Abstract
In the last years, the classical view of glial cells (in particular of astrocytes) as a simple supportive cell for neurons has been replaced by a new vision in which glial cells are active elements of the brain. Such a new vision is based on the existence of a bidirectional communication between astrocytes and neurons at synaptic level. Indeed, perisynaptic processes of astrocytes express active G-protein-coupled receptors that are able (1) to sense neurotransmitters released from the synapse during synaptic activity, (2) to increase cytosolic levels of calcium, and (3) to stimulate the release of gliotransmitters that in turn can interact with the synaptic elements. The mechanism(s) by which astrocytes can release gliotransmitter has been extensively studied during the last years. Many evidences have suggested that a fraction of astrocytes in situ release neuroactive substances both with calcium-dependent and calcium-independent mechanism(s); whether these mechanisms coexist and under what physiological or pathological conditions they occur, it remains unclear. However, the calcium-dependent exocytotic vesicular release has received considerable attention due to its potential to occur under physiological conditions via a finely regulated way. By releasing gliotransmitters in millisecond time scale with a specific vesicular apparatus, astrocytes can integrate and process synaptic information and control or modulate synaptic transmission and plasticity.
Please note the erratum to this chapter at the end of the book.
An erratum to this chapter can be found at 10.1007/978-3-7091-0932-8_26
An erratum to this chapter can be found at http://dx.doi.org/10.1007/978-3-7091-0932-8_26
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
At the beginning of the twentieth century, Camillo Golgi (1843–1926) and Santiago Ramón J Cajal (1852–1934), using various ingenious staining and microscopic techniques, discovered a huge diversity of glial cells in the brain and found the contacts formed between glial cells and blood vessels (Ramon and Cajal 1899). Further enhancements in morphological characterization of astrocytes, thanks to the improvements in cellular labeling and imaging technologies, showed that astrocytic morphology is far more complicated than previously thought (Fig. 14.1). Ultrastructural examination of the nervous system, for instance, revealed that astrocytes can be intimately associated with synapses, literally enwrapping many pre- and postsynaptic terminals (Ventura and Harris 1999). Nonetheless, for the following decades, glial cells were still considered passive elements in the central nervous system (CNS), bearing mostly supportive and nutritional roles. The fundamental difference between neurons and astrocytes lies in their electrical excitability – neurons are electrically excitable cells whereas astrocytes (as other glial cells) are nonexcitable neural cells. Neurons are able to respond to external stimuli by generation of a plasmalemmal “all-or-none” action potential, capable of propagating through the neuronal network, although not all neurons generate action potentials. Glial cells are unable to generate action potential in their plasma membrane (although they are able to express some voltage-gated channels). The advent of modern physiological techniques, most notably the patch-clamp and fluorescent calcium dyes, has dramatically changed this image of glia as “silent” brain cells.
The structural complexity of a protoplasmic astrocyte. A single astrocyte labeled with enhanced green fluorescent protein (eGFP) contacting a large blood vessel. Insert shows astrocytic processes at higher magnification (Adapted from Nedergaard et al. (2010))
Recently, by filling single astrocytes with fluorescent dyes, it has been found that astrocytes occupy nonoverlapping spatial territories in which a single astrocyte contacts hundreds of neuronal processes and multiple neuronal cell bodies (Volterra and Meldolesi 2005; Halassa et al. 2007; Bushong et al. 2005; Fig. 14.2). The processes of one astrocyte contact tens of thousands of synapses, with more than 50% of hippocampal excitatory synapses being closely opposed to an astrocytic process (Ventura and Harris 1999) at a structure called the “tripartite synapse” to recognize the structural and functional relationship between the astrocytes and the pre- and postsynaptic elements (Perea et al. 2009).
(Left) Astrocytes of the hippocampal CA1 region filled with different florescent dyes by microinjection. Yellow represents limited overlapping regions between adjacent astrocytes. (Right) Top-view reconstruction showing a biocytin-filled layer 2/3 cortical neuron in a slice from GFAP–EGFP animals. Note that a single astrocyte enwraps different dendrites of the same neuron. Left image, courtesy of E. Bushong and M. Ellisman, The National Center for Microscopy and Imaging Research, University of California, San Diego, USA; right image (Adapted from Halassa et al. (2007))
At perisynaptic sites, astrocytes exchange information with the synaptic neuronal elements, both responding to the neuron and regulating synaptic transmission. The concept of “tripartite synapse” began with a series of evidences obtained by many laboratories during the 1990s that revealed the existence of bidirectional communication between neurons and astrocytes (Bezzi and Volterra 2001; Perea et al. 2009). The signaling pathway between neurons and astrocytes at the “tripartite synapse” is reciprocal; astrocytes sense neuronal activity by increasing intracellular levels of calcium (Ca2+) and respond by releasing a variety of different molecules (the so-called gliotransmitters; Bezzi and Volterra 2001). However, tripartite synapse has been debated because not all astrocytic calcium increases cause gliotransmitter release (Fiacco et al. 2007; Petravicz et al. 2008). The possibility for perisynaptic astrocytic processes to communicate with neurons is indeed a new concept in synaptic physiology wherein, in addition to the information flow between the pre- and postsynaptic neurons, astrocytes exchange information with synaptic elements by responding to synaptic activity and possibly by modulating synaptic transmission.
2 Astrocytes Exhibit Ca2+ Excitability
The morphology and the location of astrocytes place them in a unique position to be able to listen and respond to neuronal activity. Although nonexcitable cells and thus not equipped with the cellular machinery necessary for generating action potentials, astrocytes express a wide variety of functional neurotransmitter receptors essential for sensing neuronal activity. Many of these receptors are G-protein-coupled receptors (GPCRs) that, upon activation, stimulate phospholipase C and formation of inositol (1,4,5)-triphosphate (IP3) which increases the intracellular Ca2+ concentration through the release of Ca2+ from intracellular Ca2+ stores. In the mid-1990s, the discovery of new fluorescent tools for studying intracellular ions in living cells (in particular the Ca2+ sensors), together with the advancements of microscopy imaging technologies, provided the technical background to make breakthroughs in our understanding of astrocytes functions. Indeed, the Ca2+-imaging technique allowed many laboratories to demonstrate that in vitro and in situ astrocytes can respond to neurotransmitters released from synaptic terminals during neuronal activity with GPCR-mediated intracellular Ca2+ increases (Pasti et al. 1997; Porter and McCarthy 1996; Kang et al. 1998; Araque et al. 2002; Perea and Araque 2005; Navarrete and Araque 2008; Honsek et al. 2010). More recently, activation of this signaling pathway(s) has been demonstrated to occur in vivo in response to sensory stimulation (Wang et al. 2006; Winship et al. 2007; Petzold et al. 2008; Schummers et al. 2008; Nimmerjahn et al. 2009), suggesting that astrocytes are indeed activated during physiological running of the brain circuitry. These findings are of particular relevance because they demonstrate the existence of neuron-to-astrocyte communication and that the GPCRs appear to be the first link between neuronal activity and perisynaptic astrocytes. Thus, stimulation of GPCRs and the subsequent intracellular Ca2+ rises is now widely considered a form of glial excitability, the so-called Ca2+ excitability.
Astrocytic Ca2+ signaling in vivo (both in anesthetized and in freely moving animals) is mediated by activation of glutamate or purinergic metabotropic receptors. However, depending on the brain areas and layers, and similarly to neurons, astrocytes display considerable heterogeneity, including a markedly different pattern of Ca2+ excitation (Fiacco and McCarthy 2006; Takata and Hirase 2008). For instance, in the cerebellum, an area implicated in locomotor coordination, particular astrocytes called Bergmann glia cells exhibit three forms of Ca2+ transients in awake mice (Nimmerjahn et al. 2009). One of these subtypes (named “Ca2+ flares”) was triggered during locomotion and extended over a large network of astrocytes (at least 100 microns extension). The other two forms of Ca2+ excitation (called “sparkles” and “bursts”) appear to be restricted to individual fibers or to a maximum of 40 cells, respectively. Interestingly, the dependence of flares on locomotion and the absence of flares and near abolition of sparkles under anesthesia emphasize that studies in awake animals are essential to understand the real physiological role of intracellular Ca2+ rises in astrocytes.
Intracellular Ca2+ rises in astrocytes are not stereotyped signals; there are actually multiple and varied spatiotemporal patterns of Ca2+ elevations, which probably underlie different types of function, including generation of diverse output signals. The significance of the different features of intracellular Ca2+ events generated in astrocytes by neuronal activity, e.g., amplitude, frequency, and extent of propagation, remains, however, largely unknown. The spatiotemporal characteristics of Ca2+ events in astrocytes might be heterogeneous and dependent on the location of astrocytes. For instance, in the hippocampal CA1 region, the amplitude of astrocytic Ca2+ elevations is correlated with the number of simultaneously activated synapses (Honsek et al. 2010); in the cerebellum, instead, the extent of intracellular Ca2+ propagation, rather than the amplitude, depends on the characteristics of the neuronal input. Indeed, axonal firing at low frequency generates in Bergmann glial cells’ intracellular Ca2+ rises that are spatially restricted to the cell periphery and might represent functional independent localized microdomains (Grosche et al. 1999). The existence of localized functional domain of calcium in restricted regions of astrocytes is an intriguing observation. In fact, while most of the present literature supports the general view that intracellular Ca2+ events in astrocytes are slow and long lasting (therefore, well distinct from the neuronal ones), recent evidences reports in vitro (Marchaland et al. 2008), in situ (Santello et al. 2011; Di Castro et al. 2011), and in vivo (Winship et al. 2007) the existence of astrocytic Ca2+ responses that are as fast as in neurons (time to peak within 500 ms from stimulation). The localized and fast Ca2+ events in astrocytes are evoked by endogenous synaptic activity (Chuquet et al. 2010) or by activation of metabotropic receptors (Santello et al. 2011; Marchaland et al. 2008), occur in millisecond time scale and are therefore compatible with a physiological role in fast, activity-dependent synaptic modulation (Santello and Volterra 2009; Henneberger and Rusakov 2010).
The localized variations of Ca2+ may represent a sophisticated signaling mechanism controlling a large variety of cellular process, including the release of gliotransmitters. Our group has established that such localized Ca2+ rises are implicated in the regulated exocytosis processes of glutamatergic vesicles in vitro (Marchaland et al. 2008). By mean of total internal reflection fluorescence (TIRF) microscopy, Marchaland and colleagues observed that endoplasmic reticulum (ER) tubules come in tight apposition with the plasma membrane, forming a complex structural microdomain of submicrometer volume that most likely limits diffusion of signaling molecules, including Ca2+. In this microdomain, glutamatergic vesicles lie in the in tight spatial proximity with ER structures. Moreover, like at glutamatergic synapses, the cytoskeleton could provide a structural organization of the astrocytic microdomain. Indeed, scaffold proteins like Homer(s) could provide a molecular link between IP3 receptors located at the tip of the ER tubules and the metabotropic glutamate receptors (mGluRs) on the plasma membrane (Sala et al. 2005). The activation glutamatergic GPCRs generates, in cultured astrocytes, store-dependent submicrometer Ca2+ events characterized by fast kinetics and spatial segregation. Interestingly, most of the Ca2+ events occurred at or near sites in which glutamatergic vesicles underwent exocytosis and were in strict temporal and spatial correlation with fusion events. How the subplasma membrane Ca2+ events regulate exocytosis of glutamatergic vesicles and whether this is similar to synaptic release is not clear yet. These first findings can be relevant to understand the functional role of transmitter release from astrocytes in the brain function; indeed, the fact that fast astrocytic Ca2+ events occur in intact tissue (Winship et al. 2007; Santello et al. 2011) suggests that such Ca2+ events are not peculiarity of astrocytes in cell culture but may correspond to events taking place in astrocytes of the living brain.
3 Astrocytes Release Chemical Transmitters (Gliotransmitters)
The intracellular cascade resulting in Ca2+ rise in astrocytes is the main mechanism these cells use to transduce synaptic activity. What is the functional meaning of the intracellular Ca2+ rises in astrocytes? It is now well established that GPCR-mediated Ca2+ variations in astrocytes can trigger the release of chemical substances (the so-called gliotransmitters; Bezzi and Volterra 2001; Volterra and Meldolesi 2005). The existence of communication systems based on the release of chemical transmitters from astrocytes to other brain cells was first hypothesized at the end of the 1980s based on the observation that glial cells contain, synthesize, and release a variety of compounds (Martin 1992). At that time, however, the common view of astrocytes as passive elements was dogmatic, thus the whole concept remained largely hypothetical. Nevertheless, over the last 15 years, a number of evidences have shown that astrocytes are indeed highly secretive cells, able to release different chemical transmitters in response to an active stimulus (such as the activation of GPCRs). The term “gliotransmitters” is broad and includes a huge number of neuroactive molecules, such as (1) excitatory and inhibitory amino acids (d-serine, glutamate, aspartate, GABA, glycine, and taurine), (2) ATP and related nucleotides and nucleosides (purine nucleotides ATP), (3) eicosanoids and other lipid mediators (prostaglandins), (4) neuropeptides (proenkephalin, angiotensinogen, endothelins), (5) neurotrophins (nerve growth factor, neurotrophin-3, brain-derived neurotrophic factor), (6) cytokines (interleukins (IL), interferons (IFN), tumor necrosis factors alpha (TNFα)), (7) structurally associated chemokines, and (8) growth factors (Bergami et al. 2008; Bezzi and Volterra 2001; Blum et al. 2008; Fields and Stevens 2000; Fujita et al. 2009; Hussy et al. 2000; Kang et al. 2008; Liu et al. 2008; Medhora 2000; Sanzgiri et al. 1999; Snyder and Kim 2000). Among them, compelling evidence supporting Ca2+-dependent gliotransmission has been provided for glutamate, d-serine, and ATP.
During the last 15 years, numbers of laboratories focused their studies on mechanisms of amino acid release from astrocytes (Malarkey and Parpura 2008); several different mechanisms of release from cultured astroglia have been documented, including (1) volume-sensitive organic anion channels (Haskew-Layton et al. 2008; Kimelberg et al. 1990; Mongin and Kimelberg 2002), (2) hemichannels (Cotrina et al. 1998; Stout et al. 2002; Ye et al. 2003), (3) P2X7 receptor channels (Duan et al. 2003; Kukley et al. 2001), (4) reversed operation of reuptake carriers (Attwell et al. 1993; Longuemare and Swanson 1997; Re et al. 2006; Rossi et al. 2000; Szatkowski et al. 1990; Volterra et al. 1996), or (5) exchange via the cystine–glutamate antiporter (Allen et al. 2001; Baker et al. 2002; Bender et al. 2000; Moran et al. 2003, 2005; Shanker and Aschner 2001; Tang and Kalivas 2003), occurring via Ca2+-independent processes and pimarily under pathological conditions. Recent data, however, have suggested that a fraction of astrocytes in situ release neuroactive substances with Ca2+-dependent mechanism(s) (Bezzi et al. 1998; Fiacco and McCarthy 2004; Kang et al. 1998; Lee et al. 2007; Mothet et al. 2005; Navarrete and Araque 2008; Pascual et al. 2005; Pasti et al. 1997; Serrano et al. 2006; Jourdain et al. 2007; Yang et al. 2003; Santello et al. 2011). Whether Ca2+-dependent and independent mechanisms coexist and under what physiological or pathological conditions they occur remains unclear. However, the Ca2+-dependent exocytotic vesicular release has received considerable attention due to its potential to occur under physiological conditions via a finely regulated way.
The regulated exocytosis is a process by which secretory vesicles, via formation of the soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) complex, fuse with the plasma membrane and release their content into the extracellular space. Until few years ago, the most direct evidence that Ca2+-dependent release of chemical transmitters (most specifically glutamate) occurs via exocytosis in astrocytes came from pharmacological experiments using clostridial neurotoxins and other agents that selectively interfere with neuronal exocytosis (Bezzi et al. 1998; Pascual et al. 2001; Pasti et al. 2001). If clostridial toxins blocked release of glutamate, then astrocytes must express proteins that are substrate for these toxins. Indeed, astrocytes express the core machinery proteins involved in forming the SNARE complex, such as synaptobrevin II and its homologue cellubrevin (Bezzi et al. 2004; Jourdain et al. 2007) and SNAP-23 (Hamilton and Attwell 2010). Similar to clostridial toxins, astrocytic glutamate release is inhibited by bafilomycin A1 (Baf A1). This suggests that these cells must possess organelles expressing proton-dependent vesicular glutamate transporter (VGLUT). Baf A1, in fact, interferes with H+-ATPase, leading to alkalinization of vesicular lumen and collapsing the proton gradient necessary for VGLUT to transport glutamate into glutamatergic vesicles. This hypothesis was confirmed by studies on cultured astrocytes, where SNARE proteins colocalize with a number of vesicular organelles, including small vesicles positive for VGLUT1–3 (Bezzi et al. 2004; Kreft et al. 2004; Montana et al. 2004; Zhang et al. 2004), ATP-storing vesicles (Coco et al. 2003), neuropeptide-storing granules (Ramamoorthy and Whim 2008; Prada et al. 2011), and d-serine-containing vesicles (Martineau et al. 2008), suggesting the involvement of vesicular mechanisms in the release of these gliotransmitters. Despite these indications, a conclusive demonstration of the existence of a secretory compartment in astrocytes was unresolved until few years ago when our laboratory, in collaboration with Vidar Gundersen (University of Oslo), identified in hippocampal dentate gyrus a glutamate-storing vesicular compartment with properties similar to those of synaptic vesicles in glutamatergic terminals (Bezzi et al. 2004; Jourdain et al. 2007). In intact tissue, vesicles containing glutamate in astrocytes (1) are grouped very close to plasma membrane (about 100 nm), (2) have a clear appearance (they are not electrondense), and (3) have a small diameter (about 30–50 nm of diameter).
4 Astrocytes Possess Different Exocytotic Organelles and Contain Different Gliotransmitters
Astrocytes, like specialized professional secretory cells (exocrine, endocrine, and neurons), contain at least the three major classes of secretory organelles: the small synaptic-like microvesicles (SLMV; Bezzi et al. 2004; Jourdain et al. 2007; Bergersen and Gundersen 2009); the large dense-core granules (LDCGs ; Coco et al. 2003; Ramamoorthy and Whim 2008; Prada et al. 2011), which store and release distinct cargo; and lysosomes (Jaiswal et al. 2007; Li et al. 2008; Zhang et al. 2007). In neurons and specialized secretory cells, classical neurotransmitters and peptides are located in small SLMVs and LDCGs, respectively (Kupfermann 1991). These organelles have specialized physiological functions and are typically found in different regions of the cell. For instance, in neurons, synaptic vesicles responsible for the fast release of neurotransmitters during synaptic activity are clustered at the active zones, while peptide-containing LDCGs, typical exocytic organelles involved in maintaining the tonic level of hormones and neuropeptides in endocrine cells and neurons, are diffusively distributed in axons or dendrites (Meldolesi et al. 2004; Pickel et al. 1995). In contrast to other secretory cells, morphological, molecular, and physiological properties of the two secretory processes in astrocytes are still largely unknown. SLMVs represent the best characterized secretory organelles in astrocytes. Morphologically, they strongly resemble of synaptic vesicles of nerve terminals (Bergersen and Gundersen 2009; Bezzi et al. 2004; Crippa et al. 2006; Jourdain et al. 2007), are equipped with transport proteins for uptake of transmitters (VGLUTs), and contain glutamate and possibly d-serine (Calì et al. 2009; Marchaland et al. 2008; Mothet et al. 2006). d-Serine is a small amino acid synthesized by the enzyme serine racemase that in many brain areas represents an endogenous ligand for NMDA glutamate receptor. Fusion of d-serine-containing vesicles in astrocytes is a Ca2+-dependent process mechanically similar to those of glutamatergic SLMVs. Whether glutamate and d-serine are coreleased in the same brain areas and by the same pool of vesicles is still largely unknown. SLMVs-containing glutamate have been extensively studied during the last years; in a recent work from our laboratory, we took advantage of chimerical protein VGLUT1-pHluorin (Voglmaier et al. 2006) to study in detail the characteristics of exoendocytosis and recycling processes at the single-vesicle level with TIRF illumination (Marchaland et al. 2008). The fast imaging protocol (40 Hz) applied in these sets of experiments provided some important information on both the kinetics and modalities of exocytosis and recycling. Upon GPCR stimulation, glutamatergic SLMVs undergo Ca2+-dependent-regulated exocytosis in a burst that displays a bimodal distribution (Marchaland et al. 2008): a rapid phase sustained almost exclusively by “resident” vesicles (vesicles already docked to the plasma membrane before the stimulus) mostly undergoing kiss-and-run fusion, and a relatively slower phase sustained mainly by “newcomers” vesicles (vesicles that approach the plasma membrane after the stimulus), mostly undergoing full-collapse fusion. This duality of fusion events is reminiscent of observation in neurons where only readily releasable vesicles are rapidly recycled and reused (Harata et al. 2006). Indeed, recent observations show that this bimodal fusion is essential for activation of neuronal receptors by astrocytic glutamate (Santello et al. 2011).
There is significantly less information concerning LDCGs in astrocytes. Proteins belonging to the family of granins (such as chromogranins and secretogranins) are known to be stored in LDCGs of neuroendocrine cells together with neuropeptides and hormones (Malosio et al. 2004; Meldolesi et al. 2004; Rosa and Gerdes 1994). Therefore, granins are the most useful markers to investigate the presence of LDCGs in neurons in different areas of the mammalian brain (Meldolesi et al. 2004). In 1999, Calegari and colleagues showed for the first time that secretogranin II (SgII) is also expressed in cultured hippocampal astrocytes (Calegari et al. 1999). At the ultrastructural level, SgII appeared to be packaged in LDCGs (diameter > 100 nm) located in the Golgi apparatus and near the tubular structure of the trans-Golgi networks, where biogenesis of secretory granules is known to take place. Release of intracellularly stored SgII was evoked by treatment with various secretagogues (e.g., ionomycin, dibutyryl-cAMP, and bradykinin) in a Ca2+-dependent manner. Later on, it was found that LDCGs contain ATP and does not colocalize with the SNARE synaptobrevin/VAMP2, thus representing another, distinct population of organelles (Coco et al. 2003). Recently, Prada and colleagues (2011), moreover, found that the expression LDCVs and their regulated discharge are governed by REST (otherwise called NRSF), the transcription repressor encoded by the master gene that orchestrates differentiation of nerve cells (Ballas and Mandel 2005; D’Alessandro et al. 2008). These findings suggested the possible existence of two distinct classes of secretory vesicles in astrocytes: SLMVs and LDCGs. Therefore, astrocytes, like neurons, might have a regulated secretory pathway that is responsible for the release of multiple classes of chemical transmitters. Both of these processes may be involved in the regulation of synaptic transmission by astrocyte-released molecules (see below).
Lysosomes have been considered to be a major storage site of immune-signaling substances, such as proinflammatory cytokines (Andrei et al. 2004) and adenosine (Lukashev et al. 2004; Pisoni and Thoene 1989), and have been shown to be implicated in intercellular communication at the immunological synapse (McNeil and Kirchhausen 2005). Secretory lysosomes are also enriched in certain types of glial cells like oligodendrocytes, where they are employed for myelin proteins secretion and therefore likely play a critical role in myelination (Trajkovic et al. 2006). Additionally, recent studies have revealed that elevated calcium in astrocytes does induce a special kind of regulated secretion from secretory lysosomes (Jaiswal et al. 2007; Li et al. 2008; Zhang et al. 2007). Indeed, in astrocytes, secretory lysosomes release ATP, and blockade of this release prevents the propagation of calcium waves between neighboring astrocytes (Bowser and Khakh 2007). Although these studies have been so far focused on astrocytes in culture, a similar mechanism of release is likely to occur in vivo, as acutely isolated astrocytes express some mRNAs of proteins involved in lysosome secretion (Cahoy et al. 2008). However, activated at physiological cytosolic Ca2+ concentrations, the observed Ca2+-dependent release of astrocytic secretory lysosomes operates on timescale orders of magnitude slower than neurotransmission.
5 Gliotransmitters Modulate Synaptic Transmission (Focus on Glutamate)
For a long time, intercellular signaling underlying information transfer and processing in the brain was considered to occur exclusively between neurons. Numerous studies performed during the past few years have instead established the existence of bidirectional signaling between neurons and astrocytes. Indeed, gliotransmitters released upon synaptic stimulation such as glutamate, ATP, and d-serine are able to regulate neuronal excitability (Sasaki et al. 2011) and synaptic transmission (Araque et al. 2001; Bezzi et al. 2001a; Perea et al. 2009). These findings led to the establishment of a new concept in synaptic physiology, “the tripartite synapse,” in which astrocytes exchange information with the neuronal synaptic elements (Araque et al. 1999). Consequently, astrocytes can be considered an integral part of the synapses, being involved not only in maintaining passively the homeostatic conditions for proper synaptic transmission but also participating actively in synaptic function (Santello and Volterra 2010).
Glutamate is probably the best characterized gliotransmitter, able to modulate synaptic transmission. In the hippocampal CA1 region, astrocytes of the stratum radiatum sense the activity of Schaffer collateral afferents and respond to it with Ca2+i elevations and release of glutamate. The astrocytic glutamate acts on extrasynaptic NR2B-containing NMDA receptors located on the dendrites of CA1 pyramidal cells. Activation of such receptors results in large, slow inward currents (SICs) in the pyramidal cells able to significantly depolarize the cells and even to trigger their firing (Angulo et al. 2004; Fellin et al. 2004; Perea and Araque 2005; Navarrete and Araque 2008). Astrocyte-evoked SICs have been found to occur in two or more neighboring pyramidal cells in strict temporal correlation, which has been proposed to induce their synchronous firing. In addition, astrocytic glutamate might also activate receptors localized at presynaptic level at the same synapses. Through activation of group I metabotropic glutamate receptors (mGluRs) (Perea and Araque 2007; Navarrete and Araque 2010), astrocytes enhance the frequency of spontaneous and evoked excitatory synaptic currents. Alternatively, astrocytes induce the potentiation or depression of inhibitory synaptic transmission by activation of presynaptic kainate or II/III mGlu receptors, respectively (Liu et al. 2004a, b). In addition, it has been recently shown that glutamate release from cortical astrocytes is also able to broaden action potentials and therefore to facilitate ensuing synaptic transmission (Sasaki et al. 2011). Therefore, a single gliotransmitter can exert multiple effects depending on the sites of action and the activated receptor subtypes, which provides a high degree of complexity to astrocyte–neuron communication. This complexity becomes even higher when considering that other gliotransmitters, such as GABA, ATP, adenosine (a metabolic product of ATP), or d-serine, could have converging actions on the same neuron or, on the contrary, divergently act on several cells (both neurons and glia), thus evoking distinctive responses (Perea et al. 2009).
In our lab, we demonstrated that, at perforant path–granule cell (PP–GC) synapses in the hippocampal dentate gyrus, astrocytes of the outer molecular layer sense synaptic activity, elevate their intracellular Ca2+ elevations, and release glutamate via exocytosis of SLMVs. Indeed, by using dual patch-clamp experiments on pairs of dentate GC and molecular layer astrocytes, we have shown that direct electrical stimulation of astrocytes induces strengthening of synaptic transmission. The same effect was observed when applying an agonist of the astrocytic purinergic P2Y1 receptors (P2Y1Rs). Astrocytic glutamate is released at presynaptic level in close proximity of NR2B-containing NMDA receptors: activation of these receptors results in an increased synaptic transmitter release and in the strengthening of synaptic transmission (Jourdain et al. 2007).
This is the only neuromodulatory action of astrocytes for which a precise ultrastructural correlate has been established. Thus, we found that excitatory nerve terminals in the dentate outer molecular layer express NR2B subunits and that the distribution of such NR2B subunits is particularly abundant in the extrasynaptic terminal membrane opposed to astrocytic processes containing SLMVs (Fig. 14.3). Moreover, the astrocyte input to the synapse is blocked by introducing the tetanus toxin light chain (a specific exocytosis inhibitor) through the patch pipette into the stimulated astrocyte, indicative of an obligatory role of exocytosis for the synaptic modulation. The distance separating NR2B subunits in nerve terminals from SLMVs in surrounding astrocytic processes was found to be in the majority of the cases similar to the one separating postsynaptic receptors from “readily releasable” synaptic vesicles at the active zone of nerve terminals (Gitler et al. 2004).
Electron micrographs showing NR2B (gold particles) in extrasynaptic membranes (arrows) of nerve terminals, (Ter) making asymmetric synapses with dendritic spines (Sp) in the hippocampal dentate molecular layer. NR2B particles face astrocytic processes (Ast) containing SLMVs. There is a close proximity of NR2B to astrocytic SLMVs. Insets: higher magnification showing NR2B gold particles and astrocytic SLMVs (arrowheads). Scale bars, 100 nm (Adapted from Jourdain et al. (2007), courtesy of Nature Neuroscience)
In contrast, SICs are not observed in response to the activity of PP–GC synapses.
What is the reason for this discrepancy? A possible explanation could reside in the existence of structural–functional differences between excitatory synapses in the CA1 region and in the dentate gyrus. For instance, at PP–GC synapses, activation of presynaptic ifenprodil-sensitive NMDARs seems to predominate with respect to activation of extrasynaptic (dendritic) ifenprodil-sensitive NMDARs (Dalby and Mody 2003; Jourdain et al. 2007).
Independent from the structural–functional differences, it is interesting to note that at both CA1 and dentate synapses astrocytic glutamate is able to directly activate NMDARs. This is probably because NMDARs have much higher affinity for glutamate than all other glutamate receptors; therefore, they could be particularly suited for nonsynaptic communication that implies wider diffusion and lower local accumulation of glutamate (in the synapse, glutamate reaches mM concentrations once being released).
NMDARs should open only upon membrane depolarization: this raise an apparent paradox on how astrocyte glutamate might activate these receptors. One possible explanation might be that NMDARs targeted by astrocyte-released glutamate could have a peculiar subunit composition conferring them low sensitivity to Mg2+ block, like those present on oligodendrocytes (Burzomato et al. 2010) and in presynaptic terminals in the cortex (Larsen et al. 2011), or such receptors could be located in small volume structures that might be relatively depolarized or have high input resistance: in this case, no or very small inward currents would be sufficient to induce significant depolarization and relieve the Mg2+ block. Alternatively, other reasons might explain why NMDA receptors are often particular targets for astrocyte glutamate. For instance, together with glutamate, astrocytes might corelease depolarizing agents and/or facilitatory factors with specificity for NMDARs, notably d-serine (Mothet et al. 2005; Bergersen and Gundersen 2009; Henneberger et al. 2010). d-Serine interacts with the so-called glycine-binding site of the NMDAR, allowing its transmembrane channel to open when glutamate binds. Henneberger and colleagues (2010) showed that high frequency stimulation of Schaffer collaterals in the hippocampus gives rise to astrocyte intracellular Ca2+ increase that controls the induction of long-term potentiation by releasing d-serine. d-Serine binds to NMDA receptors to promote LTP establishment when glutamate is released from the presynaptic terminal. It is therefore theoretically possible that also opening of extrasynaptic NMDARs is facilitated by d-serine release from astrocytes, although it has been reported that glycine-binding site on presynaptic NMDARs, contrarily to the synaptic one, might be already saturated (Li and Han 2007; Li et al. 2009).
6 Gliotransmission Is Controversial
Albeit multiple experimental evidences have been accumulating during the last 15 years in favor of an active role of astrocytes in some forms of synaptic plasticity, some recent studies challenged these findings. Concerns are mainly focused on actual possibility that astrocytes may not contain the machinery to exocytose glutamate and/or that glutamate in the astrocyte cytoplasm could not be sufficient for efficient vesicular loading of the transmitter (Hamilton and Attwell 2010). Moreover, regardless of the release machinery, activation of exogenous GPCRs or knock-out of IP3R in astrocytes failed to modify synaptic transmission at hippocampal CA1 pyramidal cells (Fiacco et al. 2007; Agulhon et al. 2010).
6.1 Astrocyte Glutamate Cytosolic Levels and Vesicular Filling
Glutamate that is taken up by astrocytes is converted to glutamine by the enzyme glutamine synthetase (GS), before being passed back to synaptic terminals, in which it is converted back to glutamate. Because of the high activity of GS in astrocytes, the cytoplasmic level of glutamate is substantially lower than in neurons. This raises the question of whether a sufficiently high concentration of glutamate could be accumulated in astrocytic vesicles to activate neuronal receptors when released (Barres 2008). Nevertheless, it has to be noted that the Km of vesicular glutamate transporter is lower than that of GS, proving the opportunity for glutamate transport into vesicles (Halassa and Haydon 2010). Moreover, even assuming a partial filling of the vesicles, theoretical calculations show that the organelles would contain enough glutamate to activate extrasynaptic NMDARs (Hamilton and Attwell 2010).
Studies performed in cell culture, brain slices, acutely isolated astrocytes, and tissue sections provide compelling support for the presence of vesicular machinery for glutamatergic gliotransmission (Halassa and Haydon 2010). However, in contrast, two studies, using microarrays, have not detected the message for vesicular glutamate transporters in the astrocyte transcriptome (Lovatt et al. 2007; Cahoy et al. 2008). Further work is required to determine the reasons of such discrepancy, but there is lack of consensus in data acquired by commercially available microarray platforms given the high variability in gene expression profiles obtained from different laboratories performing seemingly identical experiments (Shi et al. 2008).
6.2 Astrocyte Involvement in Synaptic Plasticity
Molecular genetic techniques have been used to express novel GPCRs in astrocytes to ask whether their selective activation triggers glutamatergic gliotransmission (Fiacco et al. 2007; Agulhon et al. 2010). One of these receptors, MrgA1, is normally expressed by dorsal root ganglion neurons but not in the central nervous system (CNS). Expression of this receptor in astrocytes results in Ca2+ transients in astrocytes in response to the peptide ligand FLRFa. However, despite a robust volume-averaged Ca2+ signal, no SIC, modulation of basal synaptic transmission or LTP gating has been detected. Moreover, knock-out of IP3R2, believed to be astrocyte specific, does not enhance or lower baseline of CA1 pyramidal neuron synaptic activity or affects LTP (Petravicz et al. 2008; Agulhon et al. 2010). Opposite results have been instead reported at the same synapses (Fellin et al. 2004; Perea and Araque 2007; Henneberger et al. 2010) (Fig. 14.4), but some of them have been criticized as obtained with nonphysiological astrocyte manipulations (such as IP3 or Ca2+ uncaging). Indeed, achieving conditions compatible with brain physiology is an issue of fundamental importance in experimental studies in vitro (Agulhon et al. 2008). Ideally, one would aim to identify and activate selectively the molecular trigger(s) of Ca2+-dependent release in astrocytes.
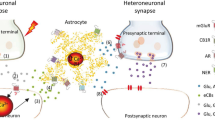
Schematic showing the current understanding of astrocytic Ca2+-signaling involvement at the synapse. (Left panel) Both in situ and in vivo studies strongly support the conclusion that synaptic release of neurotransmitters, under basal and heightened levels of stimulation, elicits Ca2+ increases in astrocytes mostly via the activation of Gq GPCRs. These astrocytic Ca2+ elevations can remain localized within small territories (microdomains) within the cell or propagate as intracellular waves into more distant compartments, depending on the level of neuronal activity. (Right panel) Whether or not astrocytic Ca2+ increases evoke the release of gliotransmitters to modulate pre- or postsynaptic metabotropic or ionotropic neuronal receptors is still under debate. To date, there are no in vivo data available, and data in situ argue both for and against the concept of gliotransmission. The potential significance of gliotransmission in neurophysiology and neuropathophysiology remains an open issue (Adapted from Agulhon et al. (2008))
One conceptual difficulty is that the current experimental techniques employed to evoke intra-astrocytic Ca2+ elevations do not reproduce the spatiotemporal aspects of physiological Ca2+ signaling. Anyway, Ca2+-dependent glutamate release from astrocytes has been associated with an action of endogenous endocannabinoids or ATP released by neurons able to modify synaptic transmission (Jourdain et al. 2007; Navarrete and Araque 2008, 2010). This disparity suggests that the origin and propagation of a physiological Ca2+ signal could depend critically on GPCR localization or, more generally, on the spatial relationships among the intracellular players involved in Ca2+ signaling in astrocytes: GPCRs, IP3Rs, Ca2+ stores, and the Ca2+-sensing molecular targets such as the trigger of transmitter release. Little is known about the intracellular distribution of such players, either on the scale of the entire astrocytic arbor or within the fine astrocyte processes that approach synaptic structures. Therefore, as a large number of information on the way astrocytes might influence neuronal functions is still not available, no definitive conclusion can be made on negative results.
7 Glutamatergic Gliotransmission and TNFα
One of the common effects of immune activation is the production of cytokines. In the CNS, cytokines are primarily produced by activated microglia, but are also generated by astrocytes and infiltrating immune cells upon brain injury (Bailey et al. 2006). They are the secreted molecules that mediate communication between immune cells and between immune system and host. Cytokines encompass a broad class of signaling molecules that have the potential to influence an immense variety of signals that regulate CNS function, including growth factor production, electrical activity, synaptic function, and axonal path finding (Carpentier and Palmer 2009).
Among the cytokine family, TNFα is well known for its proinflammatory functions in the immune system, where it is produced by a variety of cells including T cells and macrophages. In the brain, TNFα has the paradoxical ability to both protect and destroy neurons depending on a number of factors (McCoy and Tansey 2008). TNFα signals through two distinct receptors: TNF receptor 1 (TNFR1 or p55TNFR), the major mediator of proinflammatory and proapoptotic functions of TNFα, and TNFR2 (or p75TNFR), which activates more progrowth and survival pathways.
TNFα and its receptors, TNFR1 and TNFR2, are constitutively expressed in healthy brain, both in neurons and glial cells; this means that cells in the brain must be able to respond to a signaling mediated by TNFα and its receptors. For example, blocking IL-1β or TNFα by several independent means alters regulation of sleep (Imeri and Opp 2009). Other possible roles in synaptic physiology have been investigated by the labs of Malenka and Turrigiano, showing a major involvement of TNFα in synaptic plasticity and synaptic scaling (Beattie et al. 2002; Stellwagen and Malenka 2006; Kaneko et al. 2008; Steinmetz and Turrigiano 2010).
In astrocytes, recent papers reported that TNFα and its cognate receptor TNFR1 play an important role in the modulation of the regulated secretion of glutamate (Bezzi et al. 2001b; Rossi et al. 2005; Domercq et al. 2006). TNFα could directly influence glial cells potentially resulting in complex changes in the brain network. Thus, when a local inflammatory reaction is triggered in the brain, microglial cells that rapidly migrate to the injury site (Davalos et al. 2005; Nimmerjahn et al. 2005) become activated and start releasing a number of mediators such as TNFα, deeply altering the properties of glial networks (Bezzi and Volterra 2001). Indeed, TNFα at pathological concentrations appears to exert a potent control on Ca2+-dependent glutamate release from astrocytes.
The first evidence for this was reported in 2001 when Bezzi and colleagues reported two seminal observations: (1) glutamate release from astrocytes induced by the CXCR4 receptor agonist SDF1α was hampered in TNFα−/− preparations and (2) microglial TNFα release induces a massive glutamate release from astrocytes (threefold more than the one induced by other agonists) via prostaglandin PGE2 production, amplifying CXCR4-induced glutamate release (Bezzi et al. 2001b). This massive glutamate release can cause neuronal excitotoxicity both in culture and in vivo.
However, TNFα is expressed also in the normal brain, albeit at much lower levels than during inflammatory reactions and participates in homeostatic brain functions (Boulanger 2009; Vitkovic et al. 2000). In particular, constitutive TNFα has recently been implicated in control of the stability of neuronal networks in response to prolonged changes in activity via the phenomenon of synaptic scaling (Stellwagen and Malenka 2006; Turrigiano 2008) and plays a specific role in ocular dominance plasticity upon monocular visual deprivation (Kaneko et al. 2008). The TNFα released from astrocytes was able to strengthen excitatory synaptic transmission by promoting insertion of AMPA receptor subunits at the surface (Bains and Oliet 2007; Beattie et al. 2002; Stellwagen et al. 2005). The involvement of TNFα in regulating glutamate release from astrocytes during physiological conditions have been found recently by Domercq et al.; the authors showed that activation of another GPCR, the purinergic P2Y1 receptor (P2Y1R), evoked glutamate release from astrocytes via exocytosis of SLMVs (Domercq et al. 2006). Interestingly, glutamate release is impaired in TNFα knock-out and TNFR1 knock-out slices and cultures, pointing to a permissive role of the cytokine in the exocytosis of glutamate from astrocytes induced by purinergic GPCR activation (Domercq et al. 2006).
A recent paper sheds light on the way TNFα can modulate glutamate release from astrocytes and how this impinges on synaptic activity. Indeed, Santello and colleagues found that the cytokine is an essential factor for functional glutamatergic gliotransmission (Santello et al. 2011). In the hippocampal dentate gyrus, astrocytes exert a modulatory action of GC synapses via Ca2+-dependent glutamate release from astrocytes that is controlled by TNFα. In other words, the constitutive levels of the cytokine (estimated to be in the low picomolar range) need to be present for the neuromodulation to occur. Which is the mechanism(s) by which TNFα control glutamatergic gliotransmission? In astrocytes, the cytokine controls some steps of the stimulus-secretion coupling downstream of GPCR-evoked intracellular Ca2+ elevations. In particular, by using TIRF illumination, Santello and colleagues identified in cultured astrocytes obtained from TNF−/−mice, a defect in the functional docking of glutamatergic SLMVs. It appears that a constitutive TNFα level is necessary for SLMVs docking and thus of the subsequent synchronous fusion upon stimulation of metabotropic purinergic receptor (P2Y1R). Thus, in absence of the cytokine, the majority of the vesicles are far from the docking places and thus are not ready to fuse; as a consequence, the kinetics of evoked exocytosis dramatically slow down (vesicles are released over several seconds after stimulus). The asynchronous and slowly release of glutamate from astrocytes then is efficiently scavenged by the glutamate transporters (present on the astrocytic membrane) that therefore prevent presynaptic NMDARs activation and synaptic strengthening. Interestingly, TNFα absence did not alter localized Ca2+ increases in the astrocyte process, but specifically dampers vesicular fusion (Santello et al. 2011) (Fig. 14.5).
Schematic summary of the TNFα control on gliotransmission at PP–GC synapses in the hippocampal dentate gyrus. (Left) In the presence of constitutive TNFα (red diamonds), astrocyte vesicles containing glutamate (Glut, blue dots) are functionally docked at putative active zones on the plasma membrane of a perisynaptic astrocytic process. When ATP (yellow pentangles) is released (1) from GC synapses or the astrocytes (Jourdain et al. 2007), it activates P2Y1 receptors (2) and causes Ca2+ release from the internal stores in the astrocyte microcompartment (3). This in turn triggers fusion of the astrocyte vesicles in proximity of presynaptic NR2B-containing NMDARs (4), eventually causing an increase in excitatory synaptic activity (5). (Right) In the absence of TNFα, astrocytic glutamatergic vesicles are not correctly docked and ready to fuse. Therefore, when ATP triggers the usual signal transduction in astrocytes, glutamate release occurs slowly and asynchronously and is scavenged by glutamate transporters before reaching pre-NMDA receptors to induce synaptic modulation (From Santello et al. (2011))
Which could be the downstream molecule/s responsible for TNFα regulation of astrocyte exocytosis? A link could potentially exist between Rab proteins and TNFα through the protein DENN/MADD. Rab proteins, a large family of small GTPase, are central in ensuring that vesicles are delivered to their correct destinations and play an important role in the regulation of vesicle traffic and fusion in eukaryotic cells (Ferro-Novick and Novick 1993; Geppert and Südhof 1998; Stenmark 2009). Among Rab proteins, Rab3 proteins are associated with secretory vesicles of exocrine, endocrine cells (Zerial and McBride 2001), and neuronal cells (Schlüter et al. 2002) and are part of the machinery controlling exocytosis (Takai et al. 1996; Geppert and Südhof 1998; Lang and Jahn 2008). Rab3 can switch between two functionally distinct conformational states: GDP (inactive) and GTP bound (active), respectively. Rab3 GDP/GTP exchange protein (Rab3GEP also known as DENN/MADD) catalyzes the replacement of GDP by GTP and, consequently, favors the activation of Rab3.
Interestingly, it is of recent acquisition that DENN/MADD can also bind the TNFR1 through a death domain that is located at the C-terminus of the protein (Miyoshi and Takai 2004). Given that (a) when TNFα is not bound to the receptor, TNFR1–DENN/MADD interaction is extremely strong; (b) Ca2+-dependent release of glutamate from astrocytes is impaired when DENN/MADD levels are decreased (Rossi et al. 2005), an intriguing hypothesis could be that, in the absence of TNFα, DENN/MADD would be mainly bound to TNFR1 and therefore unable to function as RabGEP. Conversely, basal levels of TNFα would allow dissociation of DENN/MADD from the receptor which would promote efficient docking and fusion of SLMV in astrocytes.
References
Agulhon, C., Fiacco, T. A., & McCarthy, K. D. (2010). Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science, 327, 1250–1254.
Agulhon, C., Petravicz, J., McMullen, A. B., Sweger, E. J., Minton, S. K., Taves, S. R., Casper, K. B., Fiacco, T. A., & McCarthy, K. D. (2008). What is the role of astrocyte calcium in neurophysiology? Neuron, 59, 932–946.
Allen, J. W., Shanker, G., & Aschner, M. (2001). Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain Research, 894, 131–140.
Andrei, C., Margiocco, P., Poggi, A., Lotti, L. V., Torrisi, M. R., & Rubartelli, A. (2004). Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proceedings of the National Academy of Sciences of the United States of America, 101, 9745–9750.
Angulo, M. C., Kozlov, A. S., Charpak, S., & Audinat, E. (2004). Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. Journal of Neuroscience, 24, 6920–6927.
Araque, A., Carmignoto, G., & Haydon, P. G. (2001). Dynamic signaling between astrocytes and neurons. Annual Review of Physiology, 63, 795–813.
Araque, A., Martín, E. D., Perea, G., Arellano, J. I., & Buño, W. (2002). Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. Journal of Neuroscience, 22, 2443–2450.
Araque, A., Parpura, V., Sanzgiri, R. P., & Haydon, P. G. (1999). Tripartite synapses: Glia, the unacknowledged partner. Trends in Neurosciences, 22, 208–215.
Attwell, D., Barbour, B., & Szatkowski, M. (1993). Nonvesicular release of neurotransmitter. Neuron, 11, 401–407.
Bailey, S. L., Carpentier, P. A., McMahon, E. J., Begolka, W. S., & Miller, S. D. (2006). Innate and adaptive immune responses of the central nervous system. Critical Reviews in Immunology, 26, 149–188.
Bains, J. S., & Oliet, S. H. (2007). Glia: They make your memories stick! Trends in Neurosciences, 30, 417–424.
Baker, D. A., Xi, Z. X., Shen, H., Swanson, C. J., & Kalivas, P. W. (2002). The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of Neuroscience, 22, 9134–9141.
Ballas, N., & Mandel, G. (2005). The many faces of REST oversee epigenetic programming of neuronal genes. Current Opinion in Neurobiology, 15, 500–506.
Barres, B. A. (2008). The mystery and magic of glia: A perspective on their roles in health and disease. Neuron, 60, 430–440.
Beattie, E. C., Stellwagen, D., Morishita, W., Bresnahan, J. C., Ha, B. K., Von Zastrow, M., Beattie, M. S., & Malenka, R. C. (2002). Control of synaptic strength by glial TNFalpha. Science, 295, 2282–2285.
Bender, A. S., Reichelt, W., & Norenberg, M. D. (2000). Characterization of cystine uptake in cultured astrocytes. Neurochemistry International, 37, 269–276.
Bergami, M., Santi, S., Formaggio, E., Cagnoli, C., Verderio, C., Blum, R., Berninger, B., Matteoli, M., & Canossa, M. (2008). Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. The Journal of Cell Biology, 183, 213–221.
Bergersen, L. H., & Gundersen, V. (2009). Morphological evidence for vesicular glutamate release from astrocytes. Neuroscience, 158, 260–265.
Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S., Rossi, D., Rizzini, B. L., Pozzan, T., & Volterra, A. (1998). Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature, 391, 281–285.
Bezzi, P., Domercq, M., Brambilla, L., Galli, R., Schols, D., De Clercq, E., Vescovi, A., Bagetta, G., Kollias, G., Meldolesi, J., & Volterra, A. (2001a). CXCR4-activated astrocyte glutamate release via TNFalpha: Amplification by microglia triggers neurotoxicity. Nature Neuroscience, 4, 702–710.
Bezzi, P., Domercq, M., Vesce, S., & Volterra, A. (2001b). Neuron-astrocyte cross-talk during synaptic transmission: Physiological and neuropathological implications. Progress in Brain Research, 132, 255–265.
Bezzi, P., Gundersen, V., Galbete, J. L., Seifert, G., Steinhauser, C., Pilati, E., & Volterra, A. (2004). Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nature Neuroscience, 7, 613–620.
Bezzi, P., & Volterra, A. (2001). A neuron-glia signalling network in the active brain. Current Opinion in Neurobiology, 11, 387–394.
Blum, A. E., Joseph, S. M., Przybylski, R. J., & Dubyak, G. R. (2008). Rho-family GTPases modulate Ca(2+)-dependent ATP release from astrocytes. American Journal of Physiology: Cell Physiology, 295, C231–C241.
Boulanger, L. M. (2009). Immune proteins in brain development and synaptic plasticity. Neuron, 64, 93–109.
Bowser, D. N., & Khakh, B. S. (2007). Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. Journal of General Physiology, 129, 485–491.
Burzomato, V., Frugier, G., Pérez-Otaño, I., Kittler, J. T., & Attwell, D. (2010). The receptor subunits generating NMDA receptor mediated currents in oligodendrocytes. Journal of Physiology, 588, 3403–3414.
Bushong, E. A., Martone, M. E., Jones, Y. Z., & Ellisman, M. H. (2005). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of Neuroscience, 22, 183–192.
Cahoy, J. D., Emery, B., Kaushal, A., Foo, L. C., Zamanian, J. L., Christopherson, K. S., Xing, Y., Lubischer, J. L., Krieg, P. A., Krupenko, S. A., Thompson, W. J., & Barres, B. A. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. The Journal of Neuroscience, 28, 264–278.
Calegari, F., Coco, S., Taverna, E., Bassetti, M., Verderio, C., Corradi, N., Matteoli, M., & Rosa, P. (1999). A regulated secretory pathway in cultured hippocampal astrocytes. The Journal of Biological Chemistry, 274, 22539–22547.
Calì, C., Marchaland, J., Spagnuolo, P., Gremion, J., & Bezzi, P. (2009). Regulated exocytosis from astrocytes physiological and pathological related aspects. International Review of Neurobiology, 85, 261–293.
Carpentier, P. A., & Palmer, T. D. (2009). Immune influence on adult neural stem cell regulation and function. Neuron, 64, 79–92.
Coco, S., Calegari, F., Pravettoni, E., Pozzi, D., Taverna, E., Rosa, P., Matteoli, M., & Verderio, C. (2003). Storage and release of ATP from astrocytes in culture. The Journal of Biological Chemistry, 278, 1354–1362.
Cotrina, M. L., Lin, J. H., Alves-Rodrigues, A., Liu, S., Li, J., Azmi-Ghadimi, H., Kang, J., Naus, C. C., & Nedergaard, M. (1998). Connexins regulate calcium signaling by controlling ATP release. Proceedings of the National Academy of Sciences of the United States of America, 95, 15735–15740.
Crippa, D., Schenk, U., Francolini, M., Rosa, P., Verderio, C., Zonta, M., Pozzan, T., Matteoli, M., & Carmignoto, G. (2006). Synaptobrevin2-expressing vesicles in rat astrocytes: Insights into molecular characterization, dynamics and exocytosis. The Journal of Physiology, 570, 567–582.
D’Alessandro, R., Klajn, A., Stucchi, L., Podini, P., Malosio, M. L., & Meldolesi, J. (2008). Expression of the neurosecretory process in PC12 cells is governed by REST. Journal of Neurochemistry, 105, 1369–1383.
Dalby, N. O., & Mody, I. (2003). Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. Journal of Neurophysiology, 90, 786–797.
Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., Littman, D. R., Dustin, M. L., & Gan, W. B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience, 8, 752–758.
Di Castro MA., Chuquet J., Liaudet N., Bhaukaurally K., Santello M., Bouvier D., Tiret P., Volterra (2011). Local Ca2+ detection and modulation of synaptic release by astrocytes. Nature Neuroscience, 14, 1276–84.
Domercq, M., Brambilla, L., Pilati, E., Marchaland, J., Volterra, A., & Bezzi, P. (2006). P2Y1 receptor-evoked glutamate exocytosis from astrocytes: Control by tumor necrosis factor-alpha and prostaglandins. The Journal of Biological Chemistry, 281, 30684–30696.
Duan, S., Anderson, C. M., Keung, E. C., Chen, Y., Chen, Y., & Swanson, R. A. (2003). P2X7 receptor-mediated release of excitatory amino acids from astrocytes. The Journal of Neuroscience, 23, 1320–1328.
Fellin, T., Pascual, O., Gobbo, S., Pozzan, T., Haydon, P. G., & Carmignoto, G. (2004). Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron, 43, 729–743.
Ferro-Novick, S., & Novick, P. (1993). The role of GTP-binding proteins in transport along the exocytic pathway. Annual Review of Cell Biology, 9, 575–599.
Fiacco, T. A., Agulhon, C., Taves, S. R., Petravicz, J., Casper, K. B., Dong, X., Chen, J., & McCarthy, K. D. (2007). Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron, 54, 611–626.
Fiacco, T. A., & McCarthy, K. D. (2004). Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. The Journal of Neuroscience, 24, 722–732.
Fiacco, T. A., & McCarthy, K. D. (2006). Astrocyte calcium elevations: Properties, propagation, and effects on brain signaling. Glia, 54, 676–690.
Fields, R. D., & Stevens, B. (2000). ATP: An extracellular signaling molecule between neurons and glia. Trends in Neurosciences, 23, 625–633.
Fujita, T., Tozaki-Saitoh, H., & Inoue, K. (2009). P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia, 57, 244–257.
Geppert, M., & Südhof, T. C. (1998). RAB3 and synaptotagmin: The yin and yang of synaptic membrane fusion. Annual Review of Neuroscience, 21, 75–95.
Gitler, D., Takagishi, Y., Feng, J., Ren, Y., Rodriguiz, R. M., Wetsel, W. C., Greengard, P., & Augustine, G. J. (2004). Different presynaptic roles of synapsins at excitatory and inhibitory synapses. Journal of Neuroscience, 24, 11368–11380.
Grosche, J., Matyash, V., Möller, T., Verkhratsky, A., Reichenbach, A., & Kettenmann, H. (1999). Microdomains for neuron-glia interaction: Parallel fiber signaling to Bergmann glial cells. Nature Neuroscience, 2, 139–143.
Halassa, M. M., Fellin, T., Takano, H., Dong, J. H., & Haydon, P. G. (2007). Synaptic islands defined by the territory of a single astrocyte. The Journal of Neuroscience, 27, 6473–6477.
Halassa, M. M., & Haydon, P. G. (2010). Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annual Review of Physiology, 72, 335–355.
Hamilton, N. B., & Attwell, D. (2010). Do astrocytes really exocytose neurotransmitters? Nature Reviews Neuroscience, 11, 227–238.
Harata, N. C., Aravanis, A. M., & Tsien, R. W. (2006). Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. Journal of Neurochemistry, 97, 1546–1570.
Haskew-Layton, R. E., Rudkouskaya, A., Jin, Y., Feustel, P. J., Kimelberg, H. K., & Mongin, A. A. (2008). Two distinct modes of hypoosmotic medium-induced release of excitatory amino acids and taurine in the rat brain in vivo. PLoS One, 3, e3543.
Henneberger, C., Papouin, T., Oliet, S. H., & Rusakov, D. A. (2010). Long-term potentiation depends on release of D-serine from astrocytes. Nature, 463, 232–236.
Henneberger, C., & Rusakov, D. A. (2010). Synaptic plasticity and Ca2+ signalling in astrocytes. Neuron Glia Biology, 13, 1–6.
Honsek, S. D., Walz, C., Kafitz, K. W., & Rose, C. R. (2010). Astrocyte calcium signals at Schaffer collateral to CA1 pyramidal cell synapses correlate with the number of activated synapses but not with synaptic strength. Hippocampus. doi:10.1002/hipo.20843.
Hussy, N., Deleuze, C., Desarménien, M. G., & Moos, F. C. (2000). Osmotic regulation of neuronal activity: A new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Progress in Neurobiology, 62, 113–134.
Imeri, L., & Opp, M. R. (2009). How (and why) the immune system makes us sleep. Nature Reviews Neuroscience, 10, 199–210.
Jaiswal, J. K., Fix, M., Takano, T., Nedergaard, M., & Simon, S. M. (2007). Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proceedings of the National Academy of Sciences of the United States of America, 104, 14151–14156.
Jourdain, P., Bergersen, L. H., Bhaukaurally, K., Bezzi, P., Santello, M., Domercq, M., Matute, C., Tonello, F., Gundersen, V., & Volterra, A. (2007). Glutamate exocytosis from astrocytes controls synaptic strength. Nature Neuroscience, 10, 331–339.
Kaneko, M., Stellwagen, D., Malenka, R. C., & Stryker, M. P. (2008). Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron, 58, 673–680.
Kang, J., Jiang, L., Goldman, S. A., & Nedergaard, M. (1998). Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nature Neuroscience, 1, 683–692.
Kang, J., Kang, N., Lovatt, D., Torres, A., Zhao, Z., Lin, J., & Nedergaard, M. (2008). Connexin 43 hemichannels are permeable to ATP. Journal of Neuroscience, 28, 4702–4711.
Kimelberg, H. K., Goderie, S. K., Higman, S., Pang, S., & Waniewski, R. A. (1990). Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. The Journal of Neuroscience, 10, 1583–1591.
Kreft, M., Stenovec, M., Rupnik, M., Grilc, S., Krzan, M., Potokar, M., Pangrsic, T., Haydon, P. G., & Zorec, R. (2004). Properties of Ca(2+)-dependent exocytosis in cultured astrocytes. Glia, 46, 437–445.
Kukley, M., Barden, J. A., Steinhäuser, C., & Jabs, R. (2001). Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia, 36, 11–21.
Kupfermann, I. (1991). Functional studies of cotransmission. Physiological Reviews, 71, 683–732.
Lang, T., & Jahn, R. (2008). Core proteins of the secretory machinery. Handbook of Experimental Pharmacology, 184, 107–127.
Larsen, R. S., Corlew, R. J., Henson, M. A., Roberts, A. C., Mishina, M., Watanabe, M., Lipton, S. A., Nakanishi, N., Pérez-Otaño, I., Weinberg, R. J., & Philpot, B. D. (2011). NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nature Neuroscience, 14, 338–344.
Lee, C. J., Mannaioni, G., Yuan, H., Woo, D. H., Gingrich, M. B., & Traynelis, S. F. (2007). Astrocytic control of synaptic NMDA receptors. The Journal of Physiology, 581, 1057–1081.
Li, Y. H., & Han, T. Z. (2007). Glycine binding sites of presynaptic NMDA receptors may tonically regulate glutamate release in the rat visual cortex. Journal of Neurophysiology, 97, 817–823.
Li, P., Li, Y. H., & Han, T. Z. (2009). NR2A-containing NMDA receptors are required for LTP induction in rat dorsolateral striatum in vitro. Brain Research, 1274, 40–46.
Li, D., Ropert, N., Koulakoff, A., Giaume, C., & Oheim, M. (2008). Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. Journal of Neuroscience, 28, 7648–7658.
Liu, H. T., Sabirov, R. Z., & Okada, Y. (2008). Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal, 4, 147–154.
Liu, Q. S., Xu, Q., Arcuino, G., Kang, J., & Nedergaard, M. (2004a). Astrocyte-mediated activation of neuronal kainate receptors. Proceedings of the National Academy of Sciences of the United States of America, 101, 3172–3177.
Liu, Q. S., Xu, Q., Kang, J., & Nedergaard, M. (2004b). Astrocyte activation of presynaptic metabotropic glutamate receptors modulates hippocampal inhibitory synaptic transmission. Neuron Glia Biology, 1, 307–316.
Longuemare, M. C., & Swanson, R. A. (1997). Net glutamate release from astrocytes is not induced by extracellular potassium concentrations attainable in brain. Journal of Neurochemistry, 69, 879–882.
Lovatt, D., Sonnewald, U., Waagepetersen, H. S., Schousboe, A., He, W., Lin, J. H., Han, X., Takano, T., Wang, S., Sim, F. J., Goldman, S. A., & Nedergaard, M. (2007). The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. The Journal of Neuroscience, 27, 12255–12266.
Lukashev, D., Ohta, A., Apasov, S., Chen, J. F., & Sitkovsky, M. (2004). Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. Journal of Immunology, 173, 21–24.
Malarkey, E. B., & Parpura, V. (2008). Mechanisms of glutamate release from astrocytes. Neurochemistry International, 52, 142–154.
Malosio, M. L., Giordano, T., Laslop, A., & Meldolesi, J. (2004). Dense-core granules: A specific hallmark of the neuronal/neurosecretory cell phenotype. Journal of Cell Science, 117, 743–749.
Marchaland, J., Cali, C., Voglmaier, S. M., Li, H., Regazzi, R., Edwards, R. H., & Bezzi, P. (2008). Fast sub-plasma membrane Ca2+ transients control exo-endocytosis of SLMVs in astrocytes. Journal of Neuroscience, 28, 9122–9132.
Martin, D. L. (1992). Synthesis and release of neuroactive substances by glial cells. Glia, 5, 81–94.
Martineau, M., Galli, T., Baux, G., & Mothet, J. P. (2008). Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia, 56, 1271–1284.
McCoy, M. K., & Tansey, M. G. (2008). TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. Journal of Neuroinflammation, 5, 45.
McNeil, P. L., & Kirchhausen, T. (2005). An emergency response team for membrane repair. Nature Reviews: Molecular Cell Biology, 6, 499–505.
Medhora, M. M. (2000). Retinoic acid upregulates beta(1)-integrin in vascular smooth muscle cells and alters adhesion to fibronectin. American Journal of Physiology: Heart and Circulatory Physiology, 279, H382–H387.
Meldolesi, J., Chieregatti, E., & Luisa, M. M. (2004). Requirements for the identification of dense-core granules. Trends in Cell Biology, 14, 13–19.
Miyoshi, J., & Takai, Y. (2004). Dual role of DENN/MADD (Rab3GEP) in neurotransmission and neuroprotection. Trends in Molecular Medicine, 10, 476–480.
Mongin, A. A., & Kimelberg, H. K. (2002). ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. American Journal of Physiology: Cell Physiology, 283, C569–C578.
Montana, V., Ni, Y., Sunjara, V., Hua, X., & Parpura, V. (2004). Vesicular glutamate transporter-dependent glutamate release from astrocytes. Journal of Neuroscience, 24, 2633–2642.
Moran, M. M., McFarland, K., Melendez, R. I., Kalivas, P. W., & Seamans, J. K. (2005). Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. Journal of Neuroscience, 25, 6389–6393.
Moran, M. M., Melendez, R., Baker, D., Kalivas, P. W., & Seamans, J. K. (2003). Cystine/glutamate antiporter regulation of vesicular glutamate release. Annals of the New York Academy of Sciences, 1003, 445–447.
Mothet, J. P., Pollegioni, L., Ouanounou, G., Martineau, M., Fossier, P., & Baux, G. (2005). Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proceedings of the National Academy of Sciences of the United States of America, 102, 5606–5611.
Mothet, J. P., Rouaud, E., Sinet, P. M., Potier, B., Jouvenceau, A., Dutar, P., Videau, C., Epelbaum, J., & Billard, J. M. (2006). A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell, 5, 267–274.
Navarrete, M., & Araque, A. (2008). Endocannabinoids mediate neuron-astrocyte communication. Neuron, 57, 883–893.
Navarrete, M., & Araque, A. (2010). Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron, 68, 113–126.
Nedergaard, M., Rodríguez, J. J., & Verkhratsky, A. (2010). Glial calcium and diseases of the nervous system. Cell Calcium, 47, 140–149.
Nimmerjahn, A., Kirchhoff, F., & Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308, 1314–1318.
Nimmerjahn, A., Mukamel, E. A., & Schnitzer, M. J. (2009). Motor behavior activates Bergmann glial networks. Neuron, 62, 400–412.
Pascual, O., Casper, K. B., Kubera, C., Zhang, J., Revilla-Sanchez, R., Sul, J. Y., Takano, H., Moss, S. J., McCarthy, K., & Haydon, P. G. (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science, 310, 113–116.
Pascual, M., Climent, E., & Guerri, C. (2001). BDNF induces glutamate release in cerebrocortical nerve terminals and in cortical astrocytes. Neuroreport, 12, 2673–2677.
Pasti, L., Volterra, A., Pozzan, T., & Carmignoto, G. (1997). Intracellular calcium oscillations in astrocytes: A highly plastic, bidirectional form of communication between neurons and astrocytes in situ. Journal of Neuroscience, 17, 7817–7830.
Pasti, L., Zonta, M., Pozzan, T., Vicini, S., & Carmignoto, G. (2001). Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. Journal of Neuroscience, 21, 477–484.
Perea, G., & Araque, A. (2005). Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. Journal of Neuroscience, 25, 2192–2203.
Perea, G., & Araque, A. (2007). Astrocytes potentiate transmitter release at single hippocampal synapses. Science, 317, 1083–1086.
Perea, G., Navarrete, M., & Araque, A. (2009). Tripartite synapses: Astrocytes process and control synaptic information. Trends in Neurosciences, 32, 421–431.
Petravicz, J., Fiacco, T. A., & McCarthy, K. D. (2008). Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. Journal of Neuroscience, 28, 4967–4973.
Petzold, G. C., Albeanu, D. F., Sato, T. F., & Murthy, V. N. (2008). Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron, 58, 897–910.
Pickel, V. M., Chan, J., Veznedaroglu, E., & Milner, T. A. (1995). Neuropeptide Y and dynorphin-immunoreactive large dense-core vesicles are strategically localized for presynaptic modulation in the hippocampal formation and substantia nigra. Synapse, 19, 160–169.
Pisoni, R. L., & Thoene, J. G. (1989). Detection and characterization of a nucleoside transport system in human fibroblast lysosomes. The Journal of Biological Chemistry, 264, 4850–4856.
Porter, J. T., & McCarthy, K. D. (1996). Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. Journal of Neuroscience, 16, 5073–5081.
Prada, I., Marchaland, M., Podini, P., Magrassi, L., D’Alessandro, R., Bezzi, P., & Meldolesi, J. (2011). REST/NRSF governs the expression of dense-core vesicle gliosecretion in astrocytes. Journal of Cell Biology, 193, 537–549.
Ramamoorthy, P., & Whim, M. D. (2008). Trafficking and fusion of neuropeptide Y-containing dense-core granules in astrocytes. Journal of Neuroscience, 28, 13815–13827.
Ramon y Cajal, S. (1995). Histology of the nervous system, translated by N.Swanson and L.Swanson, Oxford University Press
Re, D. B., Nafia, I., Melon, C., Shimamoto, K., Kerkerian-Le Goff, L., & Had-Aissouni, L. (2006). Glutamate leakage from a compartmentalized intracellular metabolic pool and activation of the lipoxygenase pathway mediate oxidative astrocyte death by reversed glutamate transport. Glia, 54, 47–57.
Rosa, P., & Gerdes, H. H. (1994). The granin protein family: Markers for neuroendocrine cells and tools for the diagnosis of neuroendocrine tumors. Journal of Endocrinological Investigation, 17, 207–225.
Rossi, D., Brambilla, L., Valori, C. F., Crugnola, A., Giaccone, G., Capobianco, R., Mangieri, M., Kingston, A. E., Bloc, A., Bezzi, P., & Volterra, A. (2005). Defective tumor necrosis factor-alpha-dependent control of astrocyte glutamate release in a transgenic mouse model of Alzheimer disease. The Journal of Biological Chemistry, 280, 42088–42096.
Rossi, D. J., Oshima, T., & Attwell, D. (2000). Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature, 403, 316–321.
Sala, C., Roussignol, G., Meldolesi, J., & Fagni, L. (2005). Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. Journal of Neuroscience, 25, 4587–4592.
Santello, M., Bezzi, P., & Volterra, A. (2011). TNFa controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron, 69, 988–1001.
Santello, M., & Volterra, A. (2009). Synaptic modulation by astrocytes via Ca2+ -dependent glutamate release. Neuroscience, 158, 253–259.
Santello, M., & Volterra, A. (2010). Neuroscience: Astrocytes as aide-mémoires. Nature, 463, 169–170.
Sanzgiri, R. P., Araque, A., & Haydon, P. G. (1999). Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. Journal of Neurobiology, 41, 221–229.
Sasaki, T., Kuga, N., Namiki, S., Matsuki, N., & Ikegaya, Y. (2011). Locally synchronized astrocytes. Cerebral Cortex. [Epub ahead of print].
Schlüter, O. M., Khvotchev, M., Jahn, R., & Südhof, T. C. (2002). Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. The Journal of Biological Chemistry, 277, 40919–40929.
Schummers, J., Yu, H., & Sur, M. (2008). Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science, 320, 1638–1643.
Serrano, A., Haddjeri, N., Lacaille, J. C., & Robitaille, R. (2006). GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. Journal of Neuroscience, 26, 5370–5382.
Shanker, G., & Aschner, M. (2001). Identification and characterization of uptake systems for cystine and cysteine in cultured astrocytes and neurons: Evidence for methylmercury-targeted disruption of astrocyte transport. Journal of Neuroscience Research, 66, 998–1002.
Shi, Y., Liu, X., Gebremedhin, D., Falck, J. R., Harder, D. R., & Koehler, R. C. (2008). Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. Journal of Cerebral Blood Flow and Metabolism, 28, 111–125.
Snyder, S. H., & Kim, P. M. (2000). D-amino acids as putative neurotransmitters: Focus on D-serine. Neurochemical Research, 25, 553–560.
Steinmetz, C. C., & Turrigiano, G. G. (2010). Tumor necrosis factor-α signaling maintains the ability of cortical synapses to express synaptic scaling. The Journal of Neuroscience, 30, 14685–14690.
Stellwagen, D., Beattie, E. C., Seo, J. Y., & Malenka, R. C. (2005). Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. The Journal of Neuroscience, 25, 3219–3228.
Stellwagen, D., & Malenka, R. C. (2006). Synaptic scaling mediated by glial TNF-alpha. Nature, 440, 1054–1059.
Stenmark, H. (2009). Rab GTPases as coordinators of vesicle traffic. Nature Reviews Molecular Cell Biology, 10, 513–525.
Stout, C. E., Costantin, J. L., Naus, C. C., & Charles, A. C. (2002). Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. The Journal of Biological Chemistry, 277, 10482–10488.
Szatkowski, M., Barbour, B., & Attwell, D. (1990). Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature, 348, 443–446.
Takai, Y., Sasaki, T., Shirataki, H., & Nakanishi, H. (1996). Rab3A small GTP-binding protein in Ca(2+)-dependent exocytosis. Genes to Cells, 1, 615–632.
Takata, N., & Hirase, H. (2008). Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS One, 3, e2525.
Tang, X. C., & Kalivas, P. W. (2003). Bidirectional modulation of cystine/glutamate exchanger activity in cultured cortical astrocytes. Annals of the New York Academy of Sciences, 1003, 472–475.
Trajkovic, K., Dhaunchak, A. S., Goncalves, J. T., Wenzel, D., Schneider, A., Bunt, G., Nave, K.-A., et al. (2006). Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. The Journal of cell biology, 172(6), 937–948. doi:10.1083/jcb.200509022.
Turrigiano, G. G. (2008). The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell, 135, 422–435.
Ventura, R., & Harris, K. M. (1999). Three-dimensional relationships between hippocampal synapses and astrocytes. The Journal of Neuroscience, 19, 6897–6906.
Vitkovic, L., Bockaert, J., & Jacque, C. (2000). “Inflammatory” cytokines: Neuromodulators in normal brain? Journal of Neurochemistry, 74, 457–471.
Voglmaier, S. M., Kam, K., Yang, H., Fortin, D. L., Hua, Z., Nicoll, R. A., & Edwards, R. H. (2006). Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron, 51, 71–84.
Volterra, A., Bezzi, P., Rizzini, B. L., Trotti, D., Ullensvang, K., Danbolt, N. C., & Racagni, G. (1996). The competitive transport inhibitor L-trans-pyrrolidine-2, 4-dicarboxylate triggers excitotoxicity in rat cortical neuron-astrocyte co-cultures via glutamate release rather than uptake inhibition. European Journal of Neuroscience, 8, 2019–2028.
Volterra, A., & Meldolesi, J. (2005). Astrocytes, from brain glue to communication elements: The revolution continues. Nature Reviews Neuroscience, 6, 626–640.
Wang, X., Lou, N., Xu, Q., Tian, G. F., Peng, W. G., Han, X., Kang, J., Takano, T., & Nedergaard, M. (2006). Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nature Neuroscience, 9, 816–823.
Winship, I. R., Plaa, N., & Murphy, T. H. (2007). Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. Journal of Neuroscience, 27, 6268–6272.
Yang, Y., Ge, W., Chen, Y., Zhang, Z., Shen, W., Wu, C., Poo, M., & Duan, S. (2003). Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proceedings of the National Academy of Sciences of the United States of America, 100, 15194–15199.
Ye, Z. C., Wyeth, M. S., Baltan-Tekkok, S., & Ransom, B. R. (2003). Functional hemichannels in astrocytes: A novel mechanism of glutamate release. The Journal of Neuroscience, 23, 3588–3596.
Zerial, M., & McBride, H. (2001). Rab proteins as membrane organizers. Nature Reviews: Molecular Cell Biology, 2, 107–117.
Zhang, Z., Chen, G., Zhou, W., Song, A., Xu, T., Luo, Q., Wang, W., Gu, X. S., & Duan, S. (2007). Regulated ATP release from astrocytes through lysosome exocytosis. Nature Cell Biology, 9, 945–953.
Zhang, Q., Fukuda, M., Van Bockstaele, E., Pascual, O., & Haydon, P. G. (2004). Synaptotagmin IV regulates glial glutamate release. Proceedings of the National Academy of Sciences of the United States of America, 101, 9441–9446.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag/WIen
About this chapter
Cite this chapter
Santello, M., Calì, C., Bezzi, P. (2012). Gliotransmission and the Tripartite Synapse. In: Kreutz, M., Sala, C. (eds) Synaptic Plasticity. Advances in Experimental Medicine and Biology, vol 970. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0932-8_14
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0932-8_14
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0931-1
Online ISBN: 978-3-7091-0932-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)