Abstract—
Adsorption properties of solid solutions and binary components of the InP–ZnS system with respect to NO2 and NH3 in the temperature intervals of 250–490 K and initial pressures of 15–30 Pa have been studied for the first time. In selecting adsorbates, their unequal electron nature and toxicity is taken into account. An analysis of experimental dependences of adsorption αp = f(T), αT = f(P), and αT = f(t) and calculated values of differential heat, differential entropy, activation energy of adsorption, and electrical conductivity “behavior” under condition of adsorption is conducted. As a result, the temperature conditions for occurrence of physical and chemical activated adsorption are established. With regard to the results of IR spectroscopy, the studies of acid-base properties of the surfaces of the system components, and their charging under conditions of adsorption, the ideas are substantiated about the nature of active centers, the role of which is taken by surface coordinately unsaturated atoms (primarily In and Zn atoms) and about the donor-acceptor mechanism of gas adsorption with gases participating as acceptors. Increased activity of the surfaces of the system semiconductors with respect to basic gases, which is confirmed unambiguously by the direct adsorption studies, and also the change in electrical conductivity under conditions of adsorption even at room temperature have been the basis for recommending the studied semiconductors to be used for fabricating the corresponding low-temperature sensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
With the development of many areas of state-of-the art technology, creating novel materials, which are the more effective, the better known their properties, has been remained urgent. In this respect, there has been a growing interest in the surface and detailed information on its physicochemical properties determined by the related atomic-molecular and electron processes that necessarily start with adsorption.
Adsorption begins heterogeneous catalytic reactions; it is unavoidable even under conditions of semiconductor crystal growth, in their treatment and storage, and during fabrication and operation of devices.
From these considerations, we study adsorption properties of the novel materials, solid solutions based on binary diamond-like semiconductors of AIIIBV (InP) and AIIBVI (ZnS)–(InP)x(ZnS)1 – x type in this work.
The adsorbates (NO2, NH3) were selected on the basis of the interest, on one hand, in gases having different electron nature and, on the other hand, in toxic components of the ambient and technological media.
EXPERIMENTAL
Solid solutions of (InP)x(ZnS)1 – x (x = 3.0, 6.0, 10.0, 18.0, 85.0, 93.0, 96.0, 98.0 mol %) were obtained by the procedure developed specially for this system based on isothermal diffusion of original binary compounds (InP, ZnS) and available information on their physical and physicochemical properties by using a substantiated mode and a thermal heating program [1, 2].
The obtained solid solutions were certified according to the results of the X-ray examinations and the results of micro- and electron-microscopic studies. The studied samples were prepared as fine powders (Sspec = 0.34–1.35 m2/g) and nanofilms (d = 20–90 nm).
The X-ray examinations were conducted on a Bruker AXS D8 Advance diffractometer (Germany) in CuKα radiation (λ = 0.15406 nm, T = 293 K) by surveying in the region of far corners [3, 4] with a Lynxeye position sensitive detector, as well as using the IC-DDIPDF-2 powder diffraction database and a TOPAS 3.0 (Bruker) software for interpreting X-ray patterns (XRD patterns) and refining the parameters of the lattices, respectively; the microscopic studies were carried out on KN 8700 (Xilox, Japan) and POLAR-3 Micromed devices with a resolving capacity up to 7000 [5]; the electron-microscopic studies were made on a JSM-5700 scanning electron microscope equipped with a JED-2300 attachment for energy-dispersive analysis [6].
The adsorption properties (with respect to NO2, NH3) were studied by the piezoquartz microweighing method (sensitivity of 1.23 × 10–11 g/cm2 Hz) and by the volumetric method [7] (within the temperature of 250–490 K and pressure of 15–30 Pa) together with methods of IR spectroscopy of multiple frustrated total internal reflection (IRS MFTIR) and determination of electrical conductivity (σ). IR spectra were obtained on a InfraLum FT-02 Fourier spectrometer with a MFTIR attachment; σ was determined by the probe compensation method [1, 7].
Adsorbate gases (NO2, NH3) were obtained by the known techniques [8].
The reproducibility and accuracy of the experimental data were verified by the results of parallel measurements by means of the methods of mathematical statistics and processing of the results of quantitative analysis, as well as using Stat-2, Microsoft Excel, and Origin software.
DISCUSSION
With the required criteria met, the results of the X‑ray examinations unambiguously indicated the formation of substitutional solid solutions with a cubic sphalerite structure in the InP–ZnS system (at the assigned compositions) [2]. These results also indirectly confirm and significantly supplement the results of micro- and electron-microscopic studies, which served as the basis for determining the surface structures, the average sizes (lavg), and the average numbers (navg) of the most representative particles of the system components, their elemental compositions being almost coincident with the prescribed molar compositions [9].
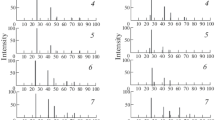
Figures 1 and 2 present the typical experimental adsorption dependences for NO2 and NH3: αp = f(T), αT = f(P), and αT = f(t). Even the appearance of the adsorption isobars (α = f(T)) suggests the occurrence of physical adsorption at T < 298 K and primarily chemically activated adsorption at higher temperatures. This suggestion is confirmed by the results of analyzing the equilibrium and kinetic adsorption isotherms and the estimated values of activation energy (Eα), differential heat (qα), and differential entropy (‒ΔS) of adsorption.
The adsorption equilibrium isotherms α = f(P) (Fig. 2a) obey the Langmuir equation (linearized in coordinates 1/α–1/P) (Fig. 3a) [7]; the adsorption kinetic isotherms α = f(t) (Fig. 2b) adhere to the Roginsky–Zeldovich–Elovich equation (linearized in coordinates α–RT log t) (Fig. 3b) [7], which is valid for the surfaces with a uniformly inhomogeneous character of distribution. Its execution was the basis for using a method for “controlling band” [7] to find the average values of activation energy of adsorption at T > 298 K and different filling of the surfaces (the values of α). They amount to ≥18 kJ/mol.
Differential heat of adsorption (qα) was calculated by the Clausius–Clapeyron equation (for the descending segments of isobars) [7] and the semiempirical equation proposed in [10] (for all segments of isobars); the differential entropies of adsorption (–ΔS) were found by the equation [7]
where qα is the differential heat of adsorption, T is the temperature, R is the molar gas constant, P is the equilibrium pressure at the specified T, and P 0 is the standard pressure (P 0 = 1 atm = 133.3 × 760 Pa).
At different temperatures (250–490 K) and the different filling of the surfaces, the values of differential heat of gas adsorption (NO2, NH3) amount to 4.2–12.4 kJ/mol, and the values of the differential entropy of adsorption (–ΔS) are ≥78.3 J/mol K.
The growth in activation energy (Eα) and the decrease in heat (qα) and entropy (i.e., the growth in ‒ΔS) of adsorption with filling of the surfaces indicate their inhomogeneity and the presence of active centers being different in force and energy state. This is confirmed by the results of nonaqueous conductometric titration: the differential curves of dependence of electrical conductivity of the equilibrium mixture (a dispersed component of the InP–ZnS + solvent + methyl-ethyl ketone system) on the volume of titranate (potassium ethylate) contain three or more peaks each [9, 11].
As for the nature of the active centers, in accordance with the results of determining pH of the isoelectric state of the component surfaces in the system, pHiso, and the results of the mechanochemical studies [2, 9, 11], coordinately unsaturated atoms, adsorbed water molecules, and OH– groups may play this role. In this respect, the absolute values of pHiso (<7), their increase under the action of main gas (NH3) and at an increase in more ionic ZnS component and consequently having a more hydrated surface in the InP–ZnS system (from 6.0 to 6.6), and also the smaller values of gas adsorption on ZnS than on InP (Fig. 1) are indicative of a relatively prevailing role of coordinately unsaturated atoms of the Lewis centers [2, 11].
It is pertinent to note the increased adsorbability of the main gas (NH3) compared to acid gas (NO2) (Fig. 1), which directly (as a result of direct adsorption studies) confirms the increased adsorption activity of the component surfaces in the InP–ZnS system with respect to the main gases, which is projected from the results of studying the acid-base properties (the calculations of pHiso) [2] and an additionally relatively dominant role of coordinately unsaturated atoms as active centers.
On the basis of the above and in accordance with the results of the adsorption studies on the other diamond-like semiconductors [1, 10, 11], it becomes evident that the adsorption of NO2 and NH3 occurs by the donor-acceptor mechanism with the involvement of surface coordinately unsaturated atoms as acceptors (primarily the A–In, Zn atoms having more pronounced metallic properties).
Hence, we logically represent the adsorption interactions of NO2 and NH3 by the schemes [1, 11]
The formation of such donor-acceptor bonds with the involvement of surface coordinately unsaturated atoms is revealed by the IR spectra of MFTIR exposed in the surface air that include the stretching bands of adsorbed coordinately bonded molecules of H2O and CO2 (H2O+δ—A–δ, \({\text{CO}}_{2}^{{ + \delta }}\)—A–δ) (Fig. 4).
As part of deepened clarification of the mechanism of gas adsorption and recognition of the role, along with a local factor, it is expedient to determine the “behavior” of electrical conductivity under conditions of gas adsorption.
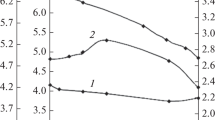
The results of such studies are presented in Fig. 5.
The temperature dependence of electrical conductivity of a solid solution in this case (InP)0.18(ZnS)0.82 in vacuum is typical of semiconductors and obeys the exponential law [7]
Under the action of adsorbed ammonia, the electrical conductivity grows (Eσ decreases accordingly), and the higher the temperature, the more noticeable the growth (Fig. 5).
The mere fact of the change in electrical conductivity under conditions of adsorption indicates the charge and the change in the electron state of the surface, which is possible during chemical interaction and consequently during the formation of a single quantum-chemical “adsorbent-adsorbate” system [1, 7, 12]. This in turn indicates the dependence of the adsorbability of NH3 molecules not only on a local factor (chemical properties of the adsorbate molecules and active centers) but also on a collective (electron) factor, which is determined by the Fermi level position and the character of the energy spectrum of the surfaces [1, 7, 12]. That is under the mentioned formation of \({\text{NH}}_{3}^{{ + \delta }}\)—A–δ donor-acceptor bonds, free carriers are necessarily involved in the elementary act of adsorption, although gas adsorption selectivity is determined by the local properties of the surface [1, 7, 10, 12].
It is pertinent to note that the concentration of free carriers (n electrons and p holes) in crystalline solids is related to the width of the band gap and the Fermi level position therein [7]:
where E1 and E2 are the energies corresponding to the upper edge of the valence band and the lower edge of the conduction band, EF is the energy corresponding to the Fermi level, K is the Boltzmann constant, and C is a constant that depends on the effective mass of carriers and temperature.
From this follows validity of the statement by F.F. Volkenstein (within the framework of the electron theory of adsorption and catalysis) about the role of the Fermi level as a regulator of adsorption and catalytic activity [7, 13].
In terms of the feasibility of using the studied semiconductors in the sensor technology, increased activity of their surfaces with respect to the main gases, which is confirmed by the results of direct adsorption studies, and the change in electrical conductivity observed even at room temperature during adsorption deserve consideration.
CONCLUSIONS
Adsorption properties of the binary and multicomponent semiconductors of the InP–ZnS system with respect to NO2 and NH3 having different electron nature and being toxic components of the surrounding and processing media were considered for the first time.
On the basis of the analysis of the experimental dependences of adsorption, the calculated values of differential heat and entropy, the activation energy of adsorption, and the results of determining electrical conductivity under conditions of adsorption, the temperature conditions were established for occurrence of physical and chemical activated adsorption.
With consideration of the results of the earlier studies of acid-base properties of the surfaces of the components of the InP–ZnS system, the suggestions about the nature of active centers and the adsorption mechanism were made and substantiated IR-spectroscopically: adsorption of NO2 and NH3 occurs by the donor-acceptor mechanism with surface coordinately unsaturated atoms (primarily In and Zn atoms) acting as acceptors.
The direct adsorption studies unambiguously confirmed the increased activity of the surfaces of the components of the InP–ZnS system with respect to the main gases, which was revealed according to the results of studying the acid-base properties. In view of this fact and the change in electrical conductivity under conditions of adsorption even at room temperature, we recommend them for fabricating the corresponding low-temperature sensor devices.
REFERENCES
Kirovskaya, I.A., Fiziko-khimicheskie svoistva binarnykh i mnogokomponentnykh almazopodobnykh poluprovodnikov (Physical and Chemical Properties of Binary and Multicomponent Diamond-Like Semiconductors), Novosibirsk: Sib. Branch Russ. Acad. Sci., 2015.
Kirovskaya, I.A., Ekkert, R.V., Ekkert, A.O., et al., New materials based on InP–ZnS system for semiconductor gas analyzers, Omsk. Nauchn. Vestn., 2019, no. 2 (164), pp. 56–61.
Gorelik, S.S., Rastorguev, L.N., and Skakov, Yu.A., Rentgenograficheskii i elektronnoopticheskii analiz (X‑Ray and Electron-Optical Analysis), Moscow: Metallurgiya, 1970.
Smyslov, E.F., Express X-ray method of nanocrystal materials lattice parameter determination, Zavod. Lab. Diagn. Mater., 2006, vol. 72, no. 5, pp. 33–35.
Clarke, A.R. and Eberhardt, C.N., Microscopy Techniques for Materials Science, Cambridge: Woodhead, 2002.
Goldstein, J.I., Newbury, D.E., Echlin, P., Joy, D.C., Fiori, C., and Lifshin, E., Scanning Electron Microscopy and X-Ray Microanalysis, New York–London: Plenum, 1981.
Kirovskaya, I.A., Adsorbtsionnye protsessy (Adsorption Processes), Irkutsk: Irkutsk. Gos. Univ., 1995.
Rapoport, F.M. and Il’inskaya, A.A., Laboratornye metody polucheniya chistykh gazov (Laboratory Methods for Preparation of Pure Gases), Moscow: Goskhimizdat, 1963.
Kirovskaya, I.A., Mironova, E.V., Kosarev, B.A., Nor, P.E., and Bukashkina, T.L., Bulk and surface properties of ZnTe–ZnS system semiconductors, Russ. J. Phys. Chem. A, 2016, vol. 90, pp. 2029–2034. https://doi.org/10.1134/S0036024416100174
Kirovskaya, I.A., Poverkhnostnye svoistva almazopodobnykh poluprovodnikov. Adsorbtsiya gazov (Surface Properties of Diamond-Like Semiconductors. Adsorption of Gases), Irkutsk: Irkutsk. Gos. Univ., 1984.
Kirovskaya, I.A., Nor, P.E., Mironova, E.V., and Kirovskaya, T.A., Adsorbenty na osnove sistem tipa A II -B VI –A II B VI —materialy dlya poluprovodnikovogo gazovogo analiza (Adsorbents Based on AIIBVI–AIIBVI Systems: Materials for Semiconductor Gas Analysis), Novosibirsk: Sib. Branch Russ. Acad. Sci., 2018.
Kiselev, V.F., Poverkhnostnye yavleniya v poluprovodnikakh i dielektrikakh (Surface Phenomena in Semiconductors and Dielectrics), Moscow: Nauka, 1970.
Vol’kenshtein, F.F., Elektronnye protsessy na poverkhnosti poluprovodnikov pri khemosorbtsii (Electron Processes on Semiconductor’s Surfaces under Chemisorption), Moscow: Nauka, 1987.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Mukhortova
Rights and permissions
About this article
Cite this article
Kirovskaya, I.A., Ekkert, R.V., Ekkert, A.O. et al. Adsorption Properties of Novel Materials Based on the InP–ZnS System. Inorg. Mater. Appl. Res. 13, 1089–1093 (2022). https://doi.org/10.1134/S2075113322040190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113322040190