved in the coordination of the functioning of synapses and neurons should be emphasized. It can be assumed that astrocytes function as bridges for multiple intersynaptic communications (Halassa and Haydon, 2010). The diversity of gliotransmitters and their targets determines the multiplicity of forms of synaptic plasticity modulation. Therefore, due to the interaction with neurons, astrocytes can affect the formation of specialized neuronal networks participating in the regulation of complex behavioral acts in healthy and diseased brains.
REFERENCES
Allen, N. and Barres, B.A., Neuroscience: Glia—more than just brain glue, Nature, 2009, vol. 457, pp. 675–677. https://doi.org/10.1038/457675a
Angulo, M.C., Kozlov, A.S., Charpak, S., and Audinat, E., Glutamate released from glial cells synchronizes neuronal activity in the hippocampus, J. Neurosci., 2004, vol. 24, pp. 6920–6927.
Araque A. and Navarrete M., Glial cells in neuronal network function, Philos. Trans. R. Soc., B, 2010, vol. 365, no. 1551, pp. 2375–2381. https://doi.org/10.1098/rstb.2009.0313
Araque, A., Parpura, V., Sanzgiri, R.P., and Haydon, P.G., Tripartite synapses: glia, the unacknowledged partner, Trends Neurosci., 1999, vol. 22, no. 5, pp. 208–215.
Araque, A., Carmignoto, G., Haydon, P.G., et al., Gliotransmitters travel in time and space, Neuron, 2014, vol. 81, no. 4, pp. 728–739.
Barnea, A., Aguila-Mansilla, N., Bigio, E.H., et al., Evidence for regulated expression of neuropeptide Y gene by rat and human cultured astrocytes, Regul. Pept., 1998, vols. 75–76, pp. 293–300.
Bass, N.H., Hess, H.H., Pope, A., and Thalheimer, C., Quantitative cytoarchitectonic distribution of neurons, glia, and DNA in rat cerebral cortex, J. Comp. Neurol., 1971, vol. 143, no. 4, pp. 481–490.
Bezzi, P. and Volterra, A.A., Neuron-glia signaling network in the active brain, Curr. Opin. Neurobiol., 2001, vol. 11, no. 3, pp. 387–394.
Bourne, J.N. and Harris, K.M., Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP, Hippocampus, 2011, vol. 21, pp. 354–373.
Bruel-Jungerman, E., Davis, S., and Laroche, S., Brain plasticity mechanisms and memory: a party of four, Neuroscientist, 2007, vol. 13, no. 5, pp. 492–505.
Bushong, E.A., Martone, M.E., Jones, Y.Z., and Ellisman, M.H., Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains, J. Neurosci., 2002, vol. 22, pp. 183–192.
Cajal, S.R., Recuerdos de mi Vida, Cambridge: MIT Press, 1937.
Copeland, C.S., Wall, T.M., Sims, R.E., et al., Astrocytes modulate thalamic sensory processing via mGlu2 receptor activation, Neuropharmacology, 2017, vol. 121, pp. 100–110. https://doi.org/10.1016/j.neuropharm.2017.04.019
Covelo, A. and Araque, A., Lateral regulation of synaptic transmission by astrocytes, Neuroscience, 2016, vol. 323, pp. 62–66. https://doi.org/10.1016/j.neuroscience.2015.02.036
Dallérac, G. and Rouach, N., Astrocytes as new targets to improve cognitive functions, Prog. Neurobiol., 2016, vol. 144, pp. 48–67.
De Roo, M., Klauser, P., Garcia, P.M., et al., Spine dynamics and synapse remodeling during LTP and memory processes, Prog. Brain Res., 2008, vol. 169, pp. 199–207.
Diniz, L.P., Almeida, J.C., Tortelli, V., et al., Astrocyte-induced synaptogenesis is mediated by transforming growth factor β signaling through modulation of D-serine levels in cerebral cortex neurons, J. Biol. Chem., 2012, vol. 287, pp. 41432–41445.
Elsayed, M. and Magistretti, P.J., A new outlook on mental illness-ses: glial involvement beyond the glue, Front. Cell Neurosci., 2015, vol. 9, p. 468. https://doi.org/10.3389/fncel.2015.00468
Fellin, T., Pascual, O., Gobbo, S., et al., Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors, Neuron, 2004, vol. 43, pp. 7429–7434.
Fellin, T., Pascual, O., and Haydon, P.G., Astrocytes coordinate synaptic networks: balanced excitation and inhibition, Physiology (Bethesda), 2006a, vol. 21, pp. 208–215.
Fellin, T., Sul, J.Y., D’Ascenzo, M., et al., Bidirectional astrocyteneuron communication: the many roles of glutamate and ATP, Novartis Found. Symp., 2006b, vol. 276, pp. 208–217.
Genoud, C., Quairiaux, C., Steiner, P., et al., Plasticity of astrocytic coverage and glutamate transporter expression in mouse cortex, PLoS Biol., 2006, vol. 4, no. 11, p. e343.
Guerra-Gomes, S., Sousa, N., Pinto, L., and Oliveira, J.F., Functional roles of astrocyte calcium elevations: from synapses to behavior, Front. Cell Neurosci., 2018, vol. 11, pp. 427. https://doi.org/10.3389/fncel.2017.00427.eCollection2017
Halassa, M.M. and Haydon, P.G., Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior, Ann. Rev. Physiol., 2010, vol. 72, pp. 335–355.
Halassa, M.M., Fellin, T., Takase, H., et al., Synaptic islands defined by the territory of a single astrocyte, J. Neurosci., 2007, vol. 27, pp. 6473–6477.
Halassa, M.M., Florian, C., Fellin, T., et al., Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss, Neuron, 2009, vol. 61, pp. 213–219.
Henneberger, C. and Rusakov, D.A., Synaptic plasticity and Ca2+ signaling in astrocytes, Neuron Glia Biol., 2010, vol. 6, no. 3, pp. 141–146. https://doi.org/10.1017/S1740925X10000153
Henneberger, C., Papouin, T., Oliet, S.H., and Rusakov, D.A., Long-term potentiation depends on release of D-serine from astrocytes, Nature, 2010, vol. 463, pp. 232–236
Jones, E.G. and Mendell, L.M., Assessing the decade of the brain, Science, 1999, vol. 284, no. 5415, p. 739. https://doi.org/10.1126/science.284.5415.739
Kang, N., Xu, J., Xu, Q., et al., Astrocytic glutamate releaseinduced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons, J. Neurophysiol., 2005, vol. 94, no. 6, pp. 4121–4130.
Khakh, B.S. and Sofroniew, M.V., Diversity of astrocyte functions and phenotypes in neural circuits, Nat. Neurosci., 2015, vol. 18, no. 7, pp. 942–952.
Khaspekov, L.G. and Frumkina, L.E., Molecular mechanisms mediating involvement of glial cells in brain plastic remodeling in epilepsy, Biochemistry (Moscow), 2017, vol. 82, no. 3, pp. 380–391.
Lee, H.S., Ghetti, A., Pinto-Duarte, A., et al., Astrocytes contribute to gamma oscillations and recognition memory, Proc. Natl. Acad. Sci. U. S. A., 2014, vol. 111, no. 32, pp. 3343–3352.
Lee, H.U., Yamazaki, Y., Tanaka, K.F., et al., Increased astrocytic ATP release results in enhanced excitability of the hippocampus, Glia, 2013, vol. 61, no. 2, pp. 210–224. https://doi.org/10.1002/glia.22427
Letellier, M., Park, Y.K., Chater, T.E., et al., Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks, Proc. Natl. Acad. Sci. U. S. A., 2016, vol. 113, no. 19, pp. 2685–2694. https://doi.org/10.1073/pnas.1523717113
Loane, D.J., Stoica, B.A., and Alan, A.I., Metabotropic glutamate receptor-mediated signaling in neuroglia, Rev. Membr. Transp. Signal., 2012, vol. 1, no. 2, pp. 136–150. https://doi.org/10.1002/wmts.30
Martin-Fernandez, M., Jamison, S., Robin, L.M., et al., Synapse-specific astrocyte gating of amygdala-related behavior, Nat. Neurosci., 2017, vol. 20, no. 11, pp. 1540–1548. https://doi.org/10.1038/nn.4649
Morris, G.P., Clark, I.A., Zinn, R., and Vissel, B., Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research, Neurobiol. Learn. Mem., 2013, vol. 105, pp. 40–53. https://doi.org/10.1016/j.nlm.2013.07.002
Neal, M. and Richardson, J.R., Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration, Biochim. Biophys. Acta, Mol. Basis Dis., 2018, vol. 1864, no. 2, pp. 432–443. https://doi.org/10.1016/j.bbadis.2017.11.004
Nedergaard, M. and Verkhratsky, A., Artifact versus reality—how astrocytes contribute to synaptic events, Glia, 2012, vol. 60, no. 7, pp. 1013–1023. https://doi.org/10.1002/glia.22288
Nedergaard, M., Ransom, B., and Goldman, S.A., New roles for astrocytes: redefining the functional architecture of the brain, Trends Neurosci., 2003, vol. 26, no. 10, pp. 523–530.
Oberheim, N.A., Goldman, S.A., and Nedergaard, M., Heterogeneity of astrocytic form and function, Methods Mol. Biol., 2012, vol. 814, pp. 23–45. https://doi.org/10.1007/978-1-61779-452-0_3
Oliet, S.H. and Mothet, J.P., Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine, Neuroscience, 2009, vol. 158, no. 1, pp. 275–283. https://doi.org/10.1016/j.neuroscience.2008.01.071
Ota, Y., Zanetti, A.T., and Hallock, R.M., The role of astrocytes in the regulation of synaptic plasticity and memory formation, Neural. Plast., 2013, vol. 2013, p. 185463. https://doi.org/10.1155/2013/185463
Pasti, L., Volterra, A., Pozzan, T., and Carmignoto, G., Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ, J. Neurosci., 1997, vol. 17, pp. 7817–7830.
Perea, G. and Araque, A., Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes, J. Neurosci., 2005, vol. 25, no. 9, pp. 2192–2203.
Perea, G. and Araque, A., Astrocytes potentiate transmitter release at single hippocampal synapses, Science, 2007, vol. 317, pp. 1083–1086.
Pirttimaki, T.M., Sims, R.E., Saunders, G., et al., Astrocytemediated neuronal synchronization properties revealed by false gliotransmitter release, J. Neurosci., 2017, vol. 37, no. 41, pp. 9859–9870.
Popov, V.I., Medvedev, N.I., Rogachevskii, V.V., et al., Three-dimensional synapses and astroglia in the hippocampus of rats and ground squirrels: New structural-functional paradigms on the functioning of the synapse, Biophysics (Moscow), 2003, vol. 48, no. 2, pp. 272–291.
Ramamoorthy, P. and Whim, M.D., Trafficking and fusion of neuropeptide Y-containing dense-core granules in astrocytes, J. Neurosci., 2008, vol. 28, pp. 13815–13827.
Sajja, V.S., Hlavac, N., and VandeVord, P.J., Role of glia in memory deficits following traumatic brain injury: biomarkers of glia dysfunction, Front Integr. Neurosci., 2016, vol. 10, p. 7. https://doi.org/10.3389/fnint.2016.00007.eCollection2016
Santello, M., Cali, C., and Bezzi, P., Gliotransmission and the tripartite synapse, Adv. Exp. Med. Biol., 2012, vol. 970, pp. 307–331. https://doi.org/10.1007/978-3-7091-0932-8_14
Sardinha, V.M., Guerra-Gomes, S., Caetano, I., et al., Astrocytic signaling supports hippocampal-prefrontal theta synchronization and cognitive function, Glia, 2017, vol. 65, no. 12, pp. 1944–1960. https://doi.org/10.1002/glia.23205
Sasaki, T., Ishikawa, T., Abe, R., et al., Astrocyte calcium signaling orchestrates neuronal synchronization in organotypic hippocampal slices, J. Physiol., 2014, vol. 592, pp. 2771–2783.
Sherrington, C.S., The Integrative Action of the Nervous System, New Haven: Yale Univ. Press, 1906, p. 18.
Sibille, J., Zapata, J., Teillon, J., and Rouach, N., Astroglial calcium signaling displays short-term plasticity and adjusts synaptic efficacy, Front. Cell Neurosci., 2015, vol. 9, p. 189. https://doi.org/10.3389/fncel.2015.00189.eCollection2015
Small, S.A., Nava, A.S., Perera, G.M., et al., Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation, Nat. Neurosci., 2001, vol. 4, pp. 442–449.
Stellwagen, D. and Malenka, R.C., Synaptic scaling mediated by glial TNF-α, Nature, 2006, vol. 440, no. 7087, pp. 1054–1059.
Tewari, S. and Parpura, V., A possible role of astrocytes in contextual memory retrieval: an analysis obtained using a quantitative framework, Front. Comput. Neurosci., 2013, vol. 7, p. 145. https://doi.org/10.3389/fncom.2013.00145
Vasile, F., Dossi, E., and Rouach, N., Human astrocytes: structure and functions in the healthy brain, Brain Struct. Funct., 2017, vol. 222, no. 5, pp. 2017–2029. https://doi.org/10.1007/s00429-017-1383-5
Wu, Y.-W., Tang, X., Arizono, M., et al., Spatiotemporal calcium dynamics in single astrocytes and its modulation by neural activity, Cell Calcium, 2014, vol. 55, no. 2, pp. 119–129.
ACKNOWLEDGMENTS
This work was supported by the Basic Research Program for State Academies of Sciences for 2013–2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Gomazkov, O.A. Astrocytes as Mediators of Integration Processes in the Brain. Biol Bull Rev 9, 157–165 (2019). https://doi.org/10.1134/S2079086419020051
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086419020051